Abstract
Sex chromosomes are typically viewed as having originated from a pair of autosomes, and differentiated as the sex-limited chromosome (e.g. Y) has degenerated by losing most genes through cessation of recombination. While often thought that degenerated sex-limited chromosomes primarily affect traits involved in sex determination and sex cell production, accumulating evidence suggests they also influence traits not sex-limited or directly involved in reproduction. Here, we provide an overview of the effects of sex-limited chromosomes on non-reproductive traits in XY, ZW or UV sex determination systems, and discuss evolutionary processes maintaining variation at sex-limited chromosomes and molecular mechanisms affecting non-reproductive traits.
Similar content being viewed by others
Sex chromosome systems and degeneration
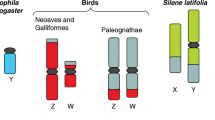
In species with genetic sex determination (GSD) via sex chromosomes, all differences between the sexes can ultimately be attributed to the sex chromosomes. There are three types of sex chromosome systems: XY and ZW in diploid organisms and UV in haploid organisms (Fig. 1).
There are three sex chromosome systems. Two systems exist in diploid organisms—XY (e.g. mammals), where male is the heterogametic sex (A), and ZW (e.g. birds), where female is the heterogametic sex (B). While in haploid organisms there is the UV system (e.g. some mosses), where the female gametophyte is U and the male is V (C)
In both the XY and ZW systems, one sex is homogametic (XX or ZZ) and the other is heterogametic (XY or ZW). The XY system, in which the male is heterogametic, is the most common [1] and well-known and is present in mammals, beetles, several flies and some fishes, reptiles, frogs and plants [2,3,4]. The female heterogametic ZW system is also widespread, being present in birds and some species of Arthropod (including crustaceans and insects), reptile (including the majority of snakes), fish, plant and amphibian [2, 4,5,6]. In both systems, the sex-limited sex chromosome—the chromosome found only in one sex, i.e. Y or W—has been lost in some species, resulting in XO males [7] and ZO females [8] respectively. In other species, multiple sex chromosomes have been observed, presumably due to neo-sex chromosome evolution involving autosome fusion or translocation [5]. Moreover, both types of heterogametic systems can occasionally coexist within the same species, such as in some frogs, houseflies, midges and fishes [9]. There can be several different reasons why some species evolve XY and others ZW systems. One factor is which kind of sterility mutation becomes fixed first during the evolution from hermaphroditism to separate sexes. If a dominant female-sterility mutation fixes, that drives the evolution of an XY system, but if a dominant male-sterility mutation fixes, then a ZW system evolves [7]. XY systems are more common, which could be due to stronger selection in males, which drives faster evolution of the Y [7] and makes the Z chromosome more male-biased, thus limiting female fitness and population growth, in ZW systems [9]. Similarly, deleterious mutations accumulating on the Y and decreasing male fertility are less harmful for a population compared to mutation accumulation on the W which decreases female fertility [10]. Finally, XY systems seem to provide protection from meiotic drive [7]. All of these phenomena could contribute to the observed higher frequency of XY sex determination compared to ZW sex determination. Species in clades with homomorphic sex chromosomes (e.g. fish and reptiles) can undergo frequent turnovers of the heterogametic systems, further increasing the observed diversity in sex determination.
The UV sex chromosome system is the least common variant and is found in haploid organisms: some mosses, liverworts, fungi and algae [4, 7], where U is female and V is male (Fig. 1C). In UV systems, diploid individuals are neuter, and haploid males and females are produced by meiosis. These haploid individuals then develop and grow to eventually produce gametes which fuse to produce a new diploid individual.
Common to all these sex chromosome systems is that the sex-limited sex chromosome, i.e. the Y, W, U and V chromosomes, often, but not always, undergoes sex-specific evolution, including degeneration and gene loss as a result of cessation of recombination, the causes of which are discussed below [7, 11]. The Y, W, U and V chromosomes are therefore also known as non-recombining sex chromosomes (NRSCs), which is how we refer to them throughout this review. Genes that remain functional on highly degenerated sex chromosomes are generally believed to code for sex-specific traits, such as sex cell production, and to have few or no other functions. Moreover, sex differences in shared traits have been traditionally attributed to sex hormones (at least in mammals). However, NRSCs have the potential to influence traits that are shared between the sexes, and a growing body of evidence shows that they can play a crucial developmental role as the loss or gain of an extra copy can dramatically affect the phenotype [12,13,14]. As the effect of these chromosomes on sex-specific traits such as sperm production is well established, it would now be valuable to switch focus to explore how these chromosomes may influence sexual dimorphism in shared traits, or even play a role in traits where sex differences are not observed. For example, it has been shown that in some cases sex hormones and sex-linked genes have opposite phenotypic effects, such that sex-linked genes reverse the effects of the sex hormones, thereby resulting in the production of an equal phenotype between males and females [15]. Thus, possible non-sexual effects of these chromosomes should not be ignored, and we show here that NRSCs can play an important role in health and disease (Table 1).
We review the literature on non-reproductive traits known (or suggested) to be affected by NRSCs. However, we believe that the list of traits is not complete, as the NRSCs may participate in other traits directly or indirectly. We also provide an overview of which mechanisms these chromosomes may influence the traits by, despite undergoing degeneration. We concentrate on all types of non-reproductive traits, except sexual behaviour and primary sex characteristics (i.e. sex-limited body structures directly involved in reproduction, such as gonads and external genitalia). We focus here on the sex-specific regions of sex chromosomes, though it should be noted that the pseudoautosomal region (PAR)—a short region of homology between sex chromosomes that behaves like an autosome and can recombine—can also play a role in sexual dimorphism. For example, there is male-biased expression in PAR genes in mammals, since one X undergoes inactivation in females [117], and in emu, since they are downregulated in females [118].
Evolution and degeneration of sex-limited chromosomes
According to the canonical model, sex chromosomes start to evolve from a pair of autosomes, when one or more genes acquire a sex-determining function. Subsequently, recombination arrest may evolve around the sex-determining region and gradually expand to encompass most of the chromosome [5]. Accumulation of male-beneficial loci on the non-recombining portions of Y and V chromosomes will be favoured, as will accumulation of female-beneficial loci on the W and U. Thus, the NRSC becomes more and more specialized to code for very specific sex characteristics, while losing most of its original gene content due to lack of recombination (Fig. 2). However, not all sex chromosomes become heteromorphic (e.g. in emu [118] and pufferfish [119]). This could be due to the fact that there are different ways of resolving sexual conflicts, situations, where sexually antagonistic loci have positive effect in one sex, but negative in the other. These conflicts can be resolved either through ensuring sex-limited inheritance of sexually antagonistic genes via recombination suppression, or simply modulating expression of these genes within each sex via sex-specific transcription factors.
Gene loss and specialization over time on NRSCs. The rate of gene loss is expected to decrease with time since recombination cessation, as non-essential genes are lost early in this process, while essential genes can be maintained through purifying selection. In contrast, the rate of specialization may be more or less constant since it will likely be dependent on mutation accumulation
NRSCs (Y, W, U and V) usually contain two distinct regions: (i) the sex-specific region where recombination is suppressed, and (ii) the PAR, which serves to ensure proper segregation during meiosis [7]. Over time, the sex-specific region tends to degrade as a result of various processes, including drift, hitchhiking effects and perhaps even selection for silencing [120]. Genes that survive this degradation process are expected to have highly important “housekeeping” functions, resulting in strong purifying selection [7]. As males usually have a higher mutation rate than females and are often subject to more intense sexual selection, male-limited sex chromosomes (Y, V) are prone to faster degeneration than female-limited sex chromosomes (W, U) [7]. Although the degeneration process results in loss of functional genes over time [11, 121, 122], it does not necessarily mean that the sex-limited region inevitably decreases in physical size, as NRSCs often acquire additional genetic material in the form of transposable elements, repetitive or organellar DNA, duplications, transpositions or autosomal translocations [11, 121, 123].
Apart from low gene content, degraded NRSCs may have up to ten times smaller nucleotide diversity than autosomes [5], leading researchers to assume that they have little standing genetic variation for phenotypic traits. As discussed in more detail below, there is now good evidence that variation on NRSCs (e.g. in gene copy number or heterochromatin length [124, 125]) can have an important influence on the phenotype [126, 127], proving that low gene content need not necessarily limit the evolutionary potential of NRSCs.
Y chromosomes
General properties of Y chromosomes
Not surprisingly, Y chromosomes are the most studied sex-limited chromosomes as they determine sex in humans and in many popular animal model organisms. Interestingly, Y chromosomes are the most prone to degeneration (compared to W, U and V chromosomes) due to small effective population size and limitation to the male line, which is associated with stronger sexual selection and higher mutation rates (via oxidative stress, lack of repair enzymes and more divisions of sex cells) [7, 128]. This reasoning is supported by the notion that the Y chromosome seems to be lost most frequently, leading to the evolution of XO systems (e.g. in several rodent and insect species [128]). Y chromosomes are often highly differentiated and degenerated, with large amounts of repetitive sequences.
This degeneration has been implicated in longevity differences between the sexes. Cross-species comparisons have shown that the heterogametic sex has a shorter lifespan on average than the homogametic sex and that this effect is exaggerated in XY systems (>20% lifespan reduction compared to <10% in ZW systems) [112]. The exact cause is unknown, but is thought to be a result of unmasking of deleterious recessive mutations in the heterogametic sex (i.e. the unguarded X hypothesis).
Mammal Y chromosomes
Mammal Y chromosomes are small, are highly repetitive and contain few coding genes, some of which exist in multiple copies, which increases their survival [129]. Most of the genes have testes-specific expression [5]. The mammalian Y chromosome is thought to have emerged 166 million years ago, when the sex-determining gene SRY arose [130]. Later on, most of the genes were lost, leaving only widely expressed and dosage-sensitive regulators of chromatin modification, transcription and splicing, translation and ubiquitination, suggesting that the surviving genes are essential and have the potential to regulate expression of target genes throughout the genome [129]. One exception to the general mammalian pattern is the platypus, which has multiple X and Y chromosomes which pair as a ring during male meiosis [131]. How this pattern has arisen is unknown, but it would be interesting to see if it results in increased recombination and gene transfer or turnover between the Xs and Ys.
The human Y chromosome is ~57 Mb large (1/3 the size of the X) and contains 64 coding and 107 non-coding genes [132]. The male-specific region of the Y chromosome (MSY) makes up 95% of the chromosome, leaving only 5% to the PAR, which occurs on both ends of the sex chromosomes (PAR1: 2.6 Mb long with ~16 genes; PAR2: 0.32 Mb long with ~5 genes) [96]. Most of the genes are ampliconic (i.e. occur in several adjacent and highly similar copies) as a result of translocation from the autosomes and Y-Y gene conversion between palindrome arms, and have testes-specific functions [133]. But several MSY genes have survived from the original ancestral autosome or have been acquired from the X or autosomes, and around half of all Y genes are expressed quite widely in the body [133, 134]. For example, two genes, RPS4Y1 and RPS4Y2, code for a ribosomal protein and are homologous to RPS4X on the X chromosome [133]. Similarly, the gene AMELY is expressed in developing tooth buds and together with TBL1Y also in the thyroid, thus possibly coding for some kind of non-reproductive sex difference, although there are homologues on the X [133, 135].
In contrast to other mammalian Y chromosomes, the mouse Y is almost entirely euchromatic except for the centromeric region and contains relatively many coding (172) and non-coding (570) genes [132]. Although it has lost more of its original genes than the human Y, the mouse Y chromosome has managed to acquire many newer testes-specific genes from autosomes due to a history of meiotic drive initiated by the X, and the similarity between the primate and mouse MSY is only 2.2% [136]. Four mouse Y chromosome genes are widely expressed throughout the body (Ddx3y, Eif2s3y, Kdm5d and Uty), but it is not clear whether these genes play a role in sex differences since they all have X-linked homologues [136].
The main function of the mammal Y chromosome depends only on a single gene—the male-determining SRY, which is a transcription factor controlling expression of numerous genes in a sex-specific manner. SRY activates another transcription factor gene, SOX9, which represses ovarian genes and activates testicular genes, determining Sertoli cell fate, thus continuing formation and maintenance of the male gonad. This eventually leads to testosterone production after the testes are fully formed. Sex hormones then act on different tissues throughout the body to produce secondary sex differences. Mammal sex determination can therefore be seen as a two-step process, where the sex chromosome content determines the fate of bipotential gonadal ridges to develop into testes or ovaries. Then these genetically determined gonads start to produce sex hormones, which in turn drive the sexual differentiation of the body (i.e. phenotypic sex) [137]. Although it has traditionally been accepted that sex hormones masculinize or feminize the body [138], during the last 30 years evidence has accumulated that direct sex chromosome effects also help to establish sex differences independently of sex hormones. During the lifespan of males, the magnitude of the effects of the Y and testosterone changes, with the Y being the most important in the very beginning of the pregnancy until start of testosterone production, and then again in the end of life, when testosterone levels drop dramatically [139].
As a result of our survey of the literature, we have identified three main mechanisms by which the Y chromosome can affect somatic traits in mammals, one of which is direct and two are indirect:
-
1.
Changes in amino acid sequence or expression level of Y-linked protein-coding genes expressed in somatic tissues (direct) (Fig. 3). Allelic variation which affects expression could occur via mutations in coding regions, promoters or regulatory regions. Because of the heterochromatic nature of the Y, expression of Y-linked genes could also be influenced by consistent differences in heterochromatin distribution between haplotypes.
-
2.
Y chromosome modulation of expression of X-linked, autosomal or Y-linked protein-coding genes (Fig. 3), for example via transcription factors, non-coding RNA genes, or heterochromatin effects (indirect). Transgenerational effects of the paternal Y on daughters (see below) would also fall into this category.
-
3.
Modulation by testosterone (indirect) (Fig. 3). Variation in Y-linked genes may result in variation in testosterone levels and associated receptors. This will have many carry-over effects on the phenotype, which are indirectly attributable to Y-linked genetic variation.
NRSC (in grey) mechanisms of action (Table 1). Direct mechanisms include (1) protein-coding gene expression in somatic tissues, while indirect mechanisms are exerted through (2) regulation of other chromosomes (via transcription factors, non-coding RNAs, heterochromatin effects or imprinting) or (3) sex hormones
These mechanisms are potentially applicable to any sex-determining system and are not exclusive to mammals. Since genes occur in interaction networks, these categories are not mutually exclusive either, and in practice, it can often be difficult to distinguish, e.g. effects mediated by testosterone from direct effects of the Y genotype. However, we feel that it is worthwhile to make a distinction between categories since the evolutionary dynamics of each type of mechanism may differ. For example, regulatory effects of Y genes are likely to be more evolutionarily labile than protein coding changes [140]. Since humans and other mammals are so well studied, they are well suited for providing a framework which can be extended to other species. Below, we first discuss the limitations of various methods of detection of the effects of NRSCs, and then move on to discussing each main mechanism of action in turn.
Methods of detection of effects of NRSCs
Which methods are used for detecting effects of NRSCs place limits on our ability to determine the mechanism of the effect (direct, indirect or primarily hormonal). Before the advent of modern methods of studying gene expression, Y chromosome copy number could be used as a proxy for expression differences in Y-linked genes, for example via natural variation in Y chromosome number via sex chromosome aneuploidies [12,13,14, 21, 141, 142] or somatic loss of the Y chromosome via aging [78, 96]. Although this method does not require modern sequencing technology, it cannot distinguish between changes in expression of protein-coding genes (category 1) versus regulatory effects (category 2), since the Y chromosome encompasses both types of genes. However, it could be possible to exclude hormonal effects (category 3) if testosterone level does not differ between individuals with different numbers of Y copies. Another simple method for detecting effects of Y-linked genes is through correlations between Y haplotype and phenotypic differences [143]. Again, without more detailed information about differences between haplotypes, it is difficult to know if differences are mainly due to direct or indirect effects, but it can be possible to exclude hormonal effects as the cause, if testosterone levels do not differ between haplotypes [144].
Expression of Y-linked genes in somatic tissues can be measured directly using standard methods such as microarrays or RNA-seq [145]. For example, seven Y genes have been found to be widely expressed in different organs in mice [136]. Depending on how well the functions of the differentially expressed genes are known, it can then be possible to assign effects to category 1 (mainly driven by protein-coding genes) or category 2 (mainly driven by regulatory effects). Similarly, RNA-seq can be useful for detecting effects of non-coding RNAs [146]. However, it is important to note that expression of Y-linked genes in somatic tissues in adults could potentially be influenced by circulating testosterone, as it has been shown that testosterone affects methylome and transcriptome of the genome [147]. These effects therefore always need to be considered, and if possible, controlled for.
The highly degenerated nature of the Y chromosome has previously made it difficult to sequence, but long-read methods have helped to overcome these issues [132]. This means that GWAS can be used to detect allelic differences in protein-coding or regulatory genes that are associated with phenotypic differences also in situations where the Y chromosome has been traditionally excluded. Proteomics could also be used for detecting protein-coding changes in Y-linked genes that affect the phenotype, although we did not discover any examples of this in our survey of the literature. Similarly, ChIP-seq (chromatin immunoprecipitation with massively parallel DNA sequencing) could be used to identify effects of Y heterochromatin conformation on the phenotype. This method has not been used for Y-linked genes in mammals to our knowledge, but has been successfully applied in Drosophila [148]. There is clearly scope for increased use of modern genomic methods to detect effects of NRSCs on non-sexual traits.
Y-linked protein-coding gene expression in somatic tissues
Several Y chromosome genes are expressed in pluripotent stem cells in vitro [146, 149], and embryonic rodent neurons (XX vs. XY) in cell cultures can undergo sexual differentiation even if there are no sex hormones present. Moreover, rodent dopaminergic neurons sustain genetically induced sex differences even after sex hormone or their inhibitor treatments in vitro; however, it is not known, if the effects are caused by the Y [150, 151]. Studies on humans with sex chromosome abnormalities (XX vs. XY vs. XXX vs. XXY vs. XYY vs. XXYY vs. XXXXY) clearly show that Y chromosome number has a significant effect on neurobiological phenotypes [12,13,14, 21, 141, 142]. For example, it seems that the Y increases brain size, possibly affecting language, emotions and other phenotypes [14]. However, as noted above, these differences are likely driven by changes in expression of both protein-coding and regulatory genes.
Other indirect evidence of an important role of expression of Y-linked protein-coding genes in somatic tissues is the association of lifetime-acquired loss-of-Y (LOY), which shows racial differences and is associated with several diseases and overall mortality [78, 96]. Surprisingly, in the case of leukaemia, LOY actually has a protective effect [97], which is very unexpected given that the protein-coding UTY gene on the Y acts as a tumour suppressor [101]. LOY is the most common somatic mutation in males and occurs through centromere dysfunction or telomere attrition; it increases with age and is accelerated by smoking and outdoor air pollution, alcohol, obesity and exposure to insecticides and polycyclic aromatic hydrocarbons [96]. Although these multifarious associations of diseases with LOY are intriguing, it must be noted that it is currently unclear whether LOY is causal in most cases. It is also unclear whether changes associated with LOY are mainly driven by the loss of protein-coding or regulatory genes. However, there is evidence that both are possible, since LOY has a direct effect on autosomal dysregulation in immune cells [152] and leads to decreased CD99 immunoprotein on leukocyte surfaces, because the CD99 gene from the PAR of the Y is lost [91].
Few studies have directly quantified how Y-linked expression variation in protein-coding genes influences somatic traits. However, there is a relatively large literature investigating how Y chromosome identity (chromosome Y consomic rodent strains or haplotypes in humans) can affect various phenotypes. For example, Y chromosome identity can affect blastocyst cell number [153] and male embryo weight [108] as well as adult body size [42, 109, 154] independent of gonadal hormones in mice. Meanwhile, vulnerability to alcohol dependence [30] and autism [35] is affected by Y chromosome haplotype in men; however, here it is impossible to disentangle direct effects of differences in expression of Y-linked genes from indirect effects of testosterone, since possible differences in testosterone levels between the haplogroups are not controlled for. It is also not clear if all of these differences in mammals are driven by different Y gene expression or other effects such as Y heterochromatin silencing genes on nearby chromosomes. Nevertheless, at least some of the variation is likely attributable to allelic differences resulting in changes in the expression of Y-linked protein-coding genes.
The best evidence of direct effects of Y-linked gene expression on phenotypic differences is in coronary artery disease, where it has been found that haplogroup I has ~50% higher age-adjusted risk than eight other haplogroups. The effect seems to be driven by downregulation of two Y chromosome genes, UTY and PRKY (both of which are protein-coding), and not by traditional cardiovascular risk factors [143]. Interestingly, it is known that the difference is not driven by steroid hormonal effects as there is no difference between the haplogroups in these traits [144].
Y-mediated regulatory effects
There is good evidence that the Y chromosome can regulate the expression of genes on other chromosomes. For example, it has been shown that male embryonic stem cells have a unique transcriptome profile, in which the Y chromosome affects expression of 294 genes in mice [149]. These early regulatory effects may have important consequences for the later development of sex differences in different organs.
SRY is of course one of the most important regulatory genes on the Y, and it is known to be differently expressed in the brain based on the Y chromosome it resides on [50]. Another study has also found that SRY expression in human cell cultures and rats can be linked to Parkinson’s disease [52]. Thus it seems that SRY participates in sexual differentiation of the brain [155, 156]. Experimental allergic encephalomyelitis and myocarditis in chromosome Y consomic mouse strains may be caused by the natural variation in copy number of the SLY and Rbmy genes, which affect expression of genes in immune cells, such that the higher the copy number the lower the expression in immune cells [82].
In some cases, the specific regulatory pathways are known. In midbrain dopamine neurons, SRY positively regulates catecholamine synthesis and metabolism, possibly explaining male bias in fight-flight behaviours and “dopamine disorders”, such as Parkinson’s disease and schizophrenia [41]. SRY also directly regulates expression of the monoamine oxidase A gene located on the X, possibly explaining sex differences in attention deficit hyperactivity disorder, depression and autism [36]. Finally, SRY can be expressed in the colon of Hirschsprung’s disease patients and explain the 5:1 male bias by repressing expression of tyrosine kinase receptor RET, a gene responsible for almost half of the cases [110].
It is also known that the Y chromosome can regulate gene expression through other epigenetic mechanisms. For example, SRY and SLY, which are present in multiple copies, can regulate chromatin structure beyond the Y [157, 158], and the Y chromosome-linked long non-coding RNA (lncRNA) lnc-KDM5D-4 decreases expression of PLIN2 located on chromosome 9 [57]. PLIN2 is involved in lipid droplet formation in hepatocytes, thus possibly protecting from fatty liver, which in turn could protect from atherosclerosis and coronary artery disease. Moreover, this lncRNA is expressed across the body and may therefore account for other discrepancies among men and women in health and disease [57]. The Y chromosome also regulates genes elsewhere in the genome through small RNAs [94].
It is worth noting that indirect intergenerational effects of the Y are also possible. The Y chromosome codes for minor histocompatibility antigens against which females can create an immune response. This immune reaction could in turn result in obstetric and neonatal complications, preterm birth or lower birth weight, and stillbirth or miscarriages, leading to a female-biased sex ratio in subsequent children [159]. Moreover, mothers seem to develop antigens to extracellular NLGN4Y (a growth factor) during each male pregnancy with additive effects, which may result in feminization of the male embryos’ brain and increase the chance that the individual will be homosexual [160]. The Y chromosome also plays a role in different transgenerational effects in mice, affecting several traits in daughters by epigenetically imprinting other chromosomes [83].
Testosterone-mediated effects of the Y chromosome
Testosterone affects many traits and has traditionally been considered the main way the Y can play a role in somatic phenotypes. Consistent with this, chromosome Y consomic mice strains have indeed shown that the Y can affect testosterone sensitivity [22, 60]. A few examples of testosterone-mediated traits where MSY identity is known to be important include discriminability of individual urine odours and serotonin levels in rodent chromosome Y consomic strains, influencing aggression [23, 24, 29]. In consomic mice strains, the Y identity also affects the size of cardiomyocytes [61] due to different responses to postpubertal testosterone [60].
From these examples, it is clear that the Y chromosome has the potential to affect a wide variety of somatic traits in multiple organ systems in mammals. In many cases, an effect of the Y can be detected, but it is not always possible to determine by which mechanism. As mentioned above, in studies that have found phenotypic variation associated with different Y haplotypes (Table 1), it is unclear whether this variation is a result of differences in Y-linked gene expression, regulatory effects, variation in testosterone production, or (most likely) some combination of these. Increased use of modern genomic and proteomic methods should help to disentangle these various mechanisms in future.
Drosophila Y chromosomes
Drosophila Y chromosomes completely lack recombination, as recombination is entirely absent in males. The Y chromosome of D. melanogaster is the best-studied and is at least 60 million years old [7]. It is almost entirely heterochromatic and contains only around 20 protein-coding genes gained from autosomes [161], while being roughly the same size as the X [162]. In fact, the current rate of gene acquisition on the Drosophila Y is eleven times higher than gene loss, so that gene content is actually increasing [163]. As many Y-linked genes in Drosophila have counterparts on autosomes, it is not clear if they have a male-specific function or are simply redundant [164]. However, a study of 22 Diptera species showed that most genes on old Y chromosomes have been hijacked from autosomes and then have undergone convergent evolution acquiring male-specific functions [165], suggesting that translocation to the Y may often be associated with the evolution of male-specific functions.
The protein-coding genes on the Drosophila Y are expressed only in the testes. The rest of the Y consists of two RNA-coding genes (the bobbed and crystal loci), long satellite DNA repeats and transposable elements [166]. The rDNA locus is the only shared one between the X and Y—no other homologues occur [165]. Dosage compensation (by male upregulation of the single X) seems to have evolved very early in the evolutionary history of Drosophila, which may be the reason why the Y degenerated so quickly [7]. Contrary to mammals, the Y chromosome does not have a sex-determining function in this group. However, it is important for male fertility as XO males are sterile [166].
Of the four potential mechanisms of action that we identified in mammals, only the second one (Y chromosome regulatory effects) seems likely to play an important role in Drosophila. Since Y-linked protein-coding genes in this species are almost exclusively related to sperm production and are limited in their expression to the testes, direct effects of Y-linked protein-coding genes via somatic expression (category 1) are unlikely, at least for non-sexual traits [166]. Sexual differentiation in Drosophila is controlled by the gene doublesex, which is alternatively spliced in males and females [7]. This means that, in contrast to mammals and birds, sex hormones do not seem to play a major role in sexual differentiation in this species [167], which suggests that hormonal mediation by Y-linked loci (category 3) is unlikely as well. We might therefore predict a priori that Y chromosome effects should mainly occur via non-coding regulatory effects in Drosophila, e.g. through small non-coding RNAs or heterochromatin effects [166].
Indeed, it has been shown that the Y chromosome may influence the expression of up to two thousand genes located on other chromosomes [168], depending on the genetic background and apparently mediated by heterochromatin formation, affecting immunity and olfaction [169]. Later results confirm that the Y chromosome seems to act as a “heterochromatin sink”—i.e. that the cell can only produce a certain amount of heterochromatin-inducing factors and that the Y chromosome seems to preferentially sequester these factors, hindering heterochromatization of repetitive regions on other chromosomes [148]. It has been shown that variation among Y chromosomes in their repetitive DNA content generates different patterns of chromatization and thus gene expression across the rest of the genome [170]. Nevertheless, it is worth bearing in mind that although the heterochromatin sink model of Y-linked regulatory variation currently has the best support, other mechanisms have not yet been ruled out.
Traits that have been shown to be influenced by the Y in D. melanogaster include longevity [162, 171], abdominal bristle number and geotaxis [172], immune gene regulation [173], same-sex sexual behaviour in males [174] and others [169, 175]. Interestingly, the paternally inherited Y chromosome and the maternally inherited mitochondria affect locomotive activity, although there is no support for any interactions between the Y and mtDNA [176]. Moreover, the Y chromosome may also affect daughters’ egg-to-adult survival rates, although the exact mechanism behind this effect is unknown [177, 178].
Y chromosomes in other species
Although non-sexual effects of the Y chromosome are best documented in model organisms, information from other taxa is increasing. For example, in several fish and insect species colouration, genes have become linked to the sex-determining locus, presumably as a way of mitigating sexual antagonism [7].
There is also evidence of Y-linked modulation of autosomal colour genes in guppies (Poecilia reticulata) [179]. And several other species of live bearing fish have alternative reproductive tactics (e.g. “courters” and “sneakers”) that have been shown to be controlled by Y-linked loci [180, 181]. These alternative morphs often differ in a suite of traits, including behaviour, colouration, cognition, life history, body size and morphology [180, 182], consistent with widespread regulatory effects of Y-linked loci.
Interestingly, a recent study in Callosobruchus seed beetles revealed that Y-linked genetic variation could explain the bulk of the response to artificial selection on body size in lines that were selected for increased sexual dimorphism [183]. This was a surprising finding since body size is a trait that is usually expected to be controlled by many small-effect autosomal loci, but is consistent with the results from live bearing fishes discussed above.
W chromosomes
General properties of W chromosomes
ZW (female heterogametic) systems are also widespread and in many aspects resemble XY systems (Fig. 1B). As mentioned above, comparative studies show that ZW systems are associated with greater longevity in males, suggesting that there may be deleterious effects of the W [112]. Similar to Y chromosomes, W chromosomes are expected to have low genetic diversity due to low effective population sizes [7], although this effect may be less exacerbated in W chromosomes since females usually have lower variance in reproductive success than males [184]. One major difference between the W and the Y is, however, the maternal co-inheritance of the W and mitochondria, which may introduce cyto-nuclear associations [185]. So far there is little data available to test for evidence of coevolution of the W and mitochondria.
Avian W chromosomes
Most data on W chromosomes come from birds. The avian ZW chromosome system is around 140 million years old, and the Z and W are highly heteromorphic. The W has lost most of its genes in most species, with the exception of some palaeognaths (e.g. ostrich) which have a large PAR [186]. Analogous to the mammalian Y, the avian W chromosome is basically a degraded counterpart of the Z chromosome. In Ficedula flycatchers, a passerine bird genus, 46 W-linked genes have been detected [187]. In chicken, the most well-studied bird species, 28 intact W genes are found [188], and all are single-copy except HINTW, which is present in multiple copies and appears to have been subject to positive selection [189, 190]. Neither the chicken W nor the flycatcher W has known acquired genes, and data from other birds or/and other independently evolved lineages (e.g. snakes) are needed to show whether this is a general feature of female-specific chromosomes. None of the 28 chicken W genes are expressed exclusively in female-specific tissues [188], and all 27 single-copy Z-W pairs are expressed in the developing chicken blastoderm, which means that the combined expression of the Z-W gene pairs in females is comparable to the expression of the two Z homologues in males [191]. Thus, the few remaining single-copy chicken W genes’ main function could be to ensure female survival by providing correct dosage (birds lack chromosome-wide dosage compensation mechanisms in contrast to mammals [192]), especially for those functioning in critical signaling pathways during early embryonic development.
It has been suggested that the relative simplicity of the W chromosome, with only broadly expressed ancestral genes and only one multicopy gene family, may be because its transmission is restricted to the female germ line. In contrast, X, Y and Z chromosomes pass through the male germ line, and all have acquired and amplified testis-expressed gene families [193]. The marked absence of acquired genes that are specifically expressed in the ovary or other female-specific tissues, even on a female-specific chromosome, suggests that, at least in amniotes, there is greater pressure to preserve or enhance male reproductive functions [193].
All three of the main mechanisms of action identified for mammalian Y chromosomes above seem plausible for avian W chromosomes as well. Because all functional W-linked genes seem to be broadly expressed, variation in amino acid sequence or expression levels of protein-coding genes (category 1) in somatic tissues could have widespread effects [194]. W-linked modulation of expression of other genes (category 2) is also possible. For example, HINTW is a truncated counterpart of the Z-linked HINTZ, and its gene product has been suggested to act as a dominant negative version, blocking a possible testis-specific function of HINTZ. Evidence for this function is however limited, as misexpression of HINTW does not disturb male gonadal development in chicken, zebra finch and emu [188]. Finally, the W could also potentially influence non-sexual traits via hormonal effects (category 3), although this mechanism may be of lesser importance in birds than in mammals. Evidence of cell-autonomous sex determination in chickens has emerged from the study of lateral gynandromorphs [194], along with sexually dimorphic gene expression that precedes gonadal differentiation [191, 194], suggesting that many sex differences are established independently of the action of sex hormones in birds. In addition, it is currently unclear whether W-linked genes have important effects on sex steroid levels in birds.
There are currently few examples of W-linked effects on non-sexual traits in birds. Genetic female (ZW) zebra finches (Taeniopygia guttata) with testes develop a feminized song system [195], suggesting that some sort of direct effect of the sex chromosomes determines this trait [196]. Apart from broad expression in the developing embryo, two W-linked genes (CHD1W and ASW) have been shown to be expressed in the adult brain, indicating possible but unknown functional roles [197]. Several colour pattern traits also seemed to be influenced by the W chromosome, including zebra finch beak colouration [198], blue egg colour in the common cuckoo (Cuculus canorus) [199] and eggshell patterning in the great tit (Parus major) [200].
W chromosomes in other species
Most Lepidoptera have a pair of differentiated ZW sex chromosomes. However, in contrast to the other systems we have discussed so far, lepidopteran W chromosomes are thought to have been acquired secondarily [201]. This is because in most lineages outside of the division Ditrysia (which comprises 98% of all species), as well as in the sister order Trichoptera, females lack a W chromosome. Pronounced heterochromatization and transposable element content suggest that lepidopteran W chromosomes consist largely or entirely of repetitive sequences [202]. Accordingly, the total number of coding sequences found on the lepidopteran W is extremely low, with little overlap between distantly related species [203,204,205]. Some families also seem to have experienced a secondary loss of the W [206], suggesting that the W chromosome is dispensable for the genome in some species, which is consistent with its heterochromatic nature and scarcity of genes [207, 208]. We can therefore speculate that, as in Drosophila, the most likely mechanism of action of lepidopteran W chromosomes is a heterochromatin “sink”. To our knowledge, no non-sexual effects of lepidopteran W chromosomes have been reported to date.
W chromosomes in other systems, including fishes, frogs and reptiles, are generally poorly studied and seem in many cases to escape extensive degeneration via either environmental sex reversal and subsequent recombination between Z and W chromosomes, or frequent turnovers [7], placing them outside the scope of this review. However, similar to the livebearers discussed above, female-benefit coloration seems to be affected by the W in cichlids [209]. In addition, snakes with heteromorphic ZW sex chromosomes generally seem to lack chromosome-wide dosage compensation [210]. This suggests that remaining W-linked genes could potentially have important effects on early embryonic development, as in birds.
U and V sex chromosomes
U and V sex chromosomes are found in organisms with haploid GSD [4, 7]. As mentioned in the introduction, cells from neuter diploid individuals undergo meiosis to produce haploid U-bearing female and V-bearing male gametophytes (Fig. 1C). U and V chromosomes have a larger effective population size (50%) relative to the autosomes compared to Y and W chromosomes (25%), since they are present in every second haploid individual (assuming equal sex ratios). Since they typically occur in species with a well-developed haploid life stage, they experience purifying selection on deleterious recessive mutations to a greater extent than Y and W. These sex chromosomes are therefore not prone to degenerate via gene loss, but rather tend to differentiate via chromosomal rearrangements (such as inversions and translocations), and accumulate sex-specific genes and transposable elements, leading to lower gene density relative to the autosomes [7]. The female U chromosome is usually larger, which is perhaps to be expected if the male-limited V chromosome is prone to faster degeneration due to higher mutation rates and more intense sexual selection in males [7].
UV sex chromosomes are the least studied of all the NRSCs and have only been well-characterized in a handful of species. In the liverwort Marchantia polymorpha the U and V chromosomes are highly diverged, despite the fact the males and females are phenotypically almost monomorphic [211, 212]. The male-specific V chromosome contains high amounts of repetitive DNA and two unique genes ORF162 and M2D3.5 [211]. In brown alga Ectocarpus sp., the sex-determining regions ceased to recombine more than 100 million years ago and there is evidence that they are now evolving rapidly [213]. Both the U and V chromosomes are similar in size and structure and moderately degenerated, containing ~20 genes with relatively low expression [213]. Interestingly, in this species, the PAR is enriched in transposable elements and has a low gene density [213], which is a pattern that is not typically seen in Y and W chromosomes. This species also exhibits a low level of sexual dimorphism [213]. Finally, the U and V sex chromosomes of the moss Ceratodon purpureus were recently characterized, revealing rather low levels of degeneration (mainly in the form of increased number of transposable elements) since their origin around 300 million years ago [214]. This suggests that recombination cessation is not sufficient in itself to drive gene loss on NRSCs.
As with W chromosomes, all three mechanisms of action are plausible in UV systems, although the importance of hormonal effects is unclear since plants (which comprise the majority of UV systems) do not have specialized masculinizing and feminizing sex hormones (even though various hormones may play an important role in sexual development) [215, 216]. Because U and V chromosomes are less prone to suffer gene loss, it seems likely that coding sequence differences and variation in somatic expression could have important effects on non-sexual traits, either directly (category 1) or indirectly (e.g. via modulation of expression of autosomal genes; category 2). However, given the low levels of sexual dimorphism in many species with UV sex chromosomes, it is unclear what traits might be affected. Nevertheless, results from C. purpureus are consistent with widespread non-sexual effects of U and V chromosomes. Using a quantitative genetic approach, significant sexual dimorphism and additive genetic variance for total mass and leaf length has been found and that male and female juvenile growth were not genetically correlated [217]. These differences were presumably driven by sex-linked loci. Similarly, another study [214] showed that >1700 U- and V-linked genes were widely expressed in somatic tissues in C. purpureus, which is substantially larger than the number of autosomal genes with sex-biased expression, suggesting that direct effects are likely to be more important than indirect regulatory effects in this species. Although genes with conserved reproductive functions were enriched among the U- and V-linked genes with somatic expression, it seems unlikely that none of these genes would affect non-sexual traits as well. There is clearly scope for further research in this area.
Conclusions and perspectives
Although mammal Y chromosomes are gene-poor, it is clear from results in Drosophila and UV systems that cessation of recombination leads to differentation, but not always to the inevitable loss of genetic material. Various processes have been linked to the maintenance of coding and non-coding variation on NRSCs, including sex reversals which allow recombination between the X and Y (or Z and W), Y recombination at palindromic sites, X-Y transposition [7], purifying selection on essential genes [218, 219], translocation from the autosomes and gene conversion [220], and duplication [221]. It is therefore far from obvious that NRSCs are an evolutionary “dead end”, and our survey of the literature clearly shows that a dearth of protein-coding genes does not necessarily mean that these chromosomes have no evolutionary or genetic potential.
Moving forward, it should be possible to use knowledge of the biology of a given species to predict by which mechanisms the NRSC could affect non-sexual traits. For example, in species with a similar biology to Drosophila—such as a lack of sex hormones and few coding genes on the Y/W/U/V—the NRSC should mainly act through epigenetic or regulatory effects. Such an approach would enable more focused and systematic detection of wide-ranging effects of NRSCs.
Our survey of the literature has revealed that almost any type of trait may be influenced by NRSC, making it challenging to find any commonalities. This is likely to some extent due to the haphazard nature of research in this area to date, but arguably also an inevitable product of the wide range of taxa that have been studied. Nevertheless, phylogenetic comparisons suggest clear links between the NRSC and longevity in diploid systems [112], and there is scope for further research in this area. For example, is there a relationship between sex differences in longevity and the relative size of the sex chromosomes compared to the rest of the genome? Or are sex differences in longevity better explained by the relative size of the non-recombining region compared to the PAR? Do ZW species experience a mosaic loss of W (LOW) similar to the LOY that has been observed in humans, and does this contribute to decreasing fitness with age or decreased longevity in females? This question was recently addressed in two long-lived bird species, where researchers did not find evidence for LOW [222]. However, we argue that the phenomenon of LOY and LOW could be found in other species. As the LOY has been shown to be accelerated by smoking [223], we suggest that LOW in birds, for example, might be detectable in urban birds, which experience toxins similar to smoking.
More speculatively, could NRSCs contribute to the genome-wide resolution of sexual antagonism, since they seem to often have extensive regulatory effects? Sexually antagonistic alleles are those which are beneficial in one sex, but deleterious in the other [224]. In the canonical model of sex chromosome evolution, it is the existence of sexually antagonistic loci in the PAR which favours the evolution of recombination cessation, in order to ensure that male-beneficial alleles are inherited together with the male-determining region, and female-beneficial alleles together with the female-determining region [7]. However, it is also possible that sex-limited chromosomes may acquire genetic variation which helps to resolve sexual antagonism post-recombination cessation [225]. Our survey of the literature found that a number of traits which have been previously associated with sexually antagonistic selection pressures can be influenced by the NRSC. For example, cholesterol levels and height in humans [226], colour genes in live bearing fish [7], same-sex sexual behaviour in Drosophila [174], and body size in Callosobruchus seed beetles [227] are all traits which have been previously shown to be sexually antagonistic, and which here were found to be affected by Y genotype. This provides some intriguing first evidence that NRSCs may play a more important role in resolving sexual antagonism than has previously been appreciated, even when highly degenerated.
Finally, we would like to highlight the unique potential of the currently understudied UV systems. In these systems, it is possible to disentangle effects of cessation of recombination from hemizygosity and sex-specific life history differences, which is not possible with XY and ZW systems [214]. They are also interesting candidates for use in experimental evolution, as they are expected to experience a faster response to sex-specific selection compared to diploid systems, since both sexes will experience selection for recombination arrest [228]. It may therefore be possible to gain new insights into the evolution of XY and ZW systems by studying UV systems.
Our survey of the literature revealed much more evidence of effects of Y, W and U and V chromosomes on non-sexual traits than we had initially anticipated. Given the fact that such effects were in many cases unexpected and unlooked for (e.g. [183]), it seems likely that we are currently only seeing the tip of the iceberg, and that many more examples of the genetic potential of NRSCs are waiting to be discovered.
Availability of data and materials
Not applicable.
References
Saunders PA, Neuenschwander S, Perrin N. Sex chromosome turnovers and genetic drift: a simulation study. J Evol Biol. 2018;31(9):1413–9.
Charlesworth D, Mank JE. The birds and the bees and the flowers and the trees: lessons from genetic mapping of sex determination in plants and animals. Genetics. 2010;186(1):9–31.
Ming R, Bendahmane A, Renner SS. Sex chromosomes in land plants. Annu Rev Plant Biol. 2011;62(1):485–514.
Bachtrog D, Mank JE, Peichel CL, Kirkpatrick M, Otto SP, Ashman T, et al. Sex determination: why so many ways of doing it? PLoS Biol. 2014;12(7):e1001899.
Ellegren H. Sex-chromosome evolution: recent progress and the influence of male and female heterogamety. Nat Rev Genet. 2011;12(3):157–66.
Parsch J, Ellegren H. The evolutionary causes and consequences of sex-biased gene expression. Nat Rev Genet. 2013;14(2):83–7.
Beukeboom LW, Perrin N. The evolution of sex determination. Oxford University Press. 2014. p. 240. https://global.oup.com/academic/product/the-evolution-of-sex-determination-9780199657148?cc=us&lang=en&.
Pennell MW, Kirkpatrick M, Otto SP, Vamosi JC, Peichel CL, Valenzuela N, et al. Y fuse? Sex chromosome fusions in fishes and reptiles. PLoS Genet. 2015;11(5):e1005237.
Scott MF, Osmond MM, Otto SP. Haploid selection, sex ratio bias, and transitions between sex-determining systems. PLoS Biol. 2018;16(6):e2005609.
Bateman AJ. Intra-sexual selection in Drosophila. Heredity (Edinb). 1948;2:349–68.
Immler S, Otto SP. The evolution of sex chromosomes in organisms with separate haploid sexes. Evolution (N Y). 2015;69(3):694–708.
Cox KH, Bonthuis PJ, Rissman EF. Mouse model systems to study sex chromosome genes and behavior: relevance to humans. Front Neuroendocrinol. 2014;35(4):405–19.
Ross JL, Tartaglia N, Merry DE, Dalva M, Zinn AR. Behavioral phenotypes in males with XYY and possible role of increased NLGN4Y expression in autism features. Genes Brain Behav. 2015;14(2):137–44.
Raznahan A, Lee NR, Greenstein D, Wallace GL, Blumenthal JD, Clasen LS, et al. Globally divergent but locally convergent X- and Y-chromosome influences on cortical development. Cereb Cortex. 2016;26(1):70–9.
Arnold AP. Sexual differentiation of brain and other tissues: five questions for the next 50 years. Horm Behav. 2020;120:104691.
Shah S, Ayub Q, Firasat S, Kaiser F, Mehdi S. Y haplogroups and aggressive behavior in a Pakistani ethnic group. Aggr Behav. 2009;35(1):68–74.
Götz MJ, Johnstone EC, Ratcliffe SG. Criminality and antisocial behaviour in unselected men with sex chromosome abnormalities. Psychol Med. 1999;29(4):953–62.
Jarvik LF, Klodin V, Matsuyama SS. Human aggression and the extra Y chromosome: Fact or fantasy? Am Psychol. 1973;28(8):674–82.
Nanko S. Personality traits of 47,XYY and 47,XXY males found among juvenile delinquents. Folia Psychiatr Neurol Jpn. 1979;33(1):29–34.
Stochholm K, Bojesen A, Jensen AS, Juul S, Gravholt CH. Criminality in men with Klinefelter’s syndrome and XYY syndrome: a cohort study. BMJ Open. 2012;2(1):e000650.
Price WH, Whatmore PB. Behaviour disorders and pattern of crime among XYY males identified at a maximum security hospital. Br Med J. 1967;1(5539):533–6.
Jutley JK, Stewart AD. Genetic analysis of the Y-chromosome of the mouse: evidence for two loci affecting androgen metabolism. Genet Res. 1985;47(1):29–34.
Monahan EJ, Maxson SC. Y chromosome, urinary chemosignals, and an agonistic behavior (offense) of mice. Physiol Behav. 1998;64(2):123–32.
Sluyter F, van Oortmerssen GA, de Ruiter AJ, Koolhaas JM. Aggression in wild house mice: current state of affairs. Behav Genet. 1996;26(5):489–96.
De Vries GJ, Rissman EF, Simerly RB, Yang L-Y, Scordalakes EM, Auger CJ, et al. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22(20):9005–14.
Guillot PV, Carlier M, Maxson SC, Roubertoux PL. Intermale aggression tested in two procedures, using four inbred strains of mice and their reciprocal congenics: Y chromosomal implications. Behav Genet. 1995;25(4):357–60.
Maxson SC. Searching for candidate genes with effects on an agonistic behavior, offense, in mice. Behav Genet. 1996;26(5):471–6.
Selmanoff MK, Abreu E, Goldman BD, Ginsburg BE. Manipulation of aggressive behavior in adult DBA/2/Bg and C57BL/10/Bg male mice implanted with testosterone in silastic tubing. Horm Behav. 1977;8(3):377–90.
Toot J, Dunphy G, Turner M, Ely D. The SHR Y-chromosome increases testosterone and aggression, but decreases serotonin as compared to the WKY Y-chromosome in the rat model. Behav Genet. 2004;34(5):515–24.
Kittles RA, Long JC, Bergen AW, Eggert M, Virkkunen M, Linnoila M, et al. Cladistic association analysis of Y chromosome effects on alcohol dependence and related personality traits. Proc Natl Acad Sci U S A. 1999;96(1):4204–9.
Dumanski JP, Lambert JC, Rasi C, Giedraitis V, Davies H, Grenier-Boley B, et al. Mosaic loss of chromosome Y in blood is associated with Alzheimer disease. Am J Hum Genet. 2016;98(6):1208–19.
Graham EJ, Vermeulen M, Vardarajan B, Bennett D, De Jager P, Pearse RV, et al. Somatic mosaicism of sex chromosomes in the blood and brain. Brain Res. 2019;1721(February):146345. https://doi.org/10.1016/j.brainres.2019.146345.
Laarakker MC, Ohl F, Van Lith HA. Chromosomal assignment of quantitative trait loci influencing modified hole board behavior in laboratory mice using consomic strains, with special reference to anxiety-related behavior and mouse chromosome 19. Behav Genet. 2008;38(2):159–84.
Nelson VR, Spezio SH, Nadeau JH. Transgenerational genetic effects of the paternal Y chromosome on daughters’ phenotypes. Epigenomics. 2010;2(4):513–21.
Serajee FJ, Huq AHMM. Association of Y chromosome haplotypes with autism. J Child Neurol. 2009;24(10):1258–61.
Wu JB, Chen K, Li Y, Lau Y-FC, Shih JC. Regulation of monoamine oxidase A by the SRY gene on the Y chromosome. FASEB J. 2009;23(11):4029–38.
Jamain S, Quach H, Quintana-Murci L, Betancur C, Philippe A, Gillberg C, et al. Y chromosome haplogroups in autistic subjects. Mol Psychiatry. 2002;7(2):217–9.
Botbol M, Roubertoux PL, Carlier M, Trabado S, Brailly-Tabard S, Perez-Diaz F, et al. Modulation of brain β-endorphin concentration by the specific part of the Y chromosome in mice. PLoS One. 2011;6(3):e16704.
Yamazaki K, Beauchamp GK, Matsuzaki O, Bard J, Thomas L, Boyse EA. Participation of the murine X and Y chromosomes in genetically determined chemosensory identity. Proc Natl Acad Sci U S A. 1986;83(June):4438–40.
Schellinck HM, Monahan E, Brown RE, Maxson SC. A comparison of the contribution of the major histocompatibility complex (MHC) and Y chromosomes to the discriminability of individual urine odors of mice by Long-Evans rats. Behav Genet. 1993;23(3):257–63.
Czech DP, Lee J, Sim H, Parish CL, Vilain E, Harley VR. The human testis-determining factor SRY localizes in midbrain dopamine neurons and regulates multiple components of catecholamine synthesis and metabolism. J Neurochem. 2012;122(2):260–71.
van Abeelen JHF, Janssens CJJG, Crusio WE, Lemmens WAJG. Y-chromosomal effects on discrimination learning and hippocampal asymmetry in mice. Behav Genet. 1989;19(4):543–9.
Hensbroek RA, Sluyter F, Guillot PV, Van Oortmerssen GA, Crusio WE. Y chromosomal effects on hippocampal mossy fiber distributions in mice selected for aggression. Brain Res. 1995;682(1–2):203–6.
Sluyter F, Bohus B, Beldhuis HJA, van Oortmerssen GA. Autosomal and Y chromosomal effects on the stereotyped response to apomorphine in wild house mice. Pharmacol Biochem Behav. 1995;52(1):17–22.
Ely D, Milsted A, Dunphy G, Boehme S, Dunmire J, Hart M, et al. Delivery of sry1, but not sry2, to the kidney increases blood pressure and sns indices in normotensive wky rats. BMC Physiol. 2009;9(1):10.
Wang Q, Xue Y, Zhang Y, Long Q, Yang F, Turner DJ, et al. Genetic basis of Y-linked hearing impairment. Am J Hum Genet. 2013;92(2):301–6.
Yan J, Feng J, Schroer R, Li W, Skinner C, Schwartz CE, et al. Analysis of the neuroligin 4Y gene in patients with autism. Psychiatr Genet. 2008;18(4):204–7.
Bardsley MZ, Kowal K, Levy C, Gosek A, Ayari N, Tartaglia N, et al. 47,XYY syndrome: clinical phenotype and timing of ascertainment. J Pediatr. 2013;163(4):1085–94.
Nielsen J, Nordland E. Length of Y chromosome and activity in boys. Clin Genet. 1975;8:291–6.
Dickey C, Toot J, Terwilliger M, Payne R, Turner M, Ely D. The SHR Y chromosome increases cardiovascular, endocrine, and behavioral responses to stress compared to the WKY Y chromosome. Physiol Behav. 2012;106(2):101–8.
Caplea A, Seachrist D, Daneshvar H, Dunphy G, Ely D. Noradrenergic content and turnover rate in kidney and heart shows gender and strain differences. J Appl Physiol. 2002;92(2):567–71.
Lee J, Pinares-Garcia P, Loke H, Ham S, Vilain E, Harley VR. Sex-specific neuroprotection by inhibition of the Y-chromosome gene, SRY, in experimental Parkinson’s disease. Proc Natl Acad Sci U S A. 2019;116(33):16577–82.
Hirata T, Hishimoto A, Otsuka I, Okazaki S, Boku S, Kimura A, et al. Investigation of chromosome Y loss in men with schizophrenia. Neuropsychiatr Dis Treat. 2018;14:2115–22.
Yoshitsugu K, Meerabux J, Asai K, Yoshikawa T. Fine mapping of an isodicentric Y chromosomal breakpoint from a schizophrenic patient. Am J Med Genet Part B. 2003;116B(1):27–31.
Dumas P, Pausová Z, Kren V, Krenová D, Pravenec M, Dumont M, et al. Contribution of autosomal loci and the Y chromosome to the stress response in rats. Hypertension. 2000;35(2):568–73.
Kimura A, Hishimoto A, Otsuka I, Okazaki S, Boku S, Horai T, et al. Loss of chromosome Y in blood, but not in brain, of suicide completers. PLoS One. 2018;13(1):e0190667.
Molina E, Chew GS, Myers SA, Clarence EM, Eales JM, Tomaszewski M, et al. A novel Y-specific long non-coding RNA associated with cellular lipid accumulation in HepG2 cells and atherosclerosis-related genes. Sci Rep. 2017;7(1):16710.
Voskarides K, Hadjipanagi D, Papazachariou L, Griffin M, Panayiotou AG. Evidence for contribution of the Y chromosome in atherosclerotic plaque occurrence in men. Genet Test Mol Biomarkers. 2014;18(8):552–6.
Eales JM, Maan AA, Xu X, Michoel T, Hallast P, Batini C, et al. Human Y chromosome exerts pleiotropic effects on susceptibility to atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39(November):2386–401.
Praktiknjo SD, Llamas B, Picard S, Robert F, Langlais D, Haibe-kains B, et al. Novel effects of chromosome Y on cardiac regulation, chromatin remodeling, and neonatal programming in male mice. Endocrinology. 2013;154(December):4746–56.
Llamas B, Bélanger S, Picard S, Deschepper CF. Cardiac mass and cardiomyocyte size are governed by different genetic loci on either autosomes or chromosome Y in recombinant inbred mice. Physiol Genomics. 2007;31(2):176–82.
Llmas B, Verdugo RA, Churchill GA, Deschepper CF. Chromosome Y variants from different inbred mouse strains are linked to differences in the morphologic and molecular responses of cardiac cells to postpubertal testosterone. BMC Genomics. 2009;10:150.
Molina E, Clarence EM, Ahmady F, Chew GS, Charchar FJ. Coronary artery disease: why we should consider the Y chromosome. Hear Lung Circ. 2016;25(8):791–801.
Yan L, Cogan JD, Hedges LK, Nunley B, Hamid R, Austin ED. The Y chromosome regulates BMPR2 expression via SRY: a possible reason “why” fewer males develop pulmonary arterial hypertension. Am J Respir Crit Care Med. 2018;198(12):1581–3.
Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, et al. Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertension. 2010;55(5):1275–82.
Umar S, Cunningham CM, Itoh Y, Moazeni S, Vaillancourt M, Sarji S, et al. The Y chromosome plays a protective role in experimental hypoxic pulmonary hypertension. Am J Respir Crit Care Med. 2018;197(7):952–5.
Davidson A, Schork N, Jaques B, Kelman A, Sutcliffe R, Reid J, et al. Blood pressure in genetically hypertensive rats. Influence of the Y chromosome. Hypertension. 1995;26(3):452–9.
Ely DL, Daneshvar H, Turner ME, Johnson ML, Salisbury RL. The hypertensive Y chromosome elevates blood pressure in F11 normotensive rats. Hypertension. 1993;21(6 Pt 2):1071–5.
Milsted A, Underwood AC, Dunmire J, Delpuerto HL, Martins AS, Ely DL, et al. Regulation of multiple renin-angiotensin system genes by Sry. J Hypertens. 2010;28(1):59–64.
Yagil C, Hubner N, Kreutz R, Ganten D, Yagil Y. Congenic strains confirm the presence of salt-sensitivity QTLs on chromosome 1 in the Sabra rat model of hypertension. Physiol Genomics. 2003;12(2):85–95.
Charchar FJ, Tomaszewski M, Lacka B, Zakrzewski J, Zukowska-Szczechowska E, Grzeszczak W, et al. Association of the human Y chromosome with cholesterol levels in the general population. Arterioscler Thromb Vasc Biol. 2004;24(2):308–12.
Russo P, Siani A, Miller MA, Karanam S, Esposito T, Gianfrancesco F, et al. Genetic variants of Y chromosome are associated with a protective lipid profile in black men. Arterioscler Thromb Vasc Biol. 2008;28(8):1569–74.
Shoji M, Tsutaya S, Shimada J, Kojima K, Kasai T, Yasujima M. Lack of association between Y chromosome Alu insertion polymorphism and hypertension. Hypertens Res Off J Japanese Soc Hypertens. 2002;25(1):501–5.
Suto JI, Satou K. Effect of the Y chromosome on plasma high-density lipoprotein-cholesterol levels in Y-chromosome-consomic mouse strains. BMC Res Notes. 2014;7(1):393.
Kren V, Qi N, Krenova D, Zidek V, Sladka M, Jachymova M, et al. Y-chromosome transfer induces changes in blood pressure and blood lipids in SHR. Hypertension. 2001;37(4):1147–52.
Strahorn P, Graham D, Charchar FJ, Sattar N, McBride MW, Dominiczak AF. Genetic determinants of metabolic syndrome components in the stroke-prone spontaneously hypertensive rat. J Hypertens. 2005;23(12):2179–86.
Ely D, Boehme S, Dunphy G, Hart M, Chiarappa F, Miller B, et al. The Sry3 Y chromosome locus elevates blood pressure and renin-angiotensin system indexes. Gend Med. 2011;8(2):126–38.
Loftfield E, Zhou W, Graubard BI, Yeager M, Chanock SJ, Freedman ND, et al. Predictors of mosaic chromosome Y loss and associations with mortality in the UK Biobank. Sci Rep. 2018;8(1):12316.
Lleo A, Oertelt-prigione S, Bianchi I, Caliari L, Finelli P, Miozzo M, et al. Y chromosome loss in male patients with primary biliary cirrhosis. J Autoimmun. 2013;41:87–91.
Persani L, Bonomi M, Lleo A, Pasini S, Civardi F, Bianchi I, et al. Increased loss of the Y chromosome in peripheral blood cells in male patients with autoimmune thyroiditis. J Autoimmun. 2012;38(2–3):J193–6.
Tang D, Han Y, Lun Y, Jiang H, Xin S, Duan Z, et al. Y chromosome loss is associated with age-related male patients with abdominal aortic aneurysms. Clin Interv Aging. 2019;14:1227–41.
Case LK, Wall EH, Dragon JA, Saligrama N, Krementsov DN, Moussawi M, et al. The Y chromosome as a regulatory element shaping immune cell transcriptomes and susceptibility to autoimmune disease. Genome Res. 2013;23:1474–85.
Case LK, Wall EH, Osmanski EE, Dragon JA, Saligrama N, Zachary JF, et al. Copy number variation in Y chromosome multicopy genes is linked to a paternal parent-of-origin effect on CNS autoimmune disease in female offspring. Genome Biol. 2015;16(1):28.
Eisenberg RA, Dixon FJ. Effect of castration on male-determined acceleration of autoimmune disease in BXSB mice. J Immunol. 1980;125(5):1959–61.
Teuscher C, Noubade R, Spach K, McElvany B, Bunn JY, Fillmore PD, et al. Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proc Natl Acad Sci U S A. 2006;103(21):8024–9.
Spach KM, Blake M, Bunn JY, McElvany B, Noubade R, Blankenhorn EP, et al. Cutting edge: the Y chromosome controls the age-dependent experimental allergic encephalomyelitis sexual dimorphism in SJL/J mice. J Immunol. 2009;182(4):1789–93.
Santiago-Raber M-L, Kikuchi S, Borel P, Uematsu S, Akira S, Kotzin BL, et al. Evidence for genes in addition to Tlr7 in the Yaa translocation linked with acceleration of systemic lupus erythematosus. J Immunol. 2008;181(August):1556–62.
Wesley JD, Tessmer MS, Paget C, Trottein F, Brossay L. A Y chromosome-linked factor impairs NK T development. J Immunol. 2007;179:3480–7.
Case LK, Toussaint L, Moussawi M, Roberts B, Saligrama N, Brossay L, et al. Chromosome Y regulates survival following murine coxsackievirus B3 infection. G3. 2012;2(1):115–21.
Sun S, Horino S, Itoh-Nakadai A, Kawabe T, Asao A, Takahashi T, et al. Y chromosome-linked B and NK cell deficiency in mice. J Immunol. 2013;190(12):6209–20.
Mattisson J, Danielsson M, Hammond M, Davies H, Gallant CJ, Nordlund J, et al. Leukocytes with chromosome Y loss have reduced abundance of the cell surface immunoprotein. Sci Rep. 2021;11:15160.
Sezgin E, Lind JM, Shrestha S, Hendrickson S, Goedert JJ, Don S, et al. Association of Y chromosome haplogroup I with HIV progression, and HAART outcome. Hum Genet. 2009;125:281–94.
Robinson DP, Huber SA, Moussawi M, Roberts B, Teuscher C, Watkins R, et al. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol Sex Differ. 2011;2(Il):8.
Krementsov DN, Case LK, Dienz O, Raza A, Fang Q, Ather JL. Genetic variation in chromosome Y regulates susceptibility to influenza A virus infection. Proc Natl Acad Sci U S A. 2017;114(13):3491–6.
Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Jacob HJ, et al. Chromosome substitution reveals the genetic basis of Dahl salt-sensitive hypertension and renal disease. Am J Physiol Physiol. 2008;295(3):F837–42.
Guo X, Dai X, Zhou T, Wang H, Ni J, Xue J, et al. Mosaic loss of human Y chromosome: what, how and why. Hum Genet. 2020;139(4):421–46.
Nomdedeu M, Pereira A, Calvo X, Colomer J, Sole F, Arias A, et al. Clinical and biological significance of isolated Y chromosome loss in myelodysplastic syndromes and chronic myelomonocytic leukemia. A report from the Spanish MDS Group. Leuk Res. 2017;63(July):85–9.
Mitelman F, Johansson B, Mertens F. Mitelman database of chromosome aberrations and gene fusions in cancer. 2019. Available from: http://cgap.nci.nih.gov/Chromosomes/Mitelman. Cited 2019 Feb 19.
Kido T, Chris Lau Y-F. Roles of the Y chromosome genes in human cancers. Asian J Androl. 2015;17(January):373–80.
Bianchi NO. Y chromosome structural and functional changes in human malignant diseases. Mutat Res - Rev Mutat Res. 2009;682(1):21–7.
Gozdecka M, Meduri E, Mazan M, Tzelepis K, Dudek M, Knights AJ, et al. UTX-mediated enhancer and chromatin remodeling suppresses myeloid leukemogenesis through noncatalytic inverse regulation of ETS and GATA programs. Nat Genet. 2018;50(6):883–94.
Murakami S, Chishima S, Uemoto H, Sakamoto E, Sato T, Kurabe N, et al. The male-specific factor Sry harbors an oncogenic function. Oncogene. 2014;33(23):2978–86.
Chen WC, Yang CC, Tsai RY, Liao CY, Yen YT, Hung CL, et al. Expression of sex-determining genes in the scalp of men with androgenetic alopecia. Dermatology. 2007;214(3):199–204.
Ellis JA, Stebbing M, Harrap SB. Significant population variation in adult male height associated with the Y chromosome and the aromatase gene. J Clin Endocrinol Metab. 2001;86(9):4147–50.
Kirsch S, Weiss B, De Rosa M, Ogata T, Lombardi G, Rappold GA. FISH deletion mapping defines a single location for the Y chromosome stature gene, GCY. J Med Genet. 2000;37(8):593–9.
Ogata T, Matsuo N. Comparison of adult height between patients with XX and XY gonadal dysgenesis: support for a Y specific growth gene(s). J Med Genet. 1992;29:539–41.
Ogata T, Matsuo N. The Y specific growth gene(s): how does it promote stature? J Med Genet. 1997;34:323–5.
Burgoyne PS, Thornhill AR, Boudrean SK, Darling SM, Bishop CE, Evans EP. The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse. Philos Trans R Soc Lond B Biol Sci. 1995;350(1333):253–60 discussion 260-261.
Suto JI. Y chromosome of the inbred mouse KK/Ta strain is associated with reduced body size in Y-consomic strains. BMC Res Notes. 2013;6(1):64.
Li Y, Kido T, Garcia-Barcelo MM, Tam PKH, Tabatabai ZL, Lau YFC. SRY interference of normal regulation of the RET gene suggests a potential role of the Y-chromosome gene in sexual dimorphism in Hirschsprung disease. Hum Mol Genet. 2015;24(3):685–97.
Grassmann F, Kiel C, den Hollander AI, Weeks DE, Lotery A, Cipriani V, et al. Y chromosome mosaicism is associated with age-related macular degeneration. Eur J Hum Genet. 2019;27(1):36–41.
Xirocostas ZA, Everingham SE, Moles AT. The sex with the reduced sex chromosome dies earlier: a comparison across the tree of life. Biol Lett. 2020;16(3):20190867.
Forsberg LA, Rasi C, Malmqvist N, Davies H, Pasupulati S, Pakalapati G, et al. Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat Genet. 2014;46(6):624–8.
Lund JB, Li S, Christensen K, Mengel-From J, Soerensen M, Marioni RE, et al. Age-dependent DNA methylation patterns on the Y chromosome in elderly males. Aging Cell. 2020;19(2):e12907.
Stochholm K, Juul S, Gravholt CH. Diagnosis and mortality in 47,XYY persons: a registry study. Orphanet J Rare Dis. 2010;5(1):15.
Alvesalo L, de la Chapelle A. Tooth sizes in two males with deletions of the long arm of the Y-chromosome. Ann Hum Genet. 1981;45(1):49–54.
Tukiainen T, Villani AC, Yen A, Rivas MA, Marshall JL, Satija R, et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550(7675):244–8.
Vicoso B, Kaiser VB, Bachtrog D. Sex-biased gene expression at homomorphic sex chromosomes in emus and its implication for sex chromosome evolution. Proc Natl Acad Sci U S A. 2013;110(16):6453–8.
Kamiya T, Kai W, Tasumi S, Oka A, Matsunaga T, Mizuno N, et al. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger Pufferfish, Takifugu rubripes (Fugu). PLoS Genet. 2012;8(7):e1002798.
Lenormand T, Fyon F, Sun E, Roze D. Sex chromosome degeneration by regulatory evolution. Curr Biol. 2020;30(15):3001–6.
van Doorn GS. Patterns and mechanisms of evolutionary transitions between genetic sex-determining systems. Cold Spring Harb Perspect Biol. 2014;6:a017681.
Bachtrog D. Y-chromosome evolution: emerging insights into processes of Y-chromosome degeneration. Nat Rev Genet. 2013;14(2):113–24.
Fraser JA, Heitman J. Chromosomal sex-determining regions in animals, plants and fungi. Curr Opin Genet Dev. 2005;15(6):645–51.
Repping S, Van Daalen SKM, Brown LG, Korver CM, Lange J, Marszalek JD, et al. High mutation rates have driven extensive structural polymorphism among human Y chromosomes. Nat Genet. 2006;38(4):463–7.
Lyckegaard EMS, Clark AG. Evolution of ribosomal RNA gene copy number on the sex chromosomes of Drosophila melanogaster. Mol Biol Evol. 1991;8(4):458–74.
Griffin RM, Le Gall D, Schielzeth H, Friberg U. Within-population Y-linked genetic variation for lifespan in Drosophila melanogaster. J Evol Biol. 2015;28(11):1940–7.
Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol. 2009;21(4):377–86.
Graves JAM. Sex chromosome specialization and degeneration in mammals. Cell. 2006;124(5):901–14.
Bellott DW, Hughes JF, Skaletsky H, Brown LG, Pyntikova T, Cho T, et al. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature. 2014;508:494–9.
Snell DM, Turner JMA. Sex chromosome effects on male–female differences in mammals. Curr Biol. 2018;28(22):R1313–24.
Gruetzner F, Ashley T, Rowell DM, Graves JAM. How did the platypus get its sex chromosome chain? A comparison of meiotic multiples and sex chromosomes in plants and animals. Chromosoma. 2006;115(2):75–88.
Howe KL, Achuthan P, Allen J, Allen J, Alvarez-Jarreta J, Ridwan Amode M, et al. Ensembl 2021. Nucleic Acids Res. 2021;49(D1):D884–91.
Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, et al. The male-specific region of the human Y chromosome is a mosic of discrete sequence classes. Nature. 2003;423:825–37.
Prokop JW, Deschepper CF. Chromosome Y genetic variants: impact in animal models and on human disease. Physiol Genomics. 2015;47(11):525–37.
Broad Institute of MIT and Harvard T. GTEx Portal, version 7. 2017. Available from: https://www.gtexportal.org/home/. Cited 2018 Apr 26.
Soh YQS, Alföldi J, Pyntikova T, Brown LG, Graves T, Minx PJ, et al. Sequencing the mouse Y chromosome reveals convergent gene acquisition and amplification on both sex chromosomes. Cell. 2014;159(4):800–13.
Jimenez R, Burgos M, Barrionuevo FJ. Sex maintenance in mammals. Genes (Basel). 2021;12(1):999.
Jost A. Hormonal factors in the development of the fetus. Cold Spring Harb Symp Quant Biol. 1954;19:167–81.
Deegan DF, Engel N. Sexual dimorphism in the age of genomics: how, when, where. Front Cell Dev Biol. 2019;7(September):186.
Dean R, Mank JE. Tissue specificity and sex-specific regulatory variation permit the evolution of sex-biased gene expression. Am Nat. 2016;188(3):E74–84.
Fryns J, Kleczkowska A, Kubień E, Van den Berghe H. XYY syndrome and other Y chromosome polysomies. Mental status and psychosocial functioning. Genet Couns. 1995;6(3):197–206.
Ratcliffe S, Butler G, Jones M. Edinburgh study of growth and development of children with sex chromosome abnormalities. In: Evans J, Hamerton J, Robinson A, editors. Children and young adults with sex chromosome aneuploidy: follow-up. New York: Wiley; 1990. p. 1–44.
Bloomer LDS, Nelson CP, Eales J, Denniff M, Christofidou P, Debiec R, et al. Male-specific region of the Y chromosome and cardiovascular risk: phylogenetic analysis and gene expression studies. Arterioscler Thromb Vasc Biol. 2013;33(7):1722–7.
Bloomer LDS, Nelson CP, Denniff M, Christofidou P, Debiec R, Thompson J, et al. Coronary artery disease predisposing haplogroup I of the Y chromosome, aggression and sex steroids - genetic association analysis. Atherosclerosis. 2014;233(1):160–4.
Mayne BT, Bianco-Miotto T, Buckberry S, Breen J, Clifton V, Shoubridge C, et al. Large scale gene expression meta-analysis reveals tissue-specific, sex-biased gene expression in humans. Front Genet. 2016;7(OCT):183.
Ronen D, Benvenisty N. Sex-dependent gene expression in human pluripotent stem cells. Cell Rep. 2014;8(4):923–32.
Ghahramani NM, Ngun TC, Chen PY, Tian Y, Krishnan S, Muir S, et al. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol Sex Differ. 2014;5(1):8.
Brown EJ, Nguyen AH, Bachtrog D. The Drosophila Y chromosome affects heterochromatin integrity genome-wide. Mol Biol Evol. 2020;37(10):2808–24.
Werner RJ, Schultz BM, Huhn JM, Jelinek J, Madzo J, Engel N. Sex chromosomes drive gene expression and regulatory dimorphisms in mouse embryonic stem cells. Biol Sex Differ. 2017;8(1):28.
Beyer C, Kolbinger W, Froehlich U, Pilgrim C, Reisert I. Sex differences of hypothalamic prolactin cells develop independently of the presence of sex steroids. Brain Res. 1992;593(2):253–6.
Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci. 2002;5(10):933–4.
Dumanski JP, Halvardson J, Davies H, Rychlicka-Buniowska E, Mattisson J, Moghadam BT, et al. Immune cells lacking Y chromosome show dysregulation of autosomal gene expression. Cell Mol Life Sci. 2021;78(8):4019–33.
Burgoyne PS. A Y-chromosomal effet on blastocyst cell number in mice. Development. 1993;117:341–5.
Chen X, McClusky R, Itoh Y, Reue K, Arnold AP. X and Y chromosome complement influence adiposity and metabolism in mice. Endocrinology. 2013;154(3):1092–104.
Kopsida E, Stergiakouli E, Lynn PM, Wilkinson LS, Davies W. The role of the Y chromosome in brain function. Open Neuroendocr J. 2009;2(0):20–30.
Swaab DF, Wolff SEC, Bao A-M. Sexual differentiation of the human hypothalamus: relationship to gender identity and sexual orientation. In: Swaab DF, Buijs RM, Lucassen PJ, Salehi A, Kreier F, editors. Handbook of clinical neurology. 3rd ed. Elsevier B.V. 2021. p. 427–43. https://www.sciencedirect.com/science/article/abs/pii/B9780128206836000312?via%3Dihub.
Cocquet J, Ellis PJI, Yamauchi Y, Mahadevaiah SK, Affara NA, Ward MA, et al. The multicopy gene Sly represses the sex chromosomes in the male mouse germline after meiosis. PLoS Biol. 2009;7(11):e1000244.
Camerino G, Parma P, Radi O, Valentini S. Sex determination and sex reversal. Curr Opin Genet Dev. 2006;16(3):289–92.
Nielsen HS. Secondary recurrent miscarriage and H-Y immunity. Hum Reprod Update. 2011;17(4):558–74.
Bogaert AF, Skorska MN, Wang C, Gabrie J, MacNeil AJ, Hoffarth MR, et al. Male homosexuality and maternal immune responsivity to the Y-linked protein NLGN4Y. Proc Natl Acad Sci U S A. 2018;115(2):302–6.
Carvalho AB, Lazzaro BP, Clark AG. Y chromosomal fertility factors kl-2 and kl-3 of Drosophila melanogaster encode dynein heavy chain polypeptides. Proc Natl Acad Sci USA. 2000;97(24):13239–44.
Brown EJ, Nguyen AH, Bachtrog D. The Y chromosome may contribute to sex-specific ageing in Drosophila. Nat Ecol Evol. 2020;4(6):853–62.
Koerich LB, Wang X, Clark AG, Carvalho AB. Low conservation of gene content in the Drosophila Y chromosome. Nature. 2008;456(7224):949–51.
Piergentili R. Multiple roles of the Y chromosome in the biology of Drosophila melanogaster. Sci World J. 2010;10:1749–67.
Mahajan S, Bachtrog D. Convergent evolution of Y chromosome gene content in flies. Nat Commun. 2017;8(1):785.
Francisco FO, Lemos B. How do Y-chromosomes modulate genome-wide epigenetic states: genome folding, chromatin sinks, and gene expression. J Genomics. 2014;2:94–103.
Uryu O, Ameku T, Niwa R. Recent progress in understanding the role of ecdysteroids in adult insects: germline development and circadian clock in the fruit fly Drosophila melanogaster. Zool Lett. 2015;1(1):32.
Sackton TB, Hartl DL. Meta-analysis reveals that genes regulated by the Y chromosome in Drosophila melanogaster are preferentially localized to repressive chromatin. Genome Biol Evol. 2013;5(1):255–66.
Jiang PP, Hartl DL, Lemos B. Y not a dead end: epistatic interactions between Y-linked regulatory polymorphisms and genetic background affect global gene expression in Drosophila melanogaster. Genetics. 2010;186(1):109–18.
Paredes S, Branco AT, Hartl DL, Maggert KA, Lemos B. Ribosomal DNA deletions modulate genome-wide gene expression: “rDNA-sensitive” genes and natural variation. PLoS Genet. 2011;7(4):e1001376.
Menon DU, Meller VH. Imprinting of the Y chromosome influences dosage compensation in roX1 roX2 Drosophila melanogaster. Genetics. 2009;183(3):811–20.
Stoltenberg SF, Hirsch J. Y-chromosome effects on Drosophila geotaxis interact with genetic or cytoplasmic background. Anim Behav. 1997;53(November):853–64.
Kutch IC, Fedorka KM. Y-linked variation for autosomal immune gene regulation has the potential to shape sexually dimorphic immunity. Proc R Soc B Biol Sci. 2015;282(1820):20151301.
Hoskins JL, Ritchie MG, Bailey NW. A test of genetic models for the evolutionary maintenance of same-sex sexual behaviour. Proc R Soc B Biol Sci. 1809;2015(282):20150429.
Lemos B, Araripe LO, Hartl DL. Polymorphic Y chromosomes harbor cryptic variation with manifold functional consequences. Science (80- ). 2008;319(5859):91–3.
Dean R, Lemos B, Dowling DK. Context-dependent effects of Y chromosome and mitochondrial haplotype on male locomotive activity in Drosophila melanogaster. J Evol Biol. 2015;28(10):1861–71.
Friberg U, Stewart AD, Rice WR. X- and Y-chromosome linked paternal effects on a life-history trait. Biol Lett. 2011;8(1):71–3.
Lund-Hansen KK, Olito C, Morrow EH, Abbott JK. Sexually antagonistic coevolution between the sex chromosomes of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2021;118(8):e2003359118.
Morris J, Darolti I, van der Bijl W, Mank JE. High-resolution characterization of male ornamentation and re-evaluation of sex linkage in guppies. Proc R Soc B Biol Sci. 2020;287(1937):20201677.
Sandkam BA, Almeida P, Darolti I, Furman BLS, van der Bijl W, Morris J, et al. Extreme Y chromosome polymorphism corresponds to five male reproductive morphs of a freshwater fish. Nat Ecol Evol. 2021;5:939–48.
Lampert KP, Schmidt C, Fischer P, Volff JN, Hoffmann C, Muck J, et al. Determination of onset of sexual maturation and mating behavior by melanocortin receptor 4 polymorphisms. Curr Biol. 2010;20(19):1729–34.
Griebling HJ, Rios-Cardenas O, Abbott J, Morris MR. A study of tactical and sexual dimorphism in cognition with insights for sexual conflict. Anim Behav. 2020;170:43–50.
Kaufmann P, Wolak ME, Husby A, Immonen E. Rapid evolution of sexual size dimorphism facilitated by Y-linked genetic variance. Nat Ecol Evol. 2021;5:1394–402.
Hosken DJ, House CM. Sexual selection. Curr Biol. 2011;21(2):R62–5.
Ågren JA, Munasinghe M, Clark AG. Sexual conflict through mother’s curse and father’s curse. Theor Popul Biol. 2019;129:9–17.
Cortez D, Marin R, Toledo-Flores D, Froidevaux L, Liechti A, Waters PD, et al. Origins and functional evolution of Y chromosomes across mammals. Nature. 2014;508(7497):488–93.
Smeds L, Warmuth V, Bolivar P, Uebbing S, Burri R, Suh A, et al. Evolutionary analysis of the female-specific avian W chromosome. Nat Commun. 2015;6(June):7330.
Hirst CE, Major AT, Ayers KL, Brown RJ, Mariette M, Sackton TB, et al. Sex reversal and comparative data undermine the W chromosome and support Z-linked DMRT1 as the regulator of gonadal sex differentiation in birds. Endocrinology. 2017;158(9):2970–87.
Smith CA. Sex determination in birds: HINTs from the W sex chromosome? Sex Dev. 2007;1(5):279–85.
Backström N, Ceplitis H, Berlin S, Ellegren H. Gene conversion drives the evolution of HINTW, an ampliconic gene on the female-specific avian W chromosome. Mol Biol Evol. 2005;22(10):1992–9.
Ayers KL, Davidson NM, Demiyah D, Roeszler KN, Grützner F, Sinclair AH, et al. RNA sequencing reveals sexually dimorphic gene expression before gonadal differentiation in chicken and allows comprehensive annotation of the W-chromosome. Genome Biol. 2013;14(3):R26.
Graves JAM. Evolution of vertebrate sex chromosomes and dosage compensation. Nat Rev Genet. 2016;17(1):33–46.
Bellott DW, Skaletsky H, Cho TJ, Brown L, Locke D, Chen N, et al. Avian W and mammalian Y chromosomes convergently retained dosage-sensitive regulators. Nat Genet. 2017;49(3):387–94.
Zhao D, McBride D, Nandi S, McQueen HA, McGrew MJ, Hocking PM, et al. Somatic sex identity is cell autonomous in the chicken. Nature. 2010;464(7286):237–42.
Wade J, Arnold P. Functional testicular tissue does not masculinize development of the zebra finch song system. Proc Natl Acad Sci U S A. 1996;93(May):5264–8.
Wade J, Swender DA, McElhinny TL. Sexual differentiation of the zebra finch song system parallels genetic, not gonadal, sex. Horm Behav. 1999;36(2):141–52.
Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, et al. Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proc Natl Acad Sci U S A. 2003;100(8):4873–8.
Evans SR, Schielzeth H, Forstmeier W, Sheldon BC, Husby A. Nonautosomal genetic variation in carotenoid coloration. Am Nat. 2014;184(3):374–83.
Fossøy F, Sorenson MD, Liang W, Ekrem T, Moksnes A, Møller AP, et al. Ancient origin and maternal inheritance of blue cuckoo eggs. Nat Commun. 2016;7:10272.
Gosler AG, Barnett PR, Reynolds SJ. Inheritance and variation in eggshell patterning in the great tit Parus major. Proc R Soc B Biol Sci. 2000;267:2469–73.
Fraïsse C, Picard MAL, Vicoso B. The deep conservation of the Lepidoptera Z chromosome suggests a non-canonical origin of the W. Nat Commun. 2017;8(1):1486.
Kaiser VB, Bachtrog D. Evolution of sex chromosomes in insects. Annu Rev Genet. 2010;44:91–112.
Gotter AL, Levine JD, Reppert SM. Sex-linked period genes in the silkmoth, Antheraea pernyi: Implications for circadian clock regulation and the evolution of sex chromosomes. Neuron. 1999;24(4):953–65.
Van’T Hof AE, Nguyen P, Dalíková M, Edmonds N, Marec F, Saccheri IJ. Linkage map of the peppered moth, Biston betularia (Lepidoptera, Geometridae): a model of industrial melanism. Heredity (Edinb). 2013;110(3):283–95.
Kiuchi T, Koga H, Kawamoto M, Shoji K, Sakai H, Arai Y, et al. A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature. 2014;509(7502):633–6.
Traut W, Marec F. Sex chromatin in Lepidoptera. Q Rev Biol. 1996;71(2):239–56.
Hejníčková M, Koutecký P, Potocký P, Provazníková I, Voleníková A, Dalíková M, et al. Absence of W chromosome in psychidae moths and implications for the theory of sex chromosome evolution in Lepidoptera. Genes (Basel). 2019;10(12):1016.
Dalíková M, Zrzavá M, Hladová I, Nguyen P, Šonský I, Flegrová M, et al. New insights into the evolution of the W chromosome in Lepidoptera. J Hered. 2017;108(7):709–19.
Roberts RB, Ser JR, Kocher TD. Sexual conflict resolved by invasion of a novel sex determiner in Lake Malawi cichlid fishes. Science. 2009;326(5955):998–1001.
Vicoso B, Emerson JJ, Zektser Y, Mahajan S, Bachtrog D. Comparative sex chromosome genomics in snakes: differentiation, evolutionary strata, and lack of global dosage compensation. PLoS Biol. 2013;11(8):e1001643.
Tanurdzic M, Banks JA. Sex-determining mechanisms in land plants. Plant Cell. 2004;16(suppl_1):S61–71.
Bowman JL, Kohchi T, Yamato KT, Jenkins J, Shu S, Ishizaki K, et al. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell. 2017;171(2):287–304.
Coelho SM, Mignerot L, Cock JM. Origin and evolution of sex-determination systems in the brown algae. New Phytol. 2019;222(4):1751–6.
Carey SB, Jenkins J, Lovell JT, Maumus F, Sreedasyam A, Payton AC, et al. Gene-rich UV sex chromosomes harbor conserved regulators of sexual development. Sci Adv. 2021;7(June):eabh2488.
Takezawa D, Komatsu K, Sakata Y. ABA in bryophytes: how a universal growth regulator in life became a plant hormone? J Plant Res. 2011;124(4):437–53.
Nath V, Bansal P. Reproductive strategies in bryophytes. In: Bahadur B, Rajam MV, Sahijram L, Krishnamurthy KV, editors. Plant biology and biotechnology. Springer (India). 2015. p. 335–47. https://link.springer.com/chapter/10.1007/978-81-322-2286-6_13.
Kollar LM, Kiel S, James AJ, Carnley CT, Scola DN, Clark TN, et al. The genetic architecture of sexual dimorphism in the moss Ceratodon purpureus. Proc R Soc B Biol Sci. 1946;2021(288):20202908.
Bachtrog D. The temporal dynamics of processes underlying Y chromosome degeneration. Genetics. 2008;179(3):1513–25.
Hughes JF, Skaletsky H, Brown LG, Pyntikova T, Graves T, Fulton RS, et al. Strict evolutionary conservation followed rapid gene loss on human and rhesus Y chromosomes. Nature. 2012;483(7387):82–7.
Marais GAB, Campos PRA, Gordo I. Can intra-Y gene conversion oppose the degeneration of the human Y chromosome? A simulation study. Genome Biol Evol. 2010;2(1):347–57.
Connallon T, Clark AG. Gene duplication, gene conversion and the evolution of the Y chromosome. Genetics. 2010;186(1):277–86.
Trujillo N, Martínez-pacheco M, Soldatini C, Young RC, Albores-barajas YV, Orta AH, et al. Lack of age-related mosaic loss of W chromosome in long-lived birds. Biol Lett. 2022;18:20210553.
Dumanski JP, Rasi C, Lonn M, Davies H, Ingelsson M, Giedraitis V, et al. Smoking is associated with mosaic loss of chromosome Y. Science (80- ). 2015;347(6217):81–3.
Rice WR, Gavrilets S, editors. The genetics and biology of sexual conflict. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2014. p. 432.
Abbott JK, Nordén AK, Hansson B. Sex chromosome evolution: historical insights and future perspectives. Proc R Soc B Biol Sci. 2017;284(1854):20162806.
Stearns SC, Govindaraju DR, Ewbank D, Byars SG. Constraints on the coevolution of contemporary human males and females. Proc R Soc B Biol Sci. 2012;279(1748):4836–44.
Berger D, Berg EC, Widegren W, Arnqvist G, Maklakov AA. Multivariate intralocus sexual conflict in seed beetles. Evolution (N Y). 2014;68(12):3457–69.
Bachtrog D, Kirkpatrick M, Mank JE, McDaniel SF, Pires JC, Rice WR, et al. Are all sex chromosomes created equal? Trends Genet. 2011;27(9):350–7.
Acknowledgements
We thank Nauris Cinovics for the help with the figures.
Funding
AC and JKA were supported by the European Research Council (ERC-2015-StG-678148) and the Swedish Research Council (VR-2015-04680) and BH by the Swedish Research Council (621-2016-689).
Author information
Authors and Affiliations
Contributions
AC conceived the idea and lead the process. AC, BH and JKA wrote the review. The authors read and approved the final version.
Authors’ information
Twitter handles: Aivars Cīrulis (@Cirulis_A), Bengt Hansson (@BengtHanssonLU) and Jessica K. Abbott (@jessicakabbott).
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cīrulis, A., Hansson, B. & Abbott, J.K. Sex-limited chromosomes and non-reproductive traits. BMC Biol 20, 156 (2022). https://doi.org/10.1186/s12915-022-01357-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12915-022-01357-5