Abstract
Background
Limited data exist regarding the potential impact of assisted reproductive technology (ART) on cardiac remodeling. In particular, whether different ART techniques are related to different cardiac alterations remains unclear. We aimed to evaluate cardiac changes in fetuses and infants arising from ART and fetal cardiac alterations in fetuses conceived by specific ART procedures.
Methods
This prospective and observational cohort study recruited 111 fetuses conceived by ART and 106 spontaneously conceived controls between December 2017 and April 2019. Echocardiography was performed between 28+0 and 32+6 weeks-of-gestation and at 0–2 and 6 months after birth.
Results
A total of 88 ART fetuses and 85 controls were included in the final analysis. Compared to controls, ART fetuses demonstrated a globular enlarged left ventricle (LV) (LV sphericity index of mid-section, 2.29 ± 0.34 vs. 2.45 ± 0.39, P = 0.006; LV area, 262.33 ± 45.96 mm2 vs. 244.25 ± 47.13 mm2, P = 0.002), a larger right ventricle (RV) (RV area, 236.10 ± 38.63 mm2 vs. 221.14 ± 42.60 mm2, P = 0.003) and reduced LV systolic deformation (LV global longitudinal strain (GLS), −19.56% ± 1.90% vs. −20.65% ± 1.88%, P = 0.013; LV GLS rate S, −3.32 ± 0.36 s-1 vs. −3.58 ± 0.39 s-1, P = 0.023). There were no significant differences between the ART and control groups at postnatal follow-ups. Furthermore, we found fetal cardiac morphometry and function were comparable between different ART procedures. Compared to controls, the fetuses derived from various ART procedures all exhibited impairments in the LV GLS and the LV GLS rate S.
Conclusions
Our analysis demonstrated that subclinical cardiac remodeling and dysfunction were evident in ART fetuses, although these alterations did not persist in early infancy. In addition, various ART procedures may cause the same unfavorable changes in the fetal heart.
Trial registration
This trial was registered at the Chinese Clinical Trial Registry (www.chictr.org.cn) (ChiCTR1900021672) on March 4, 2019, retrospectively registered.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Assisted reproductive technologies (ARTs) have allowed millions of infertile couples to achieve pregnancy. Current estimates state that over 8 million babies have been born by ART, with a significant upward trend over recent years [1]. Given the significant global uptake of ART, there are universal concerns relating to the potential impact of ART on the health of future generations.
According to the theory of fetal programming of cardiovascular disease [2, 3], there is a possibility that ART may predispose apparently healthy offspring to permanent reprogramming of cardiovascular development [4, 5]. However, only a handful of studies have focused on the effects of ART on cardiac changes. A previous cohort study of ART offspring demonstrated cardiac remodeling and dysfunction in utero that persisted into postnatal life [6, 7]. In another study, cardiac dysfunction was observed in ART children but not changes in cardiac morphometry [8]. Another study demonstrated right ventricle (RV) enlargement and diastolic dysfunction in ART pre-adolescents under high-altitude conditions [9]. It is clear that only limited data exist regarding the impact of ART on cardiac remodeling and that the exact pattern of cardiac changes remains controversial.
The evident differences between published studies could be explained by potential confounders, at least in part. For instance, ART is associated with an increased prevalence of advanced maternal age, twin pregnancy, prematurity, and low birth weight [10]; these factors are all known to be associated with cardiac remodeling or dysfunction [11,12,13,14]. On the other hand, the heterogeneity between ARTs used in different research studies might play a role in the poor consistency in previous findings. For example, Valenzuela-Alcaraz et al. [6] and von Arx et al. [9] both recruited cases that were conceived by in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI), while Zhou et al. [15] also included children that were conceived by oocyte donation and artificial insemination. Liu et al. [8] and Xu et al. [16] only studied children that were conceived by IVF. Furthermore, the proportions of subjects conceived by frozen embryo transfer (FET) have also proven to be variable in previous studies.
Accordingly, we designed a prospective cohort study that was controlled for these potential confounding factors and investigated cardiac alterations that were related to ART in fetuses and infants. We also investigated cardiac changes in fetuses conceived by specific ART processes. Because cardiac dysfunction may be subtle, we used speckle-tracking echocardiography (STE) to measure deformation of the myocardium in ART offspring.
Methods
Study
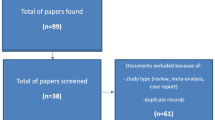
This was a prospective and observational cohort study; the detailed protocol was published previously [17]. Initially, we recruited 111 fetuses that had been conceived by ART and 106 controls at the gestational age of 28+0 to 32+6 weeks between December 2017 and April 2019 at the Department of Ultrasound, Shengjing Hospital of China Medical University, Shenyang, China. All participants were singletons, and without any structural or chromosomal anomalies, evidence of infection, or any maternal medical diseases [17]. Twenty-three ART fetuses and 21 controls were excluded due to prematurity, smoking during pregnancy, or small/large-for-gestational age (Fig. 1). Data from 88 ART conceived fetuses and 85 controls were included in the final analysis.
The time points of the two follow-up visits were set at 0–2 and 6 months after birth. At each of the three visits, the participants underwent a standard protocol to receive cardiac assessment, including conventional echocardiography and STE. The Ethics Committee of Shengjing Hospital of China Medical University approved the study, and written informed consent was obtained from all parents. The trial registration number for the present study is ChiCTR1900021672.
Fetal assessment
During prenatal evaluation, two-dimensional fetal ultrasound examinations were performed with an EPIQ 7C ultrasound system (Philips Ultrasound, Inc., Bothell, Washington) equipped with 3–5 MHz curved-array (C5-1) and 1–5 MHz sector-array (S5-1) probes. The same experienced operator (B-WJ) conducted all examinations, including the assessment of estimated fetal weight, placental Doppler, and fetal echocardiography [17].
Fetal echocardiography included a comprehensive examination to assess structural heart integrity, cardiac morphometry, and function, the latter was evaluated by both conventional echocardiography and STE analysis [17]. For STE, we acquired videos for offline analysis, including the apical or basal 4-chamber, long-axis, and 2-chamber views, as well as basal-, mid-, and apical-short-axis views. Images were analyzed by a single experienced operator (B-WJ) with QLAB software (10.8 version; Philips Healthcare, Andover, Massachusetts) [17]. The results of STE analysis included values for biventricular mid-myocardial global longitudinal strain (GLS), GLS rate, longitudinal time to peak (T2P) strain, and standard deviation of left ventricle (LV) longitudinal T2P strain, as well as LV mid-myocardial global circumferential strain (GCS), GCS rate, circumferential T2P strain, and standard deviation of circumferential T2P strain. We also generated bull’s-eye maps for LV longitudinal and circumferential strain. Global parameters were calculated by segmental averaging; for example, RV GLS was measured as the mean value of three lateral segments and the LV GLS and GCS of all 18 segments. To better compare T2P strain and LV dyssynchrony in different heart rates, all cardiac cycles were adjusted to 400ms.
Postnatal assessment
Postnatal echocardiography, including conventional morphological and functional evaluations and STE analysis, was performed by the same skilled operator (B-WJ) using an EPIQ 7C ultrasound system (Philips Ultrasound, Inc., Bothell, Washington) equipped with a 5–12 MHz sector-array (12S) probe. Infants with congenital heart defects that were not diagnosed prenatally were excluded from the follow-up visits (Fig. 1).
Statistical analysis
The IBM SPSS software (version 24; SPSS Inc., Chicago, IL) was used for analysis. Data are presented as mean ± standard deviation (SD), median (interquartile range), or percentage (number) where appropriate. Categorical variables were compared using the Pearson’s χ2 test, the continuity-adjusted χ2 test, or Fisher’s exact test. Comparisons of continuous variables between the ART and control groups were performed by independent t tests and adjusted for association to confounding factors by linear regression. Analysis of variance (ANOVA) was used to compare three groups with Bonferroni correction if P < 0.05. The Kruskal-Wallis test was used when we observed heterogeneity of variance between groups. Body surface area was calculated according to the Meban formula [18]. All P values were two-tailed, and P < 0.05 was considered statistically significant.
Inter-observer and intra-observer variability were assessed as previously described [17]. Speckle-tracking assessments were repeated in 15 prenatal and 15 postnatal randomly selected cases by a second observer (X-YJ) blinded to the group assignment and the prior measurement and by the first observer (B-WJ) after an interval of at least 3 months, respectively. Intraclass correlation coefficients (ICCs) were calculated for repeatability and reproducibility. ICCs > 0.80 were recognized as excellent and 0.60 to 0.80 as good.
Results
Cardiac changes in ART fetuses and infants
Baseline and perinatal characteristics were similar when compared between the ART and control groups, except for the higher prevalence of nulliparity (P < 0.001), lower maternal (P = 0.008) and paternal (P = 0.014) educational level, and higher rates of cesarean section (P = 0.001) in the ART group (Table 1). Maternal age, gestational age at delivery, and birth weight were all comparable between the two groups; these are all factors that are known to influence cardiac changes [11, 13, 14].
Fetal assessment results are shown in Table 2. There was no significant difference in gestational age at ultrasound examination or estimated fetal weight when compared between groups. Placental Doppler parameters were similar between groups except for a lower middle cerebral artery pulsatility index (PI) (P = 0.013) and mean uterine artery PI (P = 0.010) in the ART group. Fetuses conceived by ART were associated with larger LV (P = 0.002) and RV (P = 0.003) areas and a lower LV sphericity index of mid-section (P = 0.006) (Fig. 2). Assessments of conventional echocardiographic function were similar when compared between the two groups, except for increased mitral E′ (P = 0.046) in ART fetuses. Deformation analysis for LV revealed that fetuses conceived by ART showed significant reductions in GLS (P = 0.013) (Fig. 2) and GLS rate S (P = 0.023) after adjusting for gestational age at scan, estimated fetal weight, parity, sex of offspring, gestational diabetes, mean uterine artery PI, frame rate, and LV area. With regard to RV STE analysis, there was no significant difference between the groups with respect to systolic and diastolic deformation.
Study design and main findings. *Compared with the SC group by Bonferroni correction. ART, assisted reproductive technology; ET, embryo transfer; FET, frozen embryo transfer; GLS, global longitudinal strain; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; LV, left ventricle; RV, right ventricle; SC, spontaneous conception
The characteristics of the infants at first follow-up are shown in Additional file 1: Table S1 while the results of the anthropometry and cardiac assessment are shown in Additional file 1: Table S2. There was no significant difference in age or body surface area between groups at the time of the scan. Results derived from conventional echocardiography were comparable between the ART and control groups, with except for a thinner relative RV wall thickness (P = 0.028) and higher tricuspid E/A (P = 0.046) in infants born by ART. In addition, there were no significant differences in deformation analysis when compared between the two groups.
The characteristics of the infants at second follow-up are shown in Additional file 1: Table S3. The results derived from anthropometry and cardiac assessment are presented in Additional file 1: Table S4. The age at evaluation was similar between the two groups, whereas body surface area was higher (P < 0.001) in infants born via ART. There was no significant difference between the two groups with respect to conventional echocardiographic parameters and deformation analysis (Fig. 2).
Cardiac alterations of fetuses conceived by specific ART techniques
The baseline characteristics of fetuses conceived by specific ART procedures are summarized in Additional file 1: Tables S5 and S6. There was no significant difference in cardiac morphometry and function when compared between IVF and ICSI groups or between fresh embryo transfer (ET) and FET groups (Fig. 2 and Additional file 1: Tables S7 and S8). Compared with controls, IVF fetuses showed a lower mean uterine artery PI (P = 0.031), larger RV (P = 0.038), lower LV sphericity index of mid-section (P = 0.016) and of base-section (P = 0.035) and a decreased LV GLS (P = 0.007), LV GLS rate S (P < 0.001), and LV GCS (P = 0.039). ICSI cases demonstrated a lower mean uterine artery PI (P = 0.019), decreased mitral (P = 0.021) and tricuspid ring displacement (P = 0.027), higher mitral E’ (P = 0.036), along with decreased LV GLS (P = 0.002), LV GLS rate S (P = 0.001), and LV GCS rate A (P = 0.007) when compared to controls. Moreover, the fresh ET group was associated with a lower LV GLS (P = 0.048), LV GLS rate S (P = 0.003), and LV GCS rate S (P = 0.044), when compared to fetuses conceived spontaneously. In the FET group, fetuses showed a lower mean uterine artery PI (P = 0.001) and LV sphericity index of mid-section (P = 0.005), larger LV (P = 0.012) and RV (P = 0.035), decreased relative LV wall thickness (P = 0.031) and mitral (P = 0.017) and tricuspid ring displacement (P = 0.011), impaired LV GLS (P = 0.002), LV GLS rate S (P < 0.001), LV GLS rate A (P = 0.046), and LV GCS rate A (P = 0.022), as compared to controls. Collectively, these data show that the common features of cardiac change in ART subgroups were a reduction in LV GLS and LV GLS rate S (Fig. 2).
Intra- and inter-observer variability
With regard to the intra- and inter-observer variability of prenatal measurements, we found that the ICCs were excellent or good for LV deformation parameters. With regard to the intra- and inter-observer variability of postnatal measurements, we found that the ICCs were excellent for almost all LV deformation parameters (Additional file 1: Table S9).
Discussion
In this prospective and observational cohort study, we found that subclinical changes associated with cardiac morphology and function were detectable in ART fetuses. However, these alterations did not persist in the early infantile period (Fig. 2).
Consistent with previous studies showing cardiac morphological changes in ART fetuses [6, 19], in the present study, we demonstrated a globular enlarged LV and a larger RV in ART fetuses. From a pathophysiological perspective, a spherical dilated shape will weaken the contractile properties of the ventricle [20, 21]. Indeed, the results of STE analysis showed that there was reduced left systolic function in ART fetuses. This systolic dysfunction was presented as significant reductions in LV GLS and GLS rate S. These findings partially agreed with those of Valenzuela-Alcaraz et al. [6] who reported impaired LV and RV systolic function in fetuses conceived by ART. However, consistent with our present findings, von Arx et al. [9] revealed similar RV systolic function in ART children and controls. With regard to diastolic performance, we observed increased mitral E’ in ART fetuses. However, previous studies described unchanged or reduced mitral E’ [6, 19]. Thus, the changes in mitral E’ that are reported here need to be further validated. Taken together, these data demonstrated that ART was associated with a globular enlarged LV, a larger RV, and LV dysfunction during the prenatal period.
A previous study failed to detect cardiac remodeling in ART children at low attitude [9]. Moreover, at high attitude, Scherrer et al. [4] reported a comparable cardiac index and E/E’ ratio when compared between ART and control children. Our current data support these previous research studies. In our evaluations carried out in the first 0–2 months of life, we only noted a thinner relative RV wall thickness and a higher tricuspid E/A ratio. In view of the large number of evaluations carried out and the deformation parameters were primary outcomes, these limited findings may not be clinically important. Moreover, the cardiac data were comparable between ART infants and controls when evaluated at 6 months of life. However, our findings are inconsistent with a previous study [6] in that reported the most compelling data by far by demonstrating signs of cardiac remodeling in utero and that these changes persisted postnatally in ART offspring. When considering possible explanations for this discrepancy, it is important to acknowledge that the enrollment criteria of the present study were more stringent. According to the Barker hypothesis [2, 3], when fetuses endure adverse events, the cardiac changes may be more likely to appear later in life under the mediation of certain conditions (e.g., obesity and metabolic syndrome). It seems possible that the ART-associated cardiac remodeling disappeared after birth in our “low cardiovascular risk” infants born via ART.
Thus far, few researchers have studied cardiac changes in association with specific ART techniques. A previous study reported larger atria and globular ventricles in fetuses conceived by fresh ET compared to those by FET [22]. Most recently, Boutet et al. [19] demonstrated larger atria, thicker myocardial walls, and more impaired systolic function in a fresh ET group when compared to an FET population. In the present study, we found that cardiac morphology and function were comparable between IVF and ICSI groups as well as between fresh ET and FET groups. Compared with controls, however, all these ART subgroups were associated with a reduced LV GLS and LV GLS rate S which represents LV systolic dysfunction. Strain parameters, especially LV GLS, are considered to be independent predictors of adverse cardiovascular outcome in subjects at risk [23,24,25]. Thus, it is important and necessary to evaluate LV GLS and its potential association with ART.
The underlying mechanisms by which ART results in fetal cardiac remodeling are poorly understood. Some investigators have suggested that, as with growth restricted fetuses, the cardiac remodeling in ART fetuses may be a response to increased RV afterload with placental abnormalities [26]. Many placental abnormalities, particularly the vascular lesions, are associated with impaired utero-placental perfusion which can be noted by abnormal uterine artery Doppler waveforms [27,28,29]. In previous studies, however, no difference in utero-placental perfusion was detected between ART pregnancies and spontaneous pregnancies [30, 31]. Several recent studies demonstrated better utero-placental perfusion in pregnancies conceived by FET than those conceived spontaneously or by fresh ET [32,33,34]. In our present study, ART pregnancies also demonstrated a significantly lower mean uterine artery PI than those conceived spontaneously. After subgroup analysis, the lower mean uterine artery PI was found to be related to FET but not fresh ET. Our findings suggest that ART fetuses, especially those conceived by FET, are associated with better utero-placental perfusion. Thus, the role of placental hemodynamic function during cardiac remodeling in ART fetuses needs to be validated further.
Recently, epigenetic mechanisms have been proposed to play an important role in the cardiovascular dysfunction of ART offspring [4, 5]. In a previous study, Rexhaj et al. [35] demonstrated that vascular dysfunction in ART mice was related to altered methylation at the promoter of the gene encoding eNOS in the aorta. It has also been proposed that oxidative stress during ART procedures may also cause long-lasting cardiovascular dysfunction in the offspring [36]. A previous study detected increased levels of intracellular reactive oxygen species in the mesenteric resistance arteries of ART mice [37]. In humans, some oxidative stress-associated proteins were found to be differentially expressed in umbilical arteries collected from ART offspring [16]. However, the exact role of epigenetic modifications and oxidative stress in ART-induced cardiac alterations have yet to be elucidated. Future studies need to investigate the mechanisms that underlie these potential effects.
Several limitations in this study should be taken into consideration. First, the range of gestational ages, and the ages at scanning on each visit may be too broad. To minimize the influences of individual differences on cardiac structure and function, linear regression analysis was adjusted for confounding factors, such as gestational age, age at scan, estimated fetal weight, and body surface area. Second, we acknowledge that the present study might be underpowered as there was a 50% loss during the follow-up period. Third, we excluded a range of confounding factors during recruitment, including twin pregnancies, pregnancies with structural/chromosomal anomalies, evidence of infection or any maternal medical disease (e.g., asthma, chronic hypertension, diabetes mellitus, heart disease, HIV or hepatitis infection, lupus and thyroid disease), prematurity, small/large-for-gestational age, and maternal smoking during pregnancy. The baseline variables (e.g., maternal age, gestational age at delivery, birth weight, preeclampsia, and gestational diabetes) were comparable in ART and control fetuses. However, it remains uncertain whether the observed cardiac changes were influenced by some unknown confounding factors (e.g., vanishing twin syndrome). Finally, as a single-center cohort study with limited universality, the results should be extrapolated to the overall ART population with an appropriate degree of caution.
Conclusions
In summary, we found that fetuses conceived by ART experienced cardiac remodeling and LV dysfunction, although these cardiac alterations did not persist in early infancy. Furthermore, the fetal cardiac changes were comparable between ART subgroups. Decreased LV GLS and GLS rate S were both evident in fetuses conceived by various ART procedures. Future studies should include long-term follow-up of these cardiac changes in ART offspring, especially with regard to the lower LV GLS.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ART:
-
Assisted reproductive technology
- ET:
-
Embryo transfer
- FET:
-
Frozen embryo transfer
- GCS:
-
Global circumferential strain
- GLS:
-
Global longitudinal strain
- ICCs:
-
Intraclass correlation coefficients
- ICSI:
-
Intracytoplasmic sperm injection
- IVF:
-
In vitro fertilization
- LV:
-
Left ventricle
- PI:
-
Pulsatility index
- RV:
-
Right ventricle
- STE:
-
Speckle-tracking echocardiography
- T2P:
-
Time to peak
References
Crawford GE, Ledger WL. In vitro fertilisation/intracytoplasmic sperm injection beyond 2020. BJOG. 2019;126:237–43.
Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–4.
Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018;391:1842–52.
Scherrer U, Rimoldi SF, Rexhaj E, Stuber T, Duplain H, Garcin S, et al. Systemic and pulmonary vascular dysfunction in children conceived by assisted reproductive technologies. Circulation. 2012;125:1890–6.
Scherrer U, Rexhaj E, Allemann Y, Sartori C, Rimoldi SF. Cardiovascular dysfunction in children conceived by assisted reproductive technologies. Eur Heart J. 2015;36:1583–9.
Valenzuela-Alcaraz B, Crispi F, Bijnens B, Cruz-Lemini M, Creus M, Sitges M, et al. Assisted reproductive technologies are associated with cardiovascular remodeling in utero that persists postnatally. Circulation. 2013;128:1442–50.
Valenzuela-Alcaraz B, Serafini A, Sepulveda-Martinez A, Casals G, Rodríguez-López M, Garcia-Otero L, et al. Postnatal persistence of fetal cardiovascular remodelling associated with assisted reproductive technologies: a cohort study. BJOG. 2019;126:291–8.
Liu H, Zhang Y, Gu HT, Feng QL, Liu JY, Zhou J, et al. Association between assisted reproductive technology and cardiac alteration at age 5 years. JAMA Pediatr. 2015;169:603–5.
von Arx R, Allemann Y, Sartori C, Rexhaj E, Cerny D, de Marchi SF, et al. Right ventricular dysfunction in children and adolescents conceived by assisted reproductive technologies. J Appl Physiol (1985). 2015;118:1200–6.
Luke B. Pregnancy and birth outcomes in couples with infertility with and without assisted reproductive technology: with an emphasis on US population-based studies. Am J Obstet Gynecol. 2017;217:270–81.
Shah A, Cooke CM, Kirschenman RD, Quon AL, Morton JS, Care AS, et al. Sex-specific effects of advanced maternal age on cardiovascular function in aged adult rat offspring. Am J Physiol Heart Circ Physiol. 2018;315:H1724–34.
Valenzuela-Alcaraz B, Cruz-Lemini M, Rodríguez-López M, Goncé A, García-Otero L, Ayuso H, et al. Fetal cardiac remodeling in twin pregnancy conceived by assisted reproductive technology. Ultrasound Obstet Gynecol. 2018;51:94–100.
Goss KN, Haraldsdottir K, Beshish AG, Barton GP, Watson AM, Palta M, et al. Association between preterm birth and arrested cardiac growth in adolescents and young adults. JAMA Cardiol. 2020;5:910–9.
Patey O, Carvalho JS, Thilaganathan B. Perinatal changes in cardiac geometry and function in growth-restricted fetuses at term. Ultrasound Obstet Gynecol. 2019;53:655–62.
Zhou J, Liu H, Gu HT, Cui YG, Zhao NN, Chen J, et al. Association of cardiac development with assisted reproductive technology in childhood: a prospective single-blind pilot study. Cell Physiol Biochem. 2014;34:988–1000.
Xu GF, Zhang JY, Pan HT, Tian S, Liu ME, Yu TT, et al. Cardiovascular dysfunction in offspring of ovarian-hyperstimulated women and effects of estradiol and progesterone: a retrospective cohort study and proteomics analysis. J Clin Endocrinol Metab. 2014;99:E2494–503.
Bi WJ, Cui L, Xiao YJ, Song G, Wang X, Sun L, et al. Assessing cardiovascular remodelling in fetuses and infants conceived by assisted reproductive technologies: a prospective observational cohort study protocol. BMJ Open. 2019;9:e031452.
Meban C. The surface area and volume of the human fetus. J Anat. 1983;137:271–8.
Boutet ML, Casals G, Valenzuela-Alcaraz B, García-Otero L, Crovetto F, Cívico MS, et al. Cardiac remodeling in fetuses conceived by ARTs: fresh versus frozen embryo transfer. Hum Reprod. 2021;36:2697–708.
Athanasuleas CL, Stanley AWH, Buckberg GD. Mitral regurgitation: anatomy is destiny. Eur J Cardiothorac Surg. 2018;54:627–34.
Adhyapak SM, Parachuri VR. Architecture of the left ventricle: insights for optimal surgical ventricular restoration. Heart Fail Rev. 2010;15:73–83.
Rizzo G, Pietrolucci ME, Mappa I, Bitsadze V, Khizroeva J, Makatsariya A, et al. Fetal cardiac remodeling is affected by the type of embryo transfer in pregnancies conceived by in vitro fertilization: a prospective cohort study. Fetal Diagn Ther. 2020;47:772–8.
Sengeløv M, Jørgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T, et al. Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging. 2015;8:1351–9.
Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, et al. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132:402–14.
Oikonomou EK, Kokkinidis DG, Kampaktsis PN, Amir EA, Marwick TH, Gupta D, et al. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. 2019;4:1007–18.
Moon-Grady AJ. Re: Fetal cardiac remodeling in twin pregnancy conceived by assisted reproductive technology. B. Valenzuela-Alcaraz, M. Cruz-Lemini, M. Rodriguez-Lopez, A. Gonce, L. Garcia-Otero, H. Ayuso, M. Sitges, B. Bijnens, J. Balasch, E. Gratacos and F. Crispi. Ultrasound Obstet Gynecol 2018; 51: 94-100. Ultrasound Obstet Gynecol. 2018;51:21.
Ferrazzi E, Bulfamante G, Mezzopane R, Barbera A, Ghidini A, Pardi G. Uterine Doppler velocimetry and placental hypoxic-ischemic lesion in pregnancies with fetal intrauterine growth restriction. Placenta. 1999;20:389–94.
Brodszki J, Länne T, Laurini R, Strevens H, Wide-Swensson D, Marsál K. Vascular mechanical properties and endothelial function in pre-eclampsia with special reference to bilateral uterine artery notch. Acta Obstet Gynecol Scand. 2008;87:154–62.
Mifsud W, Sebire NJ. Placental pathology in early-onset and late-onset fetal growth restriction. Fetal Diagn Ther. 2014;36:117–28.
Prefumo F, Fratelli N, Soares SC, Thilaganathan B. Uterine artery Doppler velocimetry at 11-14 weeks in singleton pregnancies conceived by assisted reproductive technology. Ultrasound Obstet Gynecol. 2007;29:141–5.
Carbone IF, Cruz JJ, Sarquis R, Akolekar R, Nicolaides KH. Assisted conception and placental perfusion assessed by uterine artery Doppler at 11-13 weeks’ gestation. Hum Reprod. 2011;26:1659–64.
Cavoretto PI, Farina A, Gaeta G, Sigismondi C, Spinillo S, Casiero D, et al. Uterine artery Doppler in singleton pregnancies conceived after in-vitro fertilization or intracytoplasmic sperm injection with fresh vs frozen blastocyst transfer: longitudinal cohort study. Ultrasound Obstet Gynecol. 2020;56:603–10.
Inversetti A, Mandia L, Candiani M, Cetin I, Larcher A, Savasi V, et al. Uterine artery Doppler pulsatility index at 11-38 weeks in ICSI pregnancies with egg donation. J Perinat Med. 2018;46:21–7.
Choux C, Ginod P, Barberet J, Rousseau T, Bruno C, Sagot P, et al. Placental volume and other first-trimester outcomes: are there differences between fresh embryo transfer, frozen-thawed embryo transfer and natural conception? Reprod Biomed Online. 2019;38:538–48.
Rexhaj E, Paoloni-Giacobino A, Rimoldi SF, Fuster DG, Anderegg M, Somm E, et al. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J Clin Invest. 2013;123:5052–60.
Yang H, Kuhn C, Kolben T, Ma Z, Lin P, Mahner S, et al. Early life oxidative stress and long-lasting cardiovascular effects on offspring conceived by assisted reproductive technologies: a review. Int J Mol Sci. 2020;21:5175.
Schenewerk AL, Ramírez FÍ, Foote C, Ji T, Martínez-Lemus LA, Rivera RM. Effects of the use of assisted reproduction and high-caloric diet consumption on body weight and cardiovascular health of juvenile mouse offspring. Reproduction. 2013;147:111–23.
Acknowledgements
The authors wish to thank the study participants who generously gave their time and effort to the study.
Funding
This study was funded in part by the Doctoral Start-up Foundation of Liaoning Province (2021-BS-095). The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
In this study, each author has contributed significantly. Study concept and design were contributed by B-WJ and R-WD. B-WJ conducted all the ultrasound examinations, evaluated the cardiac function by STE, and drafted the manuscript. X-YJ performed measurement of cardiac function by STE for calculating inter-observer variability. X-YJ, WX, CL, and SG evaluated the conventional parameters of cardiac morphometry and function. B-WJ, Y-ZY, and ZY contributed to the data analysis and data interpretation. Additionally, the final manuscript was revised and approved by all the authors before submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of Shengjing Hospital of China Medical University approved the study, and written informed consent was obtained from all participants. The trial registration number for the present study is ChiCTR1900021672.
Consent for publication
Written informed consent for publication was obtained from the participants.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Baseline and perinatal characteristics of the study population who attended the first follow-up (0-2 months-of-age). Table S2. Anthropometric data and cardiac assessment of the study population at the first follow-up (0-2 months-of-age). Table S3. Baseline and perinatal characteristics of the study population who attended the second follow-up (6 months-of-age). Table S4. Anthropometric data and cardiac assessment of the study population at the second follow-up (6 months-of-age). Table S5. Baseline and perinatal characteristics of controls and fetuses conceived by IVF and ICSI. Table S6. Baseline and perinatal characteristics of controls and fetuses conceived by fresh ET and FET. Table S7. Fetal assessment of controls and fetuses conceived by IVF and ICSI. Table S8. Fetal assessment of controls and fetuses conceived by fresh ET and FET. Table S9. Intra-observer and inter-observer ICCs for LV deformation parameters.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bi, W., Xiao, Y., Wang, X. et al. The association between assisted reproductive technology and cardiac remodeling in fetuses and early infants: a prospective cohort study. BMC Med 20, 104 (2022). https://doi.org/10.1186/s12916-022-02303-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02303-6