Abstract
Background
Sepsis survivors experience high morbidity and mortality. Though recommended best practices have been established to address the transition and early post hospital needs and promote recovery for sepsis survivors, few patients receive recommended post-sepsis care. Our team developed the Sepsis Transition and Recovery (STAR) program, a multicomponent transition intervention that leverages virtually-connected nurses to coordinate the application of evidence-based recommendations for post-sepsis care with additional clinical support from hospitalist and primary care physicians. In this paper, we present findings from a qualitative pre-implementation study, guided by the Consolidated Framework for Implementation Research (CFIR), of factors to inform successful STAR implementation at a large learning health system prior to effectiveness testing as part of a Type I Hybrid trial.
Methods
We conducted semi-structured qualitative interviews (n = 16) with 8 administrative leaders and 8 clinicians. Interviews were transcribed and analyzed in ATLAS.ti using a combination deductive/inductive strategy based on CFIR domains and constructs and the Constant Comparison Method.
Results
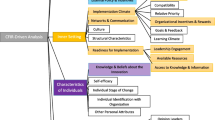
Six facilitators and five implementation barriers were identified spanning all five CFIR domains (Intervention Characteristics, Outer Setting, Inner Setting, Characteristics of Individuals and Process). Facilitators of STAR included alignment with health system goals, fostering stakeholder engagement, sharing STAR outcomes data, good communication between STAR navigators and patient care teams/PCPs, clinician promotion of STAR with patients, and good rapport and effective communication between STAR navigators and patients, caregivers, and family members. Barriers of STAR included competing demands for staff time and resources, insufficient communication and education of STAR’s value and effectiveness, underlying informational and technology gaps among patients, lack of patient access to community resources, and patient distrust of the program and/or health care.
Conclusions
CFIR proved to be a robust framework for examining facilitators and barriers for pre-implementation planning of post-sepsis care programs within diverse hospital and community settings in a large LHS. Conducting a structured pre-implementation evaluation helps researchers design with implementation in mind prior to effectiveness studies and should be considered a key component of Type I hybrid trials when feasible.
Trial registration
Clinicaltrials.gov, NCT04495946. Registered August 3, 2020.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Contributions to literature
-
This qualitative pre-implementation study of a telehealth nurse navigator-led sepsis transition and recovery (STAR) program demonstrates the Consolidated Framework for Implementation Research (CFIR) is useful to explore contextual conditions of healthcare settings as part of rigorous pre-implementation planning efforts.
-
This analysis identified actionable facilitators and barriers spanning all five CFIR domains (e.g., inner setting, outer setting) to inform and enhance initial implementation strategies of STAR.
-
These findings help to close recognized gaps in the literature on post-sepsis survivorship, including how to plan implementation of evidenced-based practices to address transition and early post hospital needs of sepsis survivors and promote recovery.
Background
Sepsis, a common and life-threatening dysregulated response to infection, remains a major cause of morbidity, mortality, and healthcare costs [1,2,3]. Although hospital survival has improved in recent years, the increasing number of sepsis survivors are vulnerable to additional health problems [4,5,6]. Fewer than one-half of sepsis survivors return to their pre-sepsis health status and many experience new or worsening physical, cognitive, and psychological impairments, along with high rates of rehospitalization and excess mortality for years after sepsis hospitalization [7,8,9]. Given increasing recognition of the substantial long-term sequelae and social determinants of health-related needs after sepsis [10], recommended best practices have been established to address the transition and early post hospital needs and promote recovery for sepsis survivors [11,12,13]. However, like the majority of other evidence-based practices (EBPs) that have yet to be successfully adopted into routine practice, few patients receive recommended post-sepsis care [14, 15].
To address the transition and early post hospital needs for sepsis survivors, our team developed the Sepsis Transition and Recovery (STAR) program, a multicomponent transition intervention that leverages centrally-located, virtually-connected nurses to coordinate the application of evidence-based recommendations for post-sepsis care with additional clinical support from hospitalist and primary care physicians [16]. The STAR program, based on the chronic care model [17], empowers patients and clinicians, via targeted education and coordinated care approaches, and was found to improve mortality and readmission outcomes among sepsis survivors [18]. There are complex barriers to translation of research findings into real-world post-sepsis care which we sought to identify and mitigate prior to effectiveness testing as part of a Type I Hybrid trial [19].
Before initiating a large-scale, pragmatic effectiveness evaluation of the STAR program (NCT04495946), we conducted a qualitative pre-implementation study with the aim to identify actionable facilitators and barriers to inform and enhance initial implementation strategies of the program across diverse hospital and community settings in a large Learning Health System (LHS). Qualitative methods are considered an integral component of implementation research and are well-known for being rigorous and efficient in the study of the hows and whys of implementation [20]. Conducting a robust pre-implementation evaluation was an intentional design choice for the overall project given the critical role of this step in the implementation process [21]. Through our qualitative investigation, we explored variations in stakeholder perspectives of the program by interviewing both administrators and clinicians.
We guided our study with the Consolidated Framework for Implementation Research (CFIR), due to its breadth, widespread use [22, 23], and expert-recommended mapping from CFIR-identified barriers to defined implementation strategies [24]. As a framework, the CFIR offers a systematic approach well-known for planning, evaluating, and supporting behavioral change for a diverse array of studies [25], using a consistent language of 39 constructs organized across five domains—Intervention Characteristics, Outer Setting, Inner Setting, Characteristics of Individuals and Process [22]. It can be used to build implementation knowledge to describe determinants of implementation [23], as well as tailor pre-implementation strategies to promote intervention success [26, 27].
Methods
For this pre-implementation study, we conducted a qualitative investigation to identify facilitators and barriers to implementing the STAR program in hospital transition care, and to elaborate and compare key stakeholder perspectives. Instrument development, data collection, analysis, and interpretation of study results were guided by the CFIR. A PhD-level trained qualitative health services researcher (TE) on the study team with experience conducting qualitative research for program evaluations and intervention development led the process of interview instrument design, data collection, and analysis. She was not known to participants of the research prior to undertaking the study. Our study team followed the Standards for Reporting Qualitative Research in the reporting of this work [28].
Study design
The pre-implementation study was conducted from March through July of 2020 in preparation for the planned implementation of the STAR program intervention in July 2020 at a large LHS. Headquartered in Charlotte, North Carolina, Atrium Health provides not-for-profit healthcare supporting over 14 million patient encounters annually across 40 hospitals and over 1,000 care locations in North Carolina, Georgia, and Alabama. We identified all stakeholders involved with post-sepsis care in this health system according to a framework for stakeholder mapping in health research [29]. With sepsis survivors and caregivers at the center of our focus for STAR, we identified stakeholder categories relevant to them to determine our recruitment approach for the pre-implementation interviews. By employing an iterative process of delineation between key individuals and groups involved in post-sepsis care at the LHS, we identified key stakeholders.
These stakeholders comprised two main groups: administrative leaders and clinicians. Administrative leaders were chief medical and nursing officers. We selected administrators due to their understanding of outer and inner setting factors and influence on organizational policy. Clinicians were hospitalists and ambulatory care providers representing diverse practice settings. We selected clinicians as representative intervention users with knowledge of intervention characteristics, outer setting, inner setting, characteristics of individuals, and process factors. We purposively sampled potential participants to reflect these organizational roles and responsibilities at the planned intervention sites. We aimed to recruit individuals to sufficiently capture a range of beliefs about post-sepsis care in these practice settings, while limiting redundancy in our data collection.
The final sample included 8 administrators (Chief Medical Officers, Nursing Executives, and a Departmental Chair; representing 7 study hospitals and leadership over post-hospital continuing care and primary care services) and 8 clinicians (with specialty areas in one or more of the following: Hospital Medicine, Internal Medicine, Infectious Disease, Family Medicine and Critical Care; representing individuals with care privileges at 6 study hospitals and primary care responsibilities in the communities served by these hospitals). See Table 1: Participant Characteristics.
Data collection
We conducted semi-structured qualitative interviews with 16 stakeholders from diverse hospitals and care settings to explore organizational support, culture, workflow processes, needs, and recommendations for STAR’s implementation. Separate and original interview guides were developed for administrator and clinician groups (See Additional file 1: Administrator Interview Guide and Additional file 2: Clinician Interview Guide) in this study, however, both guides included questions about stakeholder roles and work environments, the fit of the STAR program for their facilities which was facilitated using a printed intervention workflow diagram (See Fig. 1: Patient Trajectory through the STAR Program), and questions about the implementation of STAR. Interview guides intentionally included questions representative of all 5 of the CFIR domains (Intervention Characteristics, Outer Setting, Inner Setting, Characteristics of Individuals and Process) and were initially scripted by adapting questions from the CFIR Interview Guide Tool available at the CFIR website, www.cfguide.org [30]. Some of the sample questions from the guides are included below:
-
Do you think effectiveness data about the sepsis transition program would be needed to get team buy-in in your facility? (Intervention Characteristics)
-
How well, would you say, are new ideas (e.g., work processes, new interventions, QI projects, research) embraced and used to make improvements in your facility? (Inner Setting)
-
What, if any, barriers do you think patients will face to participate in the intervention? (Outer Setting)
-
What is your role within the organization? (Characteristics of Individuals)
-
Who would you recommend are the key individuals to speak with to make sure new interventions are successful in your practice or department? (Process)
We pilot tested (field tested) the interview guides in three rounds prior to their administration and iteratively refined the guides based on participant feedback and research team members’ perceptions of the usefulness of the data collection instruments for eliciting information we intended to capture for each stakeholder group (See Fig. 2: Diagram of Interview Guide Development at Pre-Implementation). Field testing is an established technique in qualitative research for developing interview guides as it provides researchers with the opportunity to practice asking the interview questions and identify weaknesses in the wording and order of questions when spoken aloud [31]. We then used the refined data collection instruments for the interviews reported here.
Prior to each interview, participants received standardized background information about the study topic and verbal informed consent was obtained. As an adaptation due to research restrictions during the COVID-19 pandemic, interviews were conducted telephonically. Interviews were on average 30 min in duration, which was expected given the number of questions asked of participants (13 questions for the administrators and 15 questions for the clinicians) and what was seen during the pilot testing of the interview guides prior to data collection. Participants were offered a $25 gift card for their participation. Ethical approval for this study was granted by the Advarra IRB Committee.
Data analysis
Interview recordings were transcribed and entered into ATLAS.ti X8 as text documents for thematic coding and analysis. One team member with extensive experience in qualitative research methods (TE) led the analysis of the data set using a combination deductive/inductive strategy based on CFIR domains and constructs and the Constant Comparison Method. The Constant Comparison Method is an inductive approach for developing code structure through the iterative comparison of newly coded text with previously coded text of the same theme until final thematic refinement is achieved [32]. We referred to the cfirguide.org website’s CFIR Codebook Template [33], containing domain and construct definitions and guidance for coding qualitative data with the framework and inclusion and exclusion criteria for most constructs, in our application of the framework to our codebook development and analysis. This process included creating a codebook (a complete list of codes and definitions for each code), coding the data set among team members, comparing identified codes, and merging codes when it was necessary based on analytical discussion. Each code was labeled using the following convention: 1) if it was an implementation facilitator or barrier code, 2) a simplified title indicating what the code was, and 3) and a tag of the CFIR domains and constructs that corresponded to the code. E.g., ImplFacilitator_Family support for PT: OUTSET-PT Needs & Res. Throughout the process of analyzing the qualitative interview data, our study team met bi-weekly to discuss the results and engaged with the larger stakeholder group monthly to discuss ideas for overcoming identified barriers.
To promote the reliability of the analysis and prevent interpretive bias, two study team members (TE and RB) completed inter-rater reliability (IRR) coding for 50% of the administrator interviews (n = 4). Three team members (TE, KO, and HT) completed IRR for 50% of the clinician interviews (n = 4). IRR was conducted by having additional coders (RB, KO, and HT) apart from the principal analyst (TE) apply the codebook to the data set to determine whether they agreed with the original coding of selected interview transcripts. Instances of disagreement were discussed thoroughly and, at times, resulted in the application of additional codes for selected quotations. All identified conflicts in coding were fully resolved, resulting in a final agreement of 100% between coders.
Results
Using a combination deductive/inductive coding strategy, we found 77 codes related to STAR implementation facilitators (n = 38) and barriers (n = 39) and labeled those codes with applicable CFIR domains and constructs as appropriate. The STAR implementation facilitators and barriers codes were then aggregated into 11 themes consisting of 6 facilitators (See Table 2) and 5 implementation barriers (See Table 3). STAR implementation facilitators and barriers, together, spanned all five CFIR domains (Intervention Characteristics, Outer Setting, Inner Setting, Characteristics of Individuals and Process). Administrators and clinicians reported no other sepsis-specific transition programs in their facilities at the time of data collection and indicated the STAR program would be important to address sepsis survivor needs.
Facilitators influencing the implementation of STAR
Our analysis identified six themes pertaining to implementation facilitators. See Table 2: CFIR-Guided Facilitators of STAR Implementation.
Alignment between STAR and health system goals
Participants reported that STAR’s alignment with other telehealth programs at the LHS, such as virtual hospital care, amidst surge of telehealth care during the COVID-19 pandemic would promote implementation of STAR as indicated in the administrator’s response below:
“I also think it [STAR] would be well received based on the information regarding virtual hospital and what we have been able to achieve with that. And, again with just looking for the bright spots in COVID, there have been a lot of transitions that have taken place in the last couple of months that I think you would have a much easier time implementing this in the new world of healthcare.” (A7)
Beyond virtual care, participants also described other existing infrastructure within the LHS that would align with the STAR program objectives, including sepsis work groups and sepsis champions from physicians, nurses, pharmacy, and case management. These inner setting facilitators combined demonstrate how STAR’s alignment with the implementation climate (compatibility) and structural characteristics of the LHS would influence its adoption.
Fostering engagement with stakeholders
Participants stated that fostering engagement to promote buy-in with stakeholders, including administrators, care teams, patients and caregivers, would facilitate the implementation of STAR. They recommended stakeholders be educated about what STAR is, its benefits, and for organizational stakeholders, how best to integrate STAR into their facility. See the clinician’s response below:
“I think just education [about STAR]. Just tons of education to everyone in the hospital that touches a patient. The nurses. The critical care physicians. The Hospitalists…But I think just educating the patient [about STAR] at the time of admission, just start that process. You know, this is our sepsis program, and let them know that this is going to happen at the time of discharge. And then also provide education to the providers.” (C1)
Participants also emphasized the importance of leaders heading communication about STAR with care teams and STAR navigators establishing a good rapport with clinicians who have patients enrolled in the program. See the clinician’s response below:
“Well, definitely share the information [about STAR] with their [health system leaders] teams. We have a normal leadership structure that provides the mechanism for things like this to be communicated in top down. And for sure, expecting the leaders to disseminate it from Level 2 to Level 3, Level 3 to Level 4 and on down. You know, that would be a minimum expectation…I think they should welcome you all [the STAR study team] at the meetings and give you time on the agenda to share your initiatives, at a minimum.” (C3)
These responses illustrate the relevance of the CFIR outer setting, process, and characteristics of individuals domains for the implementation of STAR, where prioritizing patient needs, attracting and involving appropriate individuals, and individual attitudes about the intervention would be facilitators of its adoption.
Share positive STAR outcomes data
Participants reported sharing positive results or impacts from the program would be helpful. They recommended using STAR performance metrics as motivation for continued buy-in and that leaders share effectiveness data. See the clinician responses below:
“I think readmission data [would be good to provide], like at 90 days, because if you are trying to get people to buy in for 90 days, cause that’s a long time, that’s about three months, I think you need to prove that it is worthwhile. If you’re trying to cut back on that 90-day readmission, because that’s what Medicare looks at, I think that would maybe entice some people to participate.” (C7)
“But, if you want to implement it as a standard process then we are going to have to see some sort of data on it before we say “yep, let’s do it”. Because there are many things that are competing for the resources that we have. So we have to on the basis on which our decisions on where the money goes, where those resources get diverted to is based on how efficiently they affect patient care, rates of readmission, and patient mortality. So we need the data to make an informed decision.” (C2)
Responses pertaining to this theme point to the significance of the CFIR intervention characteristics, inner setting and process domains in STAR’s implementation. Participants’ remarks regarding STAR’s evidence, strength and quality, shared receptivity to STAR within the LHS, and the recommendation to provide quantitative and qualitative feedback for reflecting and evaluating STAR’s quality would be facilitators of its implementation.
Good communication between STAR navigators and patient care teams/PCPs
Participants stated that good communication and recommendation-sharing between the STAR navigator and the patient’s care team and PCP will make STAR’s implementation successful. See the clinician’s response below:
“So, I think, effectively communicating with one another [the STAR navigator and clinician] what is beneficial and helping us ultimately provide for the patient from our end would be helpful. It will be a learning process, but you know, I think once we both communicate what we need from the other to be able to do our jobs, then I think that would be fine if that makes sense.” (C5)
These intervention characteristics and inner setting facilitators demonstrate the importance of intervention design, including how well STAR is bundled, presented and assembled to stakeholders, and navigator-led communication in its implementation.
Clinician promotion of STAR with patients
Our study participants emphasized the importance of clinician promotion of STAR with enrolled patients for implementation success. Specifically, our participants recommended that the LHS show patients their primary care providers and STAR navigators are in alignment to engender patient trust in the program. See the administrator’s response below:
“It always helps if they [patients] feel like it’s their own physicians or their own team that is a part of this. I think it would be important for it not to look like it was some external program that their clinicians were not involved in. So, I think, you know, trust always is important if you feel like people that you trust are endorsing something or believing it’s going to be useful.” (A8)
Similarly, one clinician said:
“I think trust, you know, would be a factor. A lot of times if patients view resources as being disconnected from their Primary Care, they may not be very accepting of them. So, if they view them as being part of “my team”, I think patients are much more likely to participate.” (C3)
Participant responses within this theme underscore the multi-domain influence of outer setting, inner setting and the process of implementation in the success of STAR, where the LHS’s prioritization of patient needs, LHS members’ and structures’ characteristics and behaviors, and the engagement of individuals with STAR would be facilitators of its implementation.
Good rapport and effective communication between STAR navigators and patients, caregivers, and family members
Participants reported that good rapport and effective communication between STAR navigators and enrolled patients and their caregivers/families would be important for implementing STAR. They emphasized the need for STAR navigators to foster a good connection with patients and their caregivers or family members. They also spoke to the integral role caregivers and family members play in patients’ post-sepsis recovery as additional points of contact who are familiar with the program if the patient does not recall what STAR is or if the patient is too ill to speak for themselves. See the clinicians’ responses below:
“I think patients get called a lot about a lot of things and they don’t always know who the person on the phone is. So, I think having that established and really something that the patient is okay with is important. And engaging, if possible, family or support members. I think that reduces barriers if they have support people available.” (C6)
“I think obviously reaching out to the family and support staff and things like that may be helpful. Some of our patients, in general, even at their baseline and at their best day aren’t going to be able to provide you the information that you need, or may not be able to provide an adequate history, or have an appropriate follow-up, and things like that, in place to be able to give you the information you need to help them as well as you would like.” (C5)
Responses within this facilitator theme highlight the importance of intervention characteristics, such as the perceived quality of STAR, and outer setting domains and constructs (patient needs and resources) in STAR navigator communication with patients and their caregivers and family members. Results show how effective navigator communication when presenting STAR to patients and their caregivers/family members, consideration of patient needs and barriers to participation, and the involvement of caregivers or family members would be facilitators of STAR’s implementation.
Barriers influencing the implementation of STAR
Our analysis identified five themes pertaining to implementation barriers. See Table 3: CFIR-Guided Barriers to STAR Implementation.
Competing demands for staff time and resources
Participants reported that competing demands for staff time and resources, including the busy state of the LHS’ facilities at the time, COVID priorities, other concurrent program implementations, and a lack of time among clinicians to engage with STAR could be barriers to its implementation. See the administrator’s response below:
“So, I think barriers would be too many implementations going on at the same time. It would fail. The other is, right now in COVID time, it’s unlikely to muster enough support or enough interest to do it. I think we need to look at what else is going on, so that there is not information overload for the front-end teammates. And the other thing we look at is, most of these programs become paper intensive or computer intensive. That means, you are just putting things there, and then, if you ask people to do too much, yes, they do too much, but they don’t really do the thing…So just be mindful of that, what you expect them to spend time on.” (A3)
Similarly, one clinician commented:
“Now, from a willingness standpoint, not that people would necessarily disagree with the overall goals and the process of your program, it’s just that if you’re in my field, and in some of my partners, if we are being pulled in ten different directions at one time, you have to prioritize what you can do in a day. So, not willingness from the standpoint of people not wanting to participate, but sometimes people not being able to weight or value that as high as something else that needs to be done.” (C5)
Participants responses pertaining to this barrier theme illustrate the role that the LHS’s inner setting, specifically its implementation climate of decreased organizational capacity to absorb change and a lack of resources dedicated for STAR, would play in hindering the implementation of the program.
Insufficient communication and education of program value and effectiveness
Participants reported that insufficient communication and education of STAR’s value and effectiveness to other clinicians could be barriers to its implementation. See the administrator’s response below:
“To me, it’s always a matter of communication. If there was, if communication didn’t work, people didn’t see it had value, they didn’t want to put any effort into it, you know, those would really be obviously the big things.” (A8)
Similarly, one clinician said:
“So, if it’s not marketed like correctly or appropriately. If we really as attending or residents don’t see the benefit. You know, is this just another checky box, or is this really going to impact our patients in the long term? Will this make a difference in their survival? Or getting them back to a base line or improvements on a base line? I think that’s probably what’s going to help make it successful or not.” (C8)
Responses related to this barrier theme show that the LHS’s inner setting and characteristics of individuals (clinicians) are important implementation domains in the adoption of STAR. Participants identified poor quality communication, and a lack of clinician knowledge and positive beliefs about STAR’s value, would be barriers to the implementation of the program.
Underlying informational and technology gaps among patients
Participants reported several patient-facing factors related to information and technology gaps among patients that could be barriers to implementing STAR. This included a patient’s health literacy or understanding of STAR, a patient’s digital literacy, and a patient’s lack of access to technology when communicating with the STAR telehealth navigator. See the clinician responses below:
“Well, I think a lot of our patients don’t have secure housing. I think our patients’ baseline social determinants of health, like consistent phone numbers, housing, health literacy around that, I think that’s a barrier that a patient would experience [to participate in the intervention].” (C6)
“I think the only barrier is that they [patients] may not understand what is going on. But that’s okay [as if not a big deal], as long as they are receptive to someone talking to them. And like I said, I want to be respectful of our patients, but some of them just do not have the medical literacy or the insight to understand….So, I think a barrier might be that the patient may not understand why you are calling and why you are asking those questions.” (C1)
“Definitely patients have to be capable of doing it uh participating with the Telehealth. At least from the perspective of a lot of my patients and during the Coronavirus pandemic, it has been difficult to get some buy in with Telehealth linkages to care. We have a very rural population and there is some adherence issues with trying to initiate, you know, telephonic or video visits that we have kind of noticed over the last several months. So, patient participation I think in some settings would be challenging.” (C4)
Participant responses within this barrier theme highlight the importance of the outer setting (external to the LHS) in the challenge of implementing STAR, where literacy and technology gaps among patients could be barriers to program enrollees’ participating in the telehealth-based intervention.
Lack of access to community resources for patients
Finally, participants reported that a patient’s lack of access to community resources, including limited primary care, paramedicine, home physical therapy, speech therapy and mental health resources in certain communities (e.g., rural communities), could pose a barrier to the implementation of the STAR program. See the clinicians’ responses below:
“I think that the idea is a good idea [pauses], but it’s just where it would work best based upon the resources of the area. I think that is going to be the major challenge.” (C7)
“Just getting plugged into community resources that can assist with their psycho-social needs as well as their comorbidities” [would be a barrier to patient participation]. (A1)
Participant responses within this theme demonstrate the relevance of intervention characteristics and the outer setting when implementing EBPs for post-sepsis care for patients who lack access to community resources. The extent to which STAR cannot adapt and meet patients’ local needs, especially those of patients who live in areas where there are insufficient resources, will be a barrier to its implementation.
Patient distrust of the program and/or healthcare
Both administrators and clinicians interviewed stated that patient distrust of the program and/or healthcare could be a potential barrier to STAR’s implementation. These reasons included patients being slow to trust a new provider, discomfort when talking with a navigator, feeling skeptical of providers who seem unaffiliated with their primary care, and general distrust of the healthcare system, particularly for patients in rural communities or impoverished areas. See the administrator and clinician responses below:
“You know, people are always a little wary of people they do not know, especially in small and rural communities.” (A1)
“Yeah, I think most of the barriers that are already well known that go with socio economic status or poverty. Trust in the healthcare system. I think those are all going to be barriers.” (C4)
Responses within this theme point to the significance of outer setting factors and the extent to which a patient’s need to trust their provider is accurately known and prioritized by the STAR navigator. Data suggests patient distrust of the STAR program or other providers would be a barrier to implementing EBPs for post-sepsis care.
Discussion
A foundation of implementation science is that intervention delivery should be tailored to local context to maximize uptake and impact [34, 35]. Formative, or pre-implementation, evaluations facilitate initial assessment of the local context and the potential determinants for implementation success within that context. Multiple theoretical frameworks have been applied to pre-implementation evaluations; the Consolidated Framework for Implementation Science (CFIR) is one of the most widely used due to its ability to comprehensively identify implementation facilitators and barriers [36]. In this study, we utilized qualitative pre-implementation interviews to identify actionable facilitators and barriers to inform and enhance initial implementation strategies of the STAR program across diverse hospital and community settings in a large LHS. From this work, our study offers several contributions to the literature on post-sepsis care.
First, our study successfully leveraged the CFIR to inform and enhance initial implementation strategies of the STAR program across diverse hospital and community settings in a large LHS. This is in line with other studies that similarly applied the CFIR during pre-implementation and found implementation determinants like ours, such as stakeholder involvement being necessary to promote buy-in and the relevance of intervention fit within the organization’s inner setting [26, 37]. While some have applied CFIR in the pre-implementation planning of a sepsis management intervention at a single site [38], to our knowledge, our team is the first to apply the CFIR at pre-implementation to inform the design and dissemination of a sepsis transition and recovery intervention for patients within a large LHS. We decided to guide our interview instrument development and subsequent analysis using the CFIR because we were interested, fundamentally, in the organizational change that will be needed to successfully implement the STAR program. By incorporating the CFIR domains and constructs into our interview instruments and intervention planning, our study was able to identify implementation partners and collect stakeholder input on the potential facilitators and barriers to the STAR program at a large LHS. One benefit of using the CFIR for pre-implementation work is the potential for direct translation to implementation strategies selection using the Expert Recommendations for Implementing Change (ERIC) mapping.
Second, study findings revealed the importance of stakeholder buy-in like other CFIR-guided pre-implementation studies [26, 39] across diverse groups, including administrators, care teams, patients, and caregivers. Implementation facilitators related to buy-in that were identified included active engagement with stakeholders, education about STAR, the sharing of positive outcomes data from STAR with clinicians, and promotion of the program’s value throughout implementation. Participants also emphasized the criticality of demonstrating alignment between clinicians and the STAR program. This included the recommendation for clinician support and promotion of STAR with patients to engender patient trust in the program. Conversely, our study found implementation barriers pertaining to lack of stakeholder buy-in as well. These included that a lack of engagement and education about the post-sepsis care program’s value and effectiveness, possible patient distrust of STAR and/or of health care, and patients’ lack of access to community resources could be potential barriers to its implementation. Together these findings point to the necessity of stakeholder buy-in for overcoming inner and outer setting barriers to implementation. They also suggest successful championing of STAR should extend beyond navigator efforts alone and include system and care team participation as well.
Third, our study found the STAR program’s fit with the LHS’s inner setting to be informative for our planning. Participants reported STAR’s compatibility with the structural characteristics and implementation climate of the LHS to likely be important considerations for implementation. At the time of this study, virtual hospital care and other telehealth programs were highly active within the LHS, in part related to the need for such programs during the surge of the COVID-19 pandemic. Additionally, STAR’s alignment with other sepsis-focused work groups and sepsis champions across the LHS was identified as another possible facilitator for implementation success. We found implementation barriers pertaining to the implementation climate of the LHS’s inner setting as well. Despite acknowledging that the program would likely align with current health system goals, participants cautioned STAR would have to compete with demands for staff time and resources. Decreased organizational capacity for a new program was another potential implementation barrier identified. Participants recommended engaging clinicians about the value and effectiveness of the program to promote support and assuage concerns. These facilitators and barriers suggest health system priorities and routine healthcare practice in the inner setting should be identified and considered carefully when making post-sepsis care program implementation decisions. They also underscore how the inner setting is not simply a background of implementation but can rather serve as an important context in implementation success.
Finally, our study findings highlighted the importance of good communication between the STAR navigator and other stakeholders, including clinicians, patients, caregivers, and family members, for successful implementation. Participants recommended clear and reciprocal communication between STAR navigators and clinicians. Similarly, they advised that navigators attempt to establish good rapport with patients, caregivers, and family members by using effective communication. Several potential implementation barriers related to communication were also reported. Participants discussed underlying patient-facing information and technology gaps that could be potential barriers to communicating with STAR navigators related to digital literacy, health literacy, or a lack of access to technology to participate in STAR. These suggest further study may be recommended to identify other patient-facing environmental conditions, such as social determinants of health, affecting sepsis recovery, as proposed in other’s work [10]. These points underscore the necessity of both effective communication and communication technology to support telehealth-based sepsis transition and recovery intervention implementation.
Study limitations
A limitation of the present research is that it is based on interviews with a small sample of employees at one, albeit large, health system. Although we carefully sampled stakeholders based on their awareness, organizational authority, and involvement in activities related to implementation of a post-sepsis care intervention at study facilities, these perspectives may not necessarily reflect the experience of all facilities within the same LHS or outside of the LHS. A second limitation is that patients were not included as participants at pre-implementation, despite later finding several facilitators and barriers related to patient needs. Third, we deliberately used the CFIR, and included all domains, to inform our approach to data collection and analysis due to its comprehensive assessment of implementation determinants and well-described associations with implementation strategies. However, using CFIR alone may have limited collection of other relevant contextual factors not represented by CFIR or specifically incorporated in our data collection. Our analysis strategy that combined inductive and deductive methods did allow for capture of themes outside of CFIR, if new information emerged from participant responses. Finally, our analysis strategy focused specifically on identifying key individual determinants; thus, additional empirical analyses examining the causal pathways or combinations of contextual factors may be helpful to advance evidence and guide decision making regarding effective implementation strategies tailored to complex determinants.
Conclusions
Our findings demonstrate effective use of the CFIR as a robust framework to examine facilitators and barriers for pre-implementation planning of post-sepsis care programs within diverse hospital and community settings in a large LHS. The comprehensive structure of the framework enabled researchers to identify key implementation determinants across external-, internal-, and program-level domains, plan for organizational change associated with implementation, and engage with relevant stakeholders. Conducting a structured pre-implementation evaluation helps researchers design with implementation in mind prior to effectiveness studies and should be considered a key component of Type I hybrid trials when feasible.
Availability of data and materials
The datasets generated and analyzed during the study are not available due to participant privacy and ethics restrictions, but the codebook and data collection tools may be available from the corresponding author on reasonable request.
Abbreviations
- STAR:
-
Sepsis transition and recovery
- LHS:
-
Learning health system
- CFIR:
-
Consolidated framework for implementation research
- EBPs:
-
Evidence-based practices
References
Buchman T, Simpson SQ, Sciarretta KL, et al. Sepsis among medicare beneficiaries: 1. The burdens of sepsis, 2012–2018*. Crit Care Med. 2020;48(3):276–88. https://doi.org/10.1097/CCM.0000000000004224. Published Online First: Epub Date.
Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–11. https://doi.org/10.1016/S0140-6736(19)32989-7. Published Online First: Epub Date.
W. MK, Marc R. Most Frequent Principal Diagnoses for Inpatient Stays in U.S. Hospitals, 2018. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs 2021. 2021. https://www.ncbi.nlm.nih.gov/books/NBK573113/.
Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018;46(6):997–1000. https://doi.org/10.1097/ccm.0000000000003119. Published Online First: Epub Date.
Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318(13):1241–9. https://doi.org/10.1001/jama.2017.13836. Published Online First: Epub Date.
Mostel Z, Perl A, Marck M, et al. Post-sepsis syndrome - an evolving entity that afflicts survivors of sepsis. Molecular medicine (Cambridge, Mass.) 2019;26(1):6. https://doi.org/10.1186/s10020-019-0132-z. Published Online First: Epub Date.
Farrah K, McIntyre L, Doig CJ, et al. Sepsis-associated mortality, resource use, and healthcare costs: a propensity-matched cohort study. Crit Care Med. 2021;49(2):215–27. https://doi.org/10.1097/ccm.0000000000004777. Published Online First: Epub Date.
Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319(1):62–75. https://doi.org/10.1001/jama.2017.17687. Published Online First: Epub Date.
Shankar-Hari M, Saha R, Wilson J, et al. Rate and risk factors for rehospitalisation in sepsis survivors: systematic review and meta-analysis. Intensive Care Med. 2020;46(4):619–36. https://doi.org/10.1007/s00134-019-05908-3. Published Online First: Epub Date.
Hilton RS, Hauschildt K, Shah M, Kowalkowski M, Taylor S. The assessment of social determinants of health in postsepsis mortality and readmission: a scoping review. Crit Care Explor. 2022;4(8):e0722. https://doi.org/10.1097/cce.0000000000000722. Published Online First: Epub Date.
Prescott HC, Iwashyna TJ, Blackwood B, et al. Understanding and enhancing sepsis survivorship. Priorities for research and practice. Am J Respir Crit Care Med. 2019;200(8):972–81. https://doi.org/10.1164/rccm.201812-2383CP. Published Online First: Epub Date.
Huang CY, Daniels R, Lembo A, et al. Life after sepsis: an international survey of survivors to understand the post-sepsis syndrome. Int J Qual Health Care. 2019;31(3):191–8. https://doi.org/10.1093/intqhc/mzy137. Published Online First: Epub Date.
Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–247. https://doi.org/10.1007/s00134-021-06506-y. published Online First: Epub Date.
Taylor SP, Chou SH, Sierra MF, et al. Association between adherence to recommended care and outcomes for adult survivors of sepsis. Ann Am Thorac Soc. 2020;17(1):89–97. https://doi.org/10.1513/AnnalsATS.201907-514OC. published Online First: Epub Date.
Watson MA, Anderson C, Karlic KJ, et al. Receipt of recovery-oriented care practices during hospitalization for sepsis. Crit Care Explor. 2022;4(9):e0766. https://doi.org/10.1097/cce.0000000000000766. Published Online First: Epub Date.
Taylor SP, Murphy S, Rios A, et al. Effect of a multicomponent sepsis transition and recovery program on mortality and readmissions after sepsis: the improving morbidity during post-acute care transitions for sepsis randomized clinical trial. Crit Care Med. 2022;50(3):469–79. https://doi.org/10.1097/ccm.0000000000005300. Published Online First: Epub Date.
Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Effective clinical practice : ECP. 1998;1(1):2–4.
Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–107. https://doi.org/10.1001/jamainternmed.2014.1608. Published Online First: Epub Date.
Kowalkowski M, Eaton T, McWilliams A, et al. Protocol for a two-arm pragmatic stepped-wedge hybrid effectiveness-implementation trial evaluating Engagement and Collaborative Management to Proactively Advance Sepsis Survivorship (ENCOMPASS). BMC Health Serv Res. 2021;21(1):544. https://doi.org/10.1186/s12913-021-06521-1. Published Online First: Epub Date.
Hamilton AB, Finley EP. Qualitative methods in implementation research: an introduction. Psychiatry Res. 2019;280:112516. https://doi.org/10.1016/j.psychres.2019.112516. Published Online First: Epub Date.
Alley ZM, Chapman JE, Schaper H, Saldana L. The relative value of Pre-Implementation stages for successful implementation of evidence-informed programs. Implement Sci. 2023;18(1):30. https://doi.org/10.1186/s13012-023-01285-0. Published Online First: Epub Date.
Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implementation science : IS. 2009;4:50.https://doi.org/10.1186/1748-5908-4-50
Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder L. A systematic review of the use of the Consolidated Framework for Implementation Research. Implement Sci. 2016;11:72. https://doi.org/10.1186/s13012-016-0437-z. Published Online First: Epub Date.
Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. https://doi.org/10.1186/s13012-015-0209-1. Published Online First: Epub Date.
Birken SA, Powell BJ, Presseau J, et al. Combined use of the Consolidated Framework for Implementation Research (CFIR) and the Theoretical Domains Framework (TDF): a systematic review. Implement Sci. 2017;12(1):2. https://doi.org/10.1186/s13012-016-0534-z. Published Online First: Epub Date.
King DK, Shoup JA, Raebel MA, et al. Planning for implementation success using RE-AIM and CFIR Frameworks: a qualitative study. Front Public Health. 2020;8:59. https://doi.org/10.3389/fpubh.2020.00059. Published Online First: Epub Date.
Dolansky MA, Pohnert A, Ball S, McCormack M, Hughes R, Pino L. Pre-Implementation of the age-friendly health systems evidence-based 4ms framework in a multi-state convenient care practice. Worldviews on evidence-based nursing. 2021;18(2):118–28. https://doi.org/10.1111/wvn.12498. Published Online First: Epub Date.
O’Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89(9):1245–51. https://doi.org/10.1097/acm.0000000000000388. Published Online First: Epub Date.
Schiller C, Winters M, Hanson HM, Ashe MC. A framework for stakeholder identification in concept mapping and health research: a novel process and its application to older adult mobility and the built environment. BMC Public Health. 2013;13:428. https://doi.org/10.1186/1471-2458-13-428. Published Online First: Epub Date.
Research CRT-CfCM. CFIR Interview guide tool. Secondary CFIR interview guide tool 2019. https://cfirguide.org/guide/app/#/.
McGrath C, Palmgren PJ, Liljedahl M. Twelve tips for conducting qualitative research interviews. Med Teach. 2019;41(9):1002–6. https://doi.org/10.1080/0142159x.2018.1497149. Published Online First: Epub Date.
Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health services research. 2007;42(4):1758–72. https://doi.org/10.1111/j.1475-6773.2006.00684.x. Published Online First: Epub Date.
Research CRT-CfCM. CFIR Codebook Template. Secondary CFIR Codebook Template 2019. https://cfirguide.org/tools/tools-and-templates/.
Baker R, Camosso-Stefinovic J, Gillies C, et al. Tailored interventions to overcome identified barriers to change: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2010;3:Cd005470. https://doi.org/10.1002/14651858.CD005470.pub2. Published Online First: Epub Date.
Mittman BS. Implementation science in health care. In: Brownson RC, Colditz GA, Proctor EK, editors. Dissemination and implementation research in health: translating science to practice. New York: Oxford; 2012. p. 400–18.
Damschroder LJ, Reardon CM, Widerquist MAO, Lowery J. The updated Consolidated Framework for Implementation Research based on user feedback. Implementation Sci. 2022;17(1):75. https://doi.org/10.1186/s13012-022-01245-0. Published Online First: Epub Date.
Piper KN, Brown LL, Tamler I, Kalokhe AS, Sales JM. Application of the consolidated framework for implementation research to facilitate delivery of trauma-informed HIV care. Ethn Dis. 2021;31(1):109–18. https://doi.org/10.18865/ed.31.1.109. Published Online First: Epub Date.
Ahmed SI, Khowaja BMH, Barolia R, et al. Adapting the FAST-M maternal sepsis intervention for implementation in Pakistan: a qualitative exploratory study. BMJ Open. 2022;12(9):e059273. https://doi.org/10.1136/bmjopen-2021-73. eCollection 2022.
Fokuo JK, McCuistian CL, Masson CL, et al. Pre-implementation assessment of tobacco cessation interventions in substance use disorder residential programs in California. Subst Use Misuse. 2022;57(9):1345–55. https://doi.org/10.1080/10826084.2022.2079139. Published Online First: Epub Date.
Acknowledgements
The authors would like to thank all of the study participants for sharing their time and insights.
Funding
Research reported in this publication was supported by the National Institute Of Nursing Research of the National Institutes of Health under Award Number R01NR018434. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
TE, ST, MK, and HT contributed to the design of the study. TE, ST, MK, RB and KO contributed to the acquisition of study data. TE, RB, KO, and HT analyzed the data. TE, ST, and MK contributed to the interpretation of the data. TE, ST, MK, RB, and HT drafted the manuscript. All authors critically revised the intellectual content of the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been approved to be conducted on behalf of all trial sites with a waiver of signed informed consent by the Advarra Institutional Review Board (#Pro00036873) on June 26, 2019. Participants were all given a study information sheet that was reviewed with them prior to their participation. All study methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Eaton, T.A., Kowalkowski, M., Burns, R. et al. Pre-implementation planning for a sepsis intervention in a large learning health system: a qualitative study. BMC Health Serv Res 24, 996 (2024). https://doi.org/10.1186/s12913-024-11344-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-024-11344-x