Abstract
Background
Breast cancer (BC) is a leading cause of premature death in women and the most expensive malignancy to treat. Since the introduction of targeted therapies has resulted in changes to BC therapy practices, health economic evaluations have become more important in this area. Taking generic medications, Aromatase Inhibitors (AIs), as a case study, we conducted a systematic review of the recent economic evaluations of AIs for estrogen receptor-positive breast cancer patients and evaluated the quality of these health economic studies.
Objective
To systematically review and examine the quality of the available economic studies of AIs in estrogen receptor-positive breast cancer.
Methods
A literature search was performed using six relevant databases (MEDLINE, Embase, Database of Abstracts of Reviews of Effects, Health Technology Assessment Database, NHS Economic Evaluation Database, and SCOPUS) from January 2010 to July 2021. All economic studies were independently assessed by two reviewers using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist to evaluate the quality of the economic evaluations. This systematic review is registered in the PROSPERO database. To compare the different currencies used in these studies, all costs were converted to international dollars (2021).
Results
A total of eight studies were included in the review; six (75%) were performed from the healthcare providers’ perspective. They were conducted in seven different countries, and all were model-based analyses using Markov models. Six (75%) considered both Quality Adjusted Life Years (QALYs) and Life Years (LY) outcomes, and all costs were derived from national databases. When compared to tamoxifen, AIs were generally cost-effective in postmenopausal women. Only half of the studies addressed the increased mortality following adverse events, and none mentioned medication adherence. For the quality assessment, six studies fulfilled 85% of the CHEERS checklist requirements and are deemed good quality.
Conclusion
AIs are generally considered cost-effective compared to tamoxifen in estrogen receptor-positive breast cancer. The overall quality of the included studies was between high and average but characterizing heterogeneity, and distributional effects should be considered in any future economic evaluation studies of AIs. Studies should include adherence and adverse effects profiles to provide evidence to facilitate decision-making among policymakers.
Highlights
-
This is a systematic review looking at Aromatase Inhibitors (AIs) in breast cancer since 2010.
-
Overall, the studies included were of high and average quality.
-
The reporting of the models has improved.
-
The structural uncertainty is still underreported and has not incorporated issues such as medication adherence and side-effect profile.
Similar content being viewed by others
Background
Breast cancer (BC) is the most diagnosed cancer in women globally. In 2020, there were an estimated 2.3 million new cases of BC and 685,000 deaths from BC worldwide [1]. It is the leading cause of cancer death in women in developing countries [1]. The high incidence and prevalence of BC impose a tremendous financial burden and carry huge socioeconomic, emotional, and public health implications. Policymakers need robust evidence on the cost-effectiveness of different treatment options to base decisions on how best to use scarce healthcare resources.
BC treatment options are determined based on the tumor’s type, stage, and grade and whether it is sensitive to hormones. Hormonal therapy is the cornerstone of adjuvant systemic treatment for patients with hormone receptor-positive BC [2]. Aromatase inhibitors (AIs) (such as letrozole, anastrozole, and exemestane) and tamoxifen are hormonal therapies used in women with breast cancer. AIs reduce recurrence and cancer mortality rates by 30% and 15%, respectively, compared with tamoxifen [3].
Several pertinent systematic reviews of economic evaluations of AIs for the treatment of hormone receptor-positive BC have been published. John-Baptiste et al. (2013) concluded that studies were overestimating the cost-effectiveness of AIs and recommended being cautious when drawing conclusions about the value of AIs versus tamoxifen [4]. Frederix et al. (2012) concluded that the included studies in their review did not demonstrate if AIs represent better value for money than tamoxifen [5]. Diaby et al. (2015) recommended additional studies to elucidate the cost-effectiveness of AIs versus tamoxifen in early-stage breast cancer [6].
Previous systematic reviews were conducted before the availability of AIs in generic formulations (patent expiration in 2011) and before long-term follow-up studies of AIs were available. This would affect the cost-effectiveness analysis of AIs due to the availability of lower cost generic drugs. Furthermore, a new checklist, Consolidated Health Economic Evaluation Reporting Standards (CHEERS), to optimize reporting of health economic evaluations was published in 2013 and updated in 2022 [7]. In addition, previous systematic reviews did not evaluate the structural uncertainty.
Economic evaluation is an essential part of the health technology assessment (HTA) process to help inform healthcare decision-makers. The quality of these studies is crucial to countries with limited HTA resources. This review will help authors from such countries to improve the quality of their studies so that policymakers will have the tools to help them make better decisions. We systematically reviewed the economic evaluation of AIs since 2010, examined the quality of these studies, and summarized the evidence on drivers of cost-effectiveness. Our aim was to look at the model structures and the input parameters and how the analyses were conducted. A comparative analysis of model structure and parametrization using a checklist and guidelines for models was conducted to improve our understanding of the quality of current evidence.
Methods
Literature search
A comprehensive literature search for economic evaluations of AIs versus tamoxifen in females with Estrogen Receptor positive BC was performed using MEDLINE (July 16, 2021), Embase (2021 July 16), Cochrane library (Database of Abstracts of Reviews of Effects, Health Technology Assessment Database, and NHS Economic Evaluation Database), and SCOPUS (July 2021).
The electronic search strategy was based on (PICOS): Population (postmenopausal females with BC), Interventions (at least one AIs), Comparators (Tamoxifen), Outcomes (health outcomes such as Quality Adjusted Life Years (QALY) or Life Years Gained (LYG) or life years saved (LYS)) and Study designs (economic study, cost-effectiveness analysis (CEA), cost-utility analysis (CUA) or cost-benefit analysis (CBA)). The exclusion criteria were: (i) descriptive costing studies as they are not considered full economic evaluations, (ii) Conference abstracts because they lack details about the methods, and (iii) economic evaluation addressing extended adjuvant therapy. No language restrictions were imposed (See supplementary appendix S1 for search strategy).
Study selection
The study selection procedure encompassed three main stages. The first stage was to import all the references to Endnote and remove duplicates. The second stage was to evaluate the remaining studies based on the title and abstract and studies that did not meet the inclusion criteria were excluded. In the third stage, the full articles of potentially relevant studies were retrieved, and those that met the inclusion criteria were included in the current review. Reviewer one (MF) and reviewer two (DC) screened the identified abstracts and full texts for eligibility.
Data extraction
We extracted the characteristics of the identified studies in two tables. A summary of the pertinent study characteristics: publication year, country, perspective, type of model, type of economic evaluation, time horizon, sponsorship, discount rate, and currency were extracted along with a summary of the model characteristics: source of data, methods of measuring outcomes, included costs, AI, type of sensitivity analysis, incremental cost-effectiveness ratio (ICER), stage of BC, line of treatment, population, and conclusion.
To allow direct comparison across countries, all costs were converted to International Dollars and then inflated to reference year (2021) using the ‘CCEMG – EPPI-Centre Cost Converter’ (v.1.6 last update: April 29, 2019), a free web-based tool for adjusting estimates of cost expressed in one currency and price year to a specific target currency and price year [8]. Data were extracted using Microsoft Excel and performed by three assessors (MF, DC, LH), and we used the help of (CFG) in extracting the two articles in Spanish.
Quality assessment
The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement was adopted to critically appraise the studies. The 28-item CHEERS checklist consists of 7 domains: Title (1 item); Abstract (1 item); Introduction (1 item); Methods (18 items); Results (4 items); Discussion (1 item); and Other relevant information (2 items) [7]. CHEERS checklist is not a scoring instrument, but we adopted the same tool based on other review studies indicating ‘yes’ when the criteria were met, ‘no’ when they were unfulfilled, and ‘‘not applicable’ when they were not required for that type of study. We divided the studies into three quality categories according to the proportion of items achieved: high (> 75%), average (50–75%) and poor (< 50%) [9].
The quality assessment was conducted by one assessor (MF) for all studies except Spanish studies, which were evaluated by (CFG), and ambiguities were resolved by consulting another assessor (DC). Sensitivity analysis was used to address uncertainty, which is divided into three categories: structural, methodological, and parameter.
Structural uncertainty
Structural uncertainty relates to whether all relevant processes are represented in the model. We abstracted the adverse events mentioned in the analysis and determined whether their effect on mortality was incorporated in the analysis or not.
Methodological uncertainty
Methodological uncertainty refers to choices about population, time horizon, and study perspective that impact how economic evaluation estimates are calculated. This includes sensitivity analysis (SA) for extrapolating beyond the follow-up time of studies, did the analysis address different subgroups such as older women, women at high risk of side effects (SE), women with comorbidities, and women at low risk of BC recurrence?
Parameter uncertainty
Parameter uncertainty concerns the numerical values of input parameters. We abstracted the data source on both BC recurrence and adverse event rates associated with AIs. Then, we determined whether the authors perform the following or not: SA on the risk of BC recurrence, SA on SE (including fracture, cardiovascular events, stroke, thromboembolic events, endometrial cancer), probabilistic sensitivity analysis (PSA), and value of information analysis (VOI) to critique the authors’ handling of parameter uncertainty.
Results
Literature search
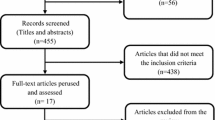
Records identified through database synthesis were 734 references, among which 185 were duplicates, 492 were excluded after screening and analysis of titles and abstracts for not matching the eligibility criteria, 47 articles were excluded due to date restriction, and two were excluded because of the comparators. A total of eight papers were retrieved and analyzed [10,11,12,13,14,15,16,17]. (Fig. 1)
Characteristics of studies included in the review
A total of eight articles were included in the final study, of which six were published in English [11,12,13,14,15,16] and two in Spanish [10, 17]. Studies were conducted in different countries including Mexico [10], China [11], Canada [12], Singapore [13], Germany [14, 15], Korea [16], and Colombia [17]. In the majority of the studies, authors conducted the analysis from the perspective of the health care system (n = 6; 75%) [10,11,12, 14, 15, 17]; only two studies were conducted from a societal perspective [13, 16]. 75% of studies (n = 6) considered both QALY and LY outcomes [11,12,13,14,15,16], while the remaining two studies used recurrence rate [10] and overall survival [17] as outcome measures. All economic evaluations involved were model-based analyses using Markov cohort models. All studies considered direct costs, except one study considered both direct and indirect costs [16]. Shih et al. involved direct costs only despite conducting their study from a societal perspective [13]. All studies clearly stated the price and currencies, and costs were derived from local sources and/or national databases. The publication years ranged from 2010 to 2018 (Table 1).
One study modeled for ten years [10], while most studies used a lifetime horizon or ranged between 20 and 35 years. Discounting of costs was made in all studies; half of the studies used a 3% discount rate, and the other half used a 5% discount rate. Three studies [11, 14, 15] justify choosing this discount rate, but others did not. Most studies compared one AI vs. Tamoxifen (n = 4), one compared letrozole vs. tamoxifen [11] and three compared anastrozole vs. tamoxifen [13, 14, 17]. The remaining studies compared anastrozole or letrozole vs. tamoxifen [15, 16] (n = 2), one comparing the three AIs (anastrozole or letrozole or exemestane) vs. tamoxifen [10], and one treated the AI drug class as a group without reference to a specific drug [12]. Efficacy data were derived from the results of clinical trials or literature. (Table 2)
Most studies report an ICER value except for two that didn’t calculate the ICER [10, 12]. The ICER values for anastrozole and letrozole after conversion to 2021 International dollars ranged between $ 40 to $ 206,256/QALY and $ 11,510 to $ 45,019/ QALY, respectively.
The Markov cycle length used in all studies was yearly except for two studies that used a 1-month [11] and a 3-month cycle [14]. Most of the studies (n = 6) concluded that when compared to tamoxifen, AIs were cost-effective at a commonly accepted threshold for cost-effectiveness (less than $50k /QALY [18]), except for two studies [10, 17] which concluded that tamoxifen is cost-effective.
Quality assessment
The quality assessment results using the CHEERS checklist per study are summarized in (Table 3). The mean number of fulfilled criteria for the CHEERS checklist was 22 out of 28. The most frequent partially or not reported items were health economic analysis plan (item 4), characterizing heterogeneity (item 18), characterizing distributional effects (item 19), approach to engagement with patients and others affected by the study (item 21), and effect of engagement with patients and others affected by the study (item 25).
The quality of the included studies is between high and average levels; 75% of the studies (n = 6) were of average quality, and 25% (n = 2) were of high quality according to our criteria.
Data sources
All the studie’s authors used one or two RCTs as a source of data to estimate the impact of hormonal therapies on breast cancer recurrence. Most data were taken from either the ATAC trial (Arimidex or Tamoxifen Alone or in combination trial) [19] and/or the BIG 1–98 trial (the breast international group trial) [20].
Costs were obtained from national databases; Ye et al. and Djalalov et al. are the only two studies that mentioned using the generic costs of the drugs [11, 12].
Handling structural uncertainty
Half of the studies (n = 4, 50%) addressed the increased mortality following adverse events (Table S1).
Handling methodological uncertainty
Few economic evaluations performed sub-group analyses to address patient heterogeneity related to older women (n = 2, 25%), and no study looked at women at low risk of breast cancer recurrence. A large proportion (75%) did not assess the impact of uncertainty arising from extrapolating beyond the trial data. Five studies (62.5%) vary the discount rate in the sensitivity analysis (Table S1).
Handling parameter uncertainty
All the studies reported sensitivity analysis on the risk of breast cancer recurrences. Two studies did not perform sensitivity analysis on the risk of adverse events (n = 2, 25%). Six studies (75%) performed PSA. One study conducted a VOI analysis. (Table S2)
Detailed information on the handling of parameter, structural and methodological uncertainty are available in (Tables S1 and S2).
Discussion
In this study, we systematically reviewed and assessed the quality of eight economic evaluations comparing AIs to tamoxifen for early-stage breast cancer published between 2010 and 2021, covering the perspectives of Chinese, Korean, German, Canadian, Singapore, Colombian and Mexican healthcare systems. When compared to tamoxifen, AIs were reported to be cost-effective in postmenopausal women with early-stage BC in most studies (75%) at a commonly accepted threshold for cost-effectiveness (less than $50k /QALY).
Two previous systematic reviews of economic evaluations conducted on AIs and tamoxifen in early-stage BC. John-Baptiste et al [4]. identified 18 cost-effectiveness studies between 2004 and 2010, while Frederix et al [5]. analyzed 20 articles about the cost-effectiveness of endocrine treatments published between 2000 and 2010. These reviews concluded that there is an overestimation of the cost-effectiveness of AIs, and there is a need for standardized models to help in decision-making. Our study now finds that AIs are cost-effective based on high to average-quality study methodology. The general evaluation approaches in all studies had a significant degree of similarity. First, all the evaluations used a Markov model. Secondly, all studies used an RCT as a source of data and national costs. But despite this fact, the reported cost-effectiveness results were not consistent across all the evaluations; this variation could be due to the difference in treatment costs in different countries.
In two studies conducted by Lux et al [14]. there were considerable differences in ICER $ 32,616/QALY and $ 206,256/QALY even though they were conducted from the German healthcare perspective, using the same discount rate (3%), the same AI (anastrozole), and similar time horizon (20–25 yrs.) and only differ in age of the participant at entry (76–80 and 64 yrs.), the higher ICER was associated with using generic drug costs.
In studies comparing letrozole to tamoxifen, the lowest ICER was associated with using generic drug prices in the latest study (2018). The ICER of the two other studies was doubled; this could be due to different discount rates, different settings, different lifetime horizons, and different ages at entry. The study in Mexico used a short time horizon of 10 years which failed to capture the full costs and effects of chronic diseases [10]. The difference between studies in the participant’s age at entry should be considered; knowing the side effects of AIs and how they affect older ages could lead to differences in the costs of side effects in different age groups.
All studies except two used a yearly Markov model cycle length without justification; the recommended cycle length is 3 months because recurrences are very relevant for the outcome and using a 3-month cycle is a better representation of the course of the disease.
Regarding the quality of reporting these evaluations, we observed that the reporting was sufficient except for reporting sub-group analysis to address heterogeneity, increase mortality following adverse events, and approaches to engage patients or others affected by the study which were partially reported. We recognized that all the studies are not following any checklists to evaluate the quality of their studies, we highly recommend using checklists to improve the reporting and hence the quality of economic evaluations.
Our review found some key drivers of cost-effectiveness that are not always discussed. First, medication adherence should be incorporated in upcoming economic evaluations. It was found that medication non-adherence places a significant cost burden on healthcare systems [21]. Second, the drug costs, whether generic or branded, would affect the cost-effectiveness.
There are some limitations of this systematic review that must be addressed. First, this review included only fully published studies, and we did not look at grey literature and excluded conference abstracts. Second, most of the studies adopted the health care system perspective rather than the societal perspective, which limits the generalizability of results. Third, comparing economic outcomes is difficult because of the variability in currencies and the health system involved in different countries.
Conclusion
Although most studies concluded that AIs are cost-effective compared to tamoxifen in early-stage BC, these results are disputable because they did not consider the adherence, the side effect profile, and the subgroup analysis. However, the overall quality of the studies included was average according to the CHEERS checklist. Characterizing heterogeneity should be considered in future studies.
Data Availability
The datasets used and/or analyzed during this study are available from the corresponding author upon reasonable request.
Abbreviations
- BC:
-
Breast cancer
- AIs:
-
Aromatase inhibitors
- CHEERS:
-
Consolidated Health Economic Evaluation Reporting Standards
- QALYs:
-
Quality Adjusted Life Years
- LYS:
-
Life years saved
- CEA:
-
Cost- effectiveness analysis
- CUA:
-
Cost utility analysis
- CBA:
-
Cost-benefit analysis
- ICER:
-
Incremental cost-effectiveness ratio
- RCT:
-
Randomized controlled trials
- SA:
-
Sensitivity analysis
- PSA:
-
Probabilistic sensitivity analysis
References
International Agency for Research on Cancer. Latest global cancer data: World Health Organiztion; 15 December 2020 [Available from: https://www.iarc.who.int/wp-content/uploads/2020/12/pr292_E.pdf.
Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KA, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast Cancer: ASCO Clinical Practice Guideline focused Update. J Clin Oncol. 2019;37(5):423–38.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus. Tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. The Lancet. 2015;386(10001):1341–52.
John-Baptiste AA, Wu W, Rochon P, Anderson GM, Bell CM. A systematic review and methodological evaluation of published cost-effectiveness analyses of aromatase inhibitors versus tamoxifen in early stage breast cancer. PLoS ONE. 2013;8(5):e62614.
Frederix GW, Severens JL, Hovels AM, Raaijmakers JA, Schellens JH. Reviewing the cost-effectiveness of endocrine early breast cancer therapies: influence of differences in modeling methods on outcomes. Value Health. 2012;15(1):94–105.
Diaby V, Tawk R, Sanogo V, Xiao H, Montero AJ. A review of systematic reviews of the cost-effectiveness of hormone therapy, chemotherapy, and targeted therapy for breast cancer. Breast Cancer Res Treat. 2015;151(1):27–40.
Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic evaluation reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ. 2022;376:e067975.
CCEMG-EPPI-Centre IS. cost converter; Version 1.6. The Campbell and Cochrane Economics Methods Group (CCEMG) and the Evidence for Policy and Practice Information and Coordinating Centre (EPPI-Centre) 2019 [Available from: https://eppi.ioe.ac.uk/costconversion/.
Meregaglia M, Cairns J. Economic evaluations of follow-up strategies for cancer survivors: a systematic review and quality appraisal of the literature. Expert Rev PharmacoEcon Outcomes Res. 2015;15(6):913–29.
Mould-Quevedo JF, Contreras-Hernandez I. Economic evaluation of adjuvant hormone therapy for postmenopausal women with hormone receptor positive early stage breast cancer. Pharmacoeconomics - Spanish Research Articles. 2011;8(2):59–70.
Ye M, Lu J, Yang F, Wu B. Economic evaluation of Letrozole for early breast Cancer in a Health Resource-Limited setting. Biomed Res Int. 2018;2018:9282646.
Djalalov S, Beca J, Amir E, Krahn M, Trudeau ME, Hoch JS. Economic evaluation of hormonal therapies for postmenopausal women with estrogen receptor-positive early breast cancer in Canada. Curr Oncol. 2015;22(2):84–96.
Shih V, Chan A, Xie F, Ko Y. Economic evaluation of Anastrozole Versus tamoxifen for early stage breast Cancer in Singapore. Value in Health Regional Issues. 2012;1(1):46–53.
Lux MP, Wockel A, Benedict A, Buchholz S, Kreif N, Harbeck N, et al. Cost-effectiveness analysis of anastrozole versus tamoxifen in adjuvant therapy for early-stage breast cancer - A health-economic analysis based on the 100-month analysis of the atac trial and the german health system. Onkologie. 2010;33(4):155–66.
Lux MP, Reichelt C, Karnon J, Tanzer TD, Radosavac D, Fasching PA et al. Cost-Benefit Analysis of Endocrine Therapy in the Adjuvant Setting for Postmenopausal Patients with Hormone Receptor-Positive Breast Cancer, Based on Survival Data and Future Prices for Generic Drugs in the Context of the German Health Care System. Breast care (Basel, Switzerland). 2011;6(5):381–9.
Lee HJ, Lee TJ, Yang BM, Min J. Cost-effectiveness analysis of adjuvant hormonal treatments for women with postmenopausal hormone-receptor positive early breast cancer in the korean context. J Breast Cancer. 2010;13(3):286–98.
Gamboa O, Diaz S, Chicaiza L, Garcia M. Cost-benefit analysis of anastrazol and tamoxifen in adjuvant treatment of hormone receptor-positive, post-menopausal breast cancer. Biomedica. 2010;30(1):46–55.
Dubois RW. Cost–effectiveness thresholds in the USA: are they coming? Are they already here? J Comp effcetiveness Res. 2015;5(1):9–11.
Cuzick JSI, Baum M, Buzdar A, Howell A, Dowsett M, Forbes JF, ATAC/LATTE investigators. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010 Dec;11:1135–41.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). A Comparison of Letrozole and Tamoxifen in Postmenopausal Women with Early Breast Cancer. N Engl J Med. 2005;353(26):2747–57.
Cutler RLF-LF, Frommer M, Benrimoj C, Garcia-Cardenas V. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open 2018 8(1).
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MF performed article search and screened the abstracts. MF and DC screened the full articles. MF, DC, LH extract data and use the help of (CFG) in extracting the 2 articles in Spanish. MF and DC provided input to the interpretation of the review and discussion. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Althuwaibi, M.F., Fernandez-Garcia, C., Hayes, L. et al. Systematic review of economic evaluations of aromatase inhibitors in estrogen receptor-positive breast cancer: quality evaluation. BMC Health Serv Res 23, 689 (2023). https://doi.org/10.1186/s12913-023-09432-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-023-09432-5