Abstract
Background
Care decision discussions are intended to align treatment with the patient’s wishes, goals and values. To overcome the numerous barriers to such discussions, physicians as well as patients need tailored support. We evaluate the effect of a physicians’ training and a conversation aid for patients about care decisions on patient and physician outcomes.
Methods
At the internal medicine outpatient clinic of the University Medical Centre Utrecht, a 1:1 randomized, parallel-group study (patient conversation aid) was combined with a pre-post intervention (physicians’ training) design. Primary outcome was patient satisfaction, secondary outcomes were patient-doctor relationship, shared-decision-making, doctor preparedness and patient appreciation of the conversation aid.
Results
Between October 2018 and February 2020 11 physicians (36% residents, 73% female) and 185 patients (median age 58 years (interquartile range (IQR) 50–68), 60% male) participated. Only 28% of the patients reported a care decision discussion during the consultation. We found no effect of the interventions on patient satisfaction (effect sizes -0.14 (95% confidence interval (CI) -0.56–0.27) for conversation aid; 0.04 (95% CI -0.40–0.48) for physician’s training), nor on the patient-doctor relationship or shared-decision-making. However, physicians felt more prepared to discuss care decisions after training (median 3 (IQR 1–4) vs 1 (IQR 0–3), p = 0.015). Patients assessed the conversation aid informative and gave an overall mark of median 7 (IQR 7–8).
Conclusions
First steps towards fruitful discussions about care decisions were made: patients considered the conversation aid informative and physicians felt better prepared to discuss care decisions after training. The low number of care decision conversations patients reported shows exactly how important it is to focus on interventions that facilitate these discussions, for both the patient and physician. Further work needs to be done to establish the best way to empower patients and physicians.
Trial registration
Dutch trial register, trial 6998 (NTR 7188), registered 04/05/2018, https://www.trialregister.nl/trial/6998.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
The nationwide ‘Choosing Wisely’ campaign started in the USA in 2012 to engage physicians and patients in conversations about unnecessary tests, treatments and procedures, hereby contributing to appropriate healthcare [1]. Following this, the Dutch Association of Internal Medicine published a list of 10 ‘Wise Choices’. One of these ‘Wise Choices’ is to discuss care decisions when talking to patients about their treatment [2].
We define care decisions as discussions to align treatment with the patient’s wishes, goals and values, in which the option could also be to waive treatment or further investigation or to put limits to this (e.g. mechanical ventilation, dialysis, tube feeding). This includes for instance code status discussions and advanced care planning (ACP). Although the international consensus definition of ACP as posed by Rietjens et al. corresponds greatly with our vision on care decisions [3], the term ACP is strongly associated with the end of life, mostly due to the extensive research in end-of-life settings [4]. To avoid this association, we choose to use the term care decisions throughout this paper.
There are numerous barriers for both physicians and patients to discuss care decisions. Barriers for physicians described in literature are for instance: feeling unskilled or inadequately trained; discomfort and fear of complaints [5]. On top of that, physicians often wrongfully assume that patients do not want to discuss care decisions [6,7,8]. Patients face other difficulties, such as a lack of knowledge, unawareness of patients of the relevance, and the expectation that physicians will initiate the discussion when needed [4, 9].
When both parties avoid talking about care decisions, these discussions do not take place in time [10]. Consequently, the opportunity to adapt treatment to align with patient’s wishes is often missed [11]. Also, this results in situations in which these discussions have to be conducted in far from ideal circumstances, such as in the acute setting at the emergency department with limited time and an acutely ill patient [12]. To overcome these barriers and make way for fruitful discussions about care decisions, physicians as well as patients need tailored support.
For this study, we aimed to evaluate the effect of a training for physicians and a conversation aid for patients about the topic of care decisions. We measured patients’ and physicians’ satisfaction during the subsequent consultation at the outpatient clinic.
Methods
Design overview
In this study, a randomized, parallel-group study was combined with a pre-post intervention design. Participating patients were randomized in a 1:1 ratio. Randomization sequence was created using Castor EDC (electronic data capture) software and was stratified by gender in a 1:1 ratio using random block sizes of 4, 6, and 8. Participating physicians were trained halfway through the study period. This resulted in 4 groups (physicians before training and patients without conversation aid (= reference group), physicians before training and patients with conversation aid, physicians after training and patients without conversation aid, physicians after training and patients with conversation aid (= intervention group) (Fig. 1). The required sample size was expected to be reached after 6–9 months, based on average number of outpatient clinic consultations per physician. 42% of eligible patient population could not be reached by phone on multiple occasions and therefore could not be approached. Furthermore, a third of the approached patients refused to participate. Due to the lower-than-expected recruitment rate and the inability to further postpone physicians’ training for logistical reasons, physicians’ training took place 7 months after the first inclusion, before half of the intended sample size was reached. The study was terminated early after 16 months because inclusion was slowing down since an increasing part of the eligible population was already approached. At this moment 80% of the attempted sample size was reached. Besides, due to the low number of actual decisions on care decisions, one of our secondary outcomes (decisional conflict) could not be properly statistically assessed. Instead, we show number of care decision discussions and decisions made. No other changes have been made to the study protocol. This study was performed in line with the principles of the Declaration of Helsinki and approved by the Medical Research Ethics Committee Utrecht (MREC 18–465) and prospectively registered 04/05/2018 at the Dutch trial register (http://www.trialregister.nl, NTR 7188).
Setting and participants
This study was conducted at the internal medicine outpatient clinic of the University Medical Centre Utrecht (UMCU), a tertiary care teaching medical centre in the Netherlands.
Physicians
Eligible physicians were residents and specialists working at one of the outpatient clinics in our university hospital. Specialties at this outpatient clinic are general internal medicine, endocrinology, diabetes, nephrology, infectious diseases, immunology, vascular disease and gastroenterology. Exclusion criteria were participation in the pilot test of the e-learning module (which was used in the training), and awareness of the purpose of the study (e.g. involvement in an earlier stage or research meeting). Eligible physicians were recruited by the research team and informed that the study was about patient-doctor communication, consultations would be video-taped, and they had to fill out a questionnaire for each participating patient. They were not informed that the focus of this study was the discussion of care decisions. Written informed consent was obtained, after which their schedules were screened for eligible patients.
Patients
Patients ≥ 18 years with a scheduled consultation with one of the participating physicians within the study period were eligible for inclusion. The time between scheduling and the actual appointment had to be ≥ 3 weeks to account for sufficient time for the patient to consider participation and the research team had to be available to obtain written informed consent before the appointment. Visits included routine visits and new patients at the outpatient clinic that were referred by their general practitioner. They visited the outpatient clinic for a variety of indications: renal insufficiency, diabetes mellitus, hypertension, etcetera. Exclusion criteria were insufficient command of the Dutch language (i.e. unable to read and understand the conversation aid and questionnaires), inability to give informed consent and a registered discussion on treatment limitations with their physician within 2 years before the visit. Patients could only participate once.
Patients were contacted by phone by the research team and informed that the study was about patient-doctor communication, half of the participants would receive an online conversation aid and the consultation would be video-taped. They were unaware of the topic of the conversation aid and focus of the study. After verbal informed consent was obtained, participants were randomized. Patients in the control group received an e-mail with the same information as discussed during the phone call, whilst the intervention group received an e-mail with information about the topic of the conversation aid, along with the web link to the conversation aid. Written informed consent was obtained directly before the outpatient clinic consultation by the research team.
The outpatient clinic consultations were video recorded for qualitative analyses, of which the results are reported in a separate publication [13]. The video camera was visible in the consultation room and both patient and physician were aware of (and consented to) the whole consultation being video recorded.
Immediately after the consultation, both the patient and the physician received a separate questionnaire (complete questionnaires in supplementary appendix 1).
Interventions
Physicians’ training
The physicians’ training consisted of an e-learning module and a hands-on training with a simulated patient (i.e. an individual trained to act as a real patient). More detailed information can be found in supplementary appendix 2. After the training, physicians were aware that care decisions were the main focus of the present study. However, physicians were instructed to do their consultations with participating patients similar to those with non-participating patients (i.e. they should not discuss care decisions solely because ‘the camera is on').
Patient education: conversation aid
The conversation aid for patients was an online application in which patients could find comprehensible information about why it is important to discuss care decisions, what certain treatments entail and what possible treatment limitations are. Written information was accompanied by visual material. Hyperlinks to additional information were included. The conversation aid was created in collaboration with the UMCU Patient Panel with special attention for the use of understandable language.
Due to the nature of the intervention, patients and physicians could not be blinded to their own intervention. However, both were unaware of each other’s intervention.
Data collection and outcomes
Baseline characteristics
Patient characteristics were extracted from the electronic patient records by the research team (age, gender, Charlson Comorbidity Index (CCI) [14]) and collected via the patient-questionnaires (marital status, educational level, work status, health perception, quality of life and social support). Health perception and quality of life were both measured on a 11-points Likert scale (0 to 10).Social support was measured with the Oslo-3-questionairre [15], translated into Dutch using the validated forward–backward method [16, 17]. Physicians’ characteristics were collected in the physician-questionnaires (age, gender, resident/specialist and years of training or work experience).
Outcome measures
The primary outcome was patient satisfaction, as a mean of 2 questions of patient satisfaction on a 11-points Likert scale (0 to 10). This scale is a frequently used outcome measure for patient satisfaction in a multitude of settings and interventions [18,19,20,21]. The two questions on patient satisfaction were:
How satisfied were you with the conversation with your physician at the outpatient clinic?
How satisfied were you with the information given before, during and after your outpatient clinic visit?
Secondary outcomes were:
The patient-doctor relationship, evaluated using the Patient Doctor Relationship Questionnaire (PDRQ-9). 9 items are scored on a 5-point Likert scale ranging from 1 (very low quality) to 5 (very high quality). The total score consists of the sum of each of the items and ranges from 9 to 45 [22, 23].
Shared-decision-making, evaluated using the Shared Decision Making Questionaire-9 for physicians (SDM-Q9-DOC). 9 items are scored on a 6-point Likert scale ranging from 0 (totally disagree) to 5 (totally agree). Items are summed and multiplied by 20/9 to provide a score with 0 indicating the lowest and 100 the highest possible level of SDM [24,25,26,27].
Doctor preparedness to discuss treatment wishes, evaluated through 8 questions ranging from very generic to care decision specific, and a mock question about medication to mask the focus of this study.
Patient appreciation of the conversation aid (intervention group only), evaluated through 10 questions on aspects of the conversation aid, an overall score, and a free text space for additional suggestions.
In summary: for each patient seen by a physician, the physician needed to complete the SDM questionnaire and physician preparedness assessment, combined in one questionnaire. All patients completed the satisfaction items, and the patient-doctor relationship questionnaire. Patients in the patient intervention group additionally completed the questions on their appreciation of the conversation aid.
Statistical analysis
We performed an intention to treat analysis. Patient characteristics are shown stratified by intervention group. Physicians’ characteristics were described narratively.
The primary outcome of mean patient satisfaction score was shown stratified by intervention using medians and interquartile ranges. The primary outcome in the intervention group (both patient and physician trained) was first compared to the reference group (neither patient nor physician trained) with a Mann–Whitney U test. Following a gatekeeping procedure to reduce the risk of a type I error, further statistical comparisons between the patient intervention-group and physician intervention-group versus the reference group would have been performed only if the primary outcome differed between the intervention group and the reference group (fixed sequence hierarchical testing). We used the same strategy for the patient-doctor relationship and shared-decision-making outcomes. To adjust for confounders while taking into account dependence between scores of patients within physicians, primary and secondary outcomes were analysed using a multilevel mixed model. Because patients within a physician might be more similar than patients from other physicians, (e.g. more satisfied, similar diseases) a random intercept for physician was added to the model. We hypothesized that the effect of the physicians training could be different for each physician due to differences in knowledge and experience, therefore we added a random slope for physicians training. Analyses were adjusted for patients’ age, gender, CCI, quality of life, and physicians’ gender and level (resident or specialist) based on previous literature [28,29,30]. An interaction term between patient intervention and physician intervention was added to assess whether the effect of either intervention differed depending on the other intervention. The non-significant interaction term indicated that the effects of both interventions were independent. Therefore, the interaction term was subsequently removed from further analyses and, because the sample size calculated for the fixed hierarchical testing was not met, we additionally analysed the data as being a two-by-two factorial design.
To evaluate preparedness of the physician, results of physicians’ questionnaires before and after training were compared and tested for statistical significance using Mann–Whitney U test. Patient appreciation of the conversation aid was described narratively.
All analyses were performed using IBM SPSS Statistics 25.0.0.2 software. P values < 0.05 were considered statistically significant.
Sample size calculation
An a priori sample size calculation for the comparison of the main intervention group (physician trained, patient informed) and reference group (physician not trained, patient uninformed) on the primary outcome was performed using the statistic program G*Power. In previous studies, patient satisfaction on an 11-point Likert scale (0 to 10) was found to be between 5 and 9, with standard deviations between 1.2 and 3.2 [18,19,20,21]. Hence, we assumed the mean patient satisfaction score to be 7.0 (reference group) and 8.0 (intervention group) with a standard deviation of 2 (i.e. a Cohen’s effect size 0.5). To achieve a power of > 80% with a (one-sided) alpha of 0.05, 51 patients per group were needed. To enable stratified analysis by gender and a loss to follow-up of 10%, the required sample size would be 232 patients.
Results
Study participants
Eleven physicians participated in this study, including 4 residents (educational year 3–6) and 7 specialists from different areas of specialization (nephrology, vascular medicine, immunology, endocrinology, gastroenterology). The majority were female (8/11, 73%), responsible for 71% of all consultations in this study. One physician was not able to participate due to lack of time. Between October 2018 and February 2020, a total of 185 patients participated in the study. Figure 1 shows a diagram of the patient-participant flow.
The physicians’ training took place when 77 patients were included (33% of the attempted total sample size). Table 1 shows the baseline characteristics of the patients stratified by intervention group. The overall median age was 58 years (IQR 50–68), 60% were men, and the median CCI was 3.0 (IQR 1.0–5.0). A total of nine patients were lost to follow-up. These were equally divided amongst the four groups and the characteristics we had from these patients (age, gender and CCI) were similar to the overall values.
Patient satisfaction, patient-doctor relationship and shared decision making
Table 2 shows the mean patient satisfaction, patient-doctor relationship and shared-decision-making stratified by intervention group. The number of patient-reported care decision discussions during the outpatient clinic visit and in which a decision was made are shown as well. Only 45/161 (28%) patients reported to have discussed care decisions during the outpatient clinic visit, of which 25 (56%) made a decision.
After adjusting for patient-related (age, gender, quality of life, CCI) and physician-related (specialist/resident, gender) confounders, no statistically significant association between conversation aid and physician’s training and mean satisfaction score was found (effect sizes -0.14 (95% CI -0.56 to 0.27) for conversation aid; -0.04 (95% CI -0.48 to 0.40) for physician’s training). Similarly, for the secondary outcomes, patient-doctor relationship (effect sizes -0.45 (95% CI -2.85 to 1.95) for conversation aid; 1.28 (95% CI -1.04 to 3.60) for physician’s training) and shared-decision-making (-0.01 (95% CI -5.96 to 5.94) for conversation aid; -0.23 (95% CI -8.89 to 8.42) for physician’s training) no statistically significant association was found (supplementary appendix 3). When looking at the interventions separately, no significant difference in median satisfaction score with and without the intervention was observed (before physicians’ training 8.0 (IQR 8.0–9.0), after training 8.5 (IQR 8.0–9.5), p = 0.476; without conversation aid 8.5 (IQR 8.0–9.25), with conversation aid 8.0 (IQR 8.0–9.0), p = 0.106).
Preparedness of the physician
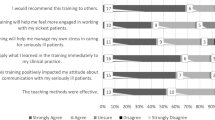
Physicians felt more prepared to discuss care decisions after training (median 3 (IQR 1–4) vs 1 (IQR 0–3), p = 0.015). There were no differences in general aspects of the consultation (i.e. overall satisfaction and preparedness to answer questions of the patient) or factors related to discussing care decisions between before and after the training.
Patients appreciation of the conversation aid
Most patients appraised the conversation aid with an overall mark of 8 (median 7, IQR 7–8).
Most patients consider the conversation aid to be clear, informative, impartial, understandable and not too time-consuming. Most patients stated not to feel insecure or sad after reading the aid. When asked whether the conversation aid helped to form an opinion on care decisions, the majority of patients did not express a clear opinion.
In summary, neither of the interventions had a statistically significant effect on patient satisfaction, patient-doctor relationship and extend of shared-decision-making experienced by physicians. Physicians felt statistically significant more prepared to discuss care decisions after training and patients evaluated the conversation aid positively.
Discussion
Neither of the interventions had a statistically significant effect on patient satisfaction, patient-doctor relationship and extent of shared decision-making experienced by physicians. Patients considered the conversation aid to be informative and easy to understand without causing insecurity or anxiety. Furthermore, physicians felt better prepared to discuss treatment decisions after the training.
With the interventions under study, we aimed to stimulate patient empowerment, patient-centred care and meaningful discussions on care decisions, all hopefully resulting in more satisfied patients. We deliberately refrained from using number of care discussions as study outcome as discussing care decisions just to ‘check off a box’ does not improve patient-centred care and leads to frustration and pressure in physicians [31]. We aimed to plant a seed, to stimulate patients to think about their preferences and to create common ground to start the conversation; not to force them to reach an immediate decision.
Although it was not the focus of our study, the low proportion of patients reporting to have discussed care decisions (28%), of which about half made a decision during the outpatient clinic visit, is remarkable. These low numbers might be explained by the perception that it is ‘too soon’ or ‘not yet relevant’ to discuss care decisions [32,33,34]. Besides, previous research showed physicians often miss openers from patients that could have prompted these discussions [11]. Our qualitative analysis shows that care decisions is a precarious topic for which there is no obvious interactional slot. Therefore, effort is needed to introduce the topic and create common ground [13].
There are several possible explanations for the absence of a statistically significant effect of the interventions on the mean patient satisfaction. First, we did not reach the intended sample size. This could have resulted in insufficient power to detect a possible effect. Another explanation may be the low number of care decision conversations, possibly diluting any effect. Furthermore, patient satisfaction is influenced by many factors [35]. We tried to minimize the influence of unrelated aspects by specifically directing the two questions on patient satisfaction to the conversation with the physician and the information given rather than measuring overall satisfaction, but this does not completely exclude other influences. Moreover, patient satisfaction scores without any of the interventions were high, with a median of 8.5 (IQR 8.0–9.0). It is harder to improve satisfaction, if satisfaction is already very high [36]. Finally, it could be the case that the interventions under study are not sufficient in improving care decision discussions, and therefore did not result in a statistically significant effect.
Patients’ general attitude towards the conversation aid was positive. Patients considered the conversation aid informative. Yet, they did not assess it as helpful in forming an opinion about care decisions or discussing them. A potential explanation can be that processing the information and forming an opinion requires more time, in which case the conversation aid might still have planted a seed for further consideration. Physicians are often afraid that introducing the topic of care decisions makes patients anxious or insecure [5]. It is reassuring that most patients reported that they did not feel insecure, sad or anxious when being provided with information about care decisions in the conversation aid.
Physicians indicated they felt more prepared to discuss care decisions after the training. The fact that this difference was not seen in separate important components of care decision discussions raises questions about whether the physicians actually were better prepared for these conversations, especially as it is known self-assessment has a poor agreement with externally assessed performances [37, 38]. However, the feeling of being unskilled or inadequately trained is a known barrier to discuss care decisions [5]. Therefore, we consider the feeling of being better prepared an important step to remove this barrier and thereby improving care decision conversations.
The strength of our study lies in the outpatient clinic setting we studied. Most research on care decisions is conducted in end-of-life settings, although it is considered essential to start discussing this in an earlier stage [2].
We are aware that our research may have several limitations. The earlier termination of the study and low participation rate could have led to selection bias. The presence of the video camera in the consultation room could have influenced the conversations and thereby patient satisfaction. A sense of familiarity between the patient and physician could have influenced care decision discussions and patient satisfaction. This ‘familiarity’ might depend on many factors (e.g. number of visits, content of those visits) which makes it impossible to control or correct for. Furthermore, the conversation aid and questionnaires were in Dutch. Our results are therefore not extendable to patients with low literacy or language barriers. Finally, reasons for why neither the physician nor the patient introduced the topic of care decisions was not asked in the questionnaires. Further work needs to be done to establish the best way to remove the remaining barriers to care decision discussions and motivate physicians and patients to engage in these discussions.
Conclusion
Although the conversation aid for patients and training for physicians did not improve patient satisfaction in this study, these interventions can eliminate some barriers to discuss care decisions: physicians feel more prepared to discuss care decisions and patients are more informed without feeling anxious or sad. The low number of care decision discussions shows there is still a lot of work to be done. Further work needs to be done to establish the best way to remove the barriers to care decision discussions and motivate physicians and patients to engage in these discussions.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. Metadata is available in the DataverseNL repository, https://doi.org/10.34894/YFB1S1.
Abbreviations
- ACP:
-
Advanced care planning
- UMCU:
-
University Medical Centre Utrecht
- CCI:
-
Charlson Comorbidity Index
- PDRQ-9:
-
Patient-doctor relationship questionnaire
- SDM-Q9-DOC:
-
Shared Decision Making Questionaire-9 for physicians
- OSS-3:
-
Oslo-3-questionairre
- DES:
-
Decisional Evaluation Scale
- IQR:
-
Interquartile range
References
Levinson W, Kallewaard M, Bhatia S, Wolfson D, Shortt S, Kerr EA. “Choosing Wisely”: a growing international campaign On behalf of the Choosing Wisely International Working Group. https://doi.org/10.1136/bmjqs-2014-003821.
Nederlandse Internisten Vereniging (Netherlands Association of Internal Medicine ). No Title. Verstandige keuzes bij interne geneeskunde (Wise Choices in Internal Medicine).
Rietjens JAC, Sudore RL, Connolly M, van Delden JJ, Drickamer MA, Droger M, et al. Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol. 2017;18(9):e543-51. https://doi.org/10.1016/S1470-2045(17)30582-X.
Houben CHM, Spruit MA, Groenen MTJ, Wouters EFM, Janssen DJA. Efficacy of advance care planning: A systematic review and meta-analysis. J Am Med Dir Assoc. 2014;15(7):477–89. https://doi.org/10.1016/j.jamda.2014.01.008.
Mockford C, Fritz Z, George R, Court R, Grove A, Clarke B, et al. Do not attempt cardiopulmonary resuscitation (DNACPR) orders: A systematic review of the barriers and facilitators of decision-making and implementation. Vol. 88, Resuscitation. Elsevier Ireland Ltd; 2015. p. 99–113.
Curtis JR, Patrick DL, Caldwell ES, Collier AC. Why don’t patients and physicians talk about end-of-life care? Arch Intern Med. 2000;160:1690–6.
Patel K, Janssen DJA, Curtis JR. Advance care planning in COPD. Respirology. 2012;17(1):72–8.
Selman L, Harding R, Beynon T, Hodson F, Coady E, Hazeldine C, et al. Improving end-of-life care for patients with chronic heart failure: “Let’s hope it’ll get better, when I know in my heart of hearts it won’t.” Heart. 2007;93(8):963–7.
Ouchi K, George N, Schuur JD, Aaronson EL, Lindvall C, Bernstein E, et al. Goals-of-Care Conversations for Older Adults With Serious Illness in the Emergency Department: Challenges and Opportunities. Ann Emerg Med. 2019;74(2):276–84. https://doi.org/10.1016/j.annemergmed.2019.01.003.
Walczak A, Butow PN, Davidson PM, Bellemore FA, Tattersall MHN, Clayton JM, et al. Patient perspectives regarding communication about prognosis and end-of-life issues: How can it be optimised? Patient Educ Couns [Internet]. 2013;90(3):307–14. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0738399111004587
Ahluwalia SC, Levin JR, Lorenz KA, Gordon HS. Missed opportunities for advance care planning communication during outpatient clinic visits. J Gen Intern Med. 2012;27(4):445–51.
Cruz-Carreras MT, Chaftari P, Viets-Upchurch J. Advance care planning: challenges at the emergency department of a cancer care center. Support Care Cancer. 2018;26:585.
Briedé S, van Charldorp TC, Kaasjager KAH. Discussing care decisions at the internal medicine outpatient clinic: A conversation analysis. Patient Educ Couns [Internet]. 2021;(xxxx):1–8. https://doi.org/10.1016/j.pec.2021.11.029
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–83.
Kocalevent R, Berg L, Beutel ME, Hinz A, Zenger M, Härter M. Social support in the general population : standardization of the Oslo social support scale ( OSSS-3 ). BMC Psychol. 2018;6:4–11.
Dalgard OS, Dowrick C, Lehtinen V, Vazquez-Barquero JL, Casey P, Wilkinson G, et al. Negative life events, social support and gender difference in depression. A multinational community survey with data from the ODIN study. Soc Psychiatry Psychiatr Epidemiol. 2006;41:444.
Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. 2000;25:3186.
Timmers T, Janssen L, Pronk Y, van der Zwaard BC, Koëter S, van Oostveen D, et al. Assessing the efficacy of an educational smartphone or tablet app with subdivided and interactive content to increase patients’ medical knowledge: Randomized controlled trial. JMIR mHealth uHealth. 2018;6:e10742.
van Berckel MMG, Bosma NH, Hageman MGJS, Ring D, Vranceanu AM. The Correlation Between a Numerical Rating Scale of Patient Satisfaction With Current Management of an Upper Extremity Disorder and a General Measure of Satisfaction With the Medical Visit. Hand. 2017;12:202.
Ackermans L, Hageman MG, Bos AH, Haverkamp D, Scholtes VAB, Poolman RW. Feedback to patients about patient-reported outcomes does not improve empowerment or satisfaction. Clin Orthop Relat Res. 2018;476:716.
Bashour HN, Kanaan M, Kharouf MH, Abdulsalam AA, Tabbaa MA, Cheikha SA. The effect of training doctors in communication skills on women’s satisfaction with doctor-woman relationship during labour and delivery: A stepped wedge cluster randomised trial in Damascus. BMJ Open. 2013;3:e002674.
Van Der Feltz-Cornelis CM, Van Oppen P, Van Marwijk HWJ, De Beurs E, Van Dyck R. A patient-doctor relationship questionnaire (PDRQ-9) in primary care: Development and psychometric evaluation. Gen Hosp Psychiatry. 2004;26(2):115–20.
Qiao T, Fan Y, Geater AF, Chongsuvivatwong V, McNeil EB. <p>Factors associated with the doctor–patient relationship: doctor and patient perspectives in hospital outpatient clinics of Inner Mongolia Autonomous Region China. Patient Prefer Adherence. 2019;13:1125–43.
Attribution-noncommercial-noderivatives CC, License I, Loon K, Stiggelbout AM, Shared I, Making D, et al. Vragenlijst Over Gezamenlijke Besluitvorming ( SDM-Q-Doc ). 2012;(February):4–5.
Rodenburg-Vandenbussche S, Pieterse AH, Kroonenberg PM, Scholl I, van der Weijden T, Luyten GPM, et al. Dutch Translation and Psychometric Testing of the 9-Item Shared Decision Making Questionnaire (SDM-Q-9) and Shared Decision Making Questionnaire-Physician Version (SDM-Q-Doc) in Primary and Secondary Care. PLoS ONE. 2015;10(7):e0132158.
Kriston L, Scholl I, Hölzel L, Simon D, Loh A, Härter M. The 9-item Shared Decision Making Questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Educ Couns. 2010;80(1):94–9. https://doi.org/10.1016/j.pec.2009.09.034.
Scholl I, Kriston L, Dirmaier J, Buchholz A, Härter M. Development and psychometric properties of the Shared Decision Making Questionnaire - physician version (SDM-Q-Doc). Patient Educ Couns. 2012;88(2):284–90.
Heidegger T, Husemann Y, Nuebling M, Morf D, Sieber T, Huth A, et al. Patient satisfaction with anaesthesia care: Development of a psychometric questionnaire and benchmaking among six hospitals in Switzerland and Austria. Br J Anaesth. 2002;89:863.
Hall MC, Elliott KM, Stiles GW. Hospital patient satisfaction: Correlates, dimensionality, and determinants. J Hosp Mark. 1993;7:77.
Curtis JR, Downey L, Back AL, Nielsen EL, Paul S, Lahdya AZ, et al. Effect of a patient and clinician communication-priming intervention on patient-reported goals-of-care discussions between patients with serious illness and clinicians: A randomized clinical trial. JAMA Intern Med. 2018;178:930.
Smith AK, Ries AP, Zhang B, Tulsky JA, Prigerson HG, Block SD. Resident approaches to advance care planning on the day of hospital admission. Arch Intern Med. 2006;166(15):1597–602.
Simon J, Porterfield P, Bouchal SR, Heyland D. “Not yet” and “Just ask”: Barriers and facilitators to advance care planning-a qualitative descriptive study of the perspectives of seriously ill, older patients and their families. BMJ Support Palliat Care. 2015;5(1):54–62.
Schickedanz AD, Schillinger D, Landefeld CS, Knight SJ, Williams BA, Sudore RL. A clinical framework for improving the advance care planning process: Start with patients’ self-identified barriers. J Am Geriatr Soc. 2009;57(1):31–9.
Johnson S, Butow P, Kerridge I, Tattersall M. Advance care planning for cancer patients: A systematic review of perceptions and experiences of patients, families, and healthcare providers. Psychooncol. 2016;25(4):362–86.
Trout A, Magnusson AR, Hedges JR. Patient satisfaction investigations and the emergency department: What does the literature say? Acad Emerg Med. 2000;7:695.
Friesner D, Neufelder D, Raisor J, Bozman CS. How to improve patient satisfaction when patients are already satisfied: a continuous process-improvement approach. Hosp Top. 2009;87:24.
Gordon MJ. A review of the validity and accuracy of self-assessments in health professions training. Acad Med. 1991;66:762.
Woolliscroft JO, TenHaken J, Smith J, Calhoun JG. Medical students’ clinical self-assessments: comparisons with external measures of performance and the students’ self-assessments of overall performance and effort. Acad Med. 1993;68:285.
Acknowledgements
We would like to thank all physicians and patients that participated in this study. Furthermore, we would like to thank B. Silvius, I.P Klaassen and C.A.M Joosten (research nurses) and C. van Ginkel (physician) for assistance in recruiting participants and obtaining informed consents.
Funding
This work was funded by the Netherlands Organization for Health Research and Development (ZonMw), grant number 516000504 (80–83900-98–753). The funding foundation had no role in study design, data collection, data analysis, preparation of the article, or decision to publish.
Author information
Authors and Affiliations
Contributions
Saskia Briedé: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Project Administration, Visualization, Writing – Original Draft Preparation.
Maria A. de Winter: Conceptualization, Formal Analysis, Funding Acquisition, Methodology, Writing – Review & Editing.
Tessa C. van Charldorp: Conceptualization, Supervision, Writing – Review & Editing.
Karin A.H. Kaasjager: Conceptualization, Funding Acquisition, Methodology, Project Administration, Resources, Supervision, Writing – Review & Editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Medical Research Ethics Committee Utrecht (MREC 18–465) and written informed consent was obtained from all participants before study participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Briedé, S., de Winter, M.A., van Charldorp, T.C. et al. The effect of physician training and patient education on the discussion of care decisions at the internal medicine outpatient clinic. BMC Health Serv Res 22, 1569 (2022). https://doi.org/10.1186/s12913-022-08901-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-022-08901-7