Abstract
Background
Prostate cancer is the fifth cause of cancer mortality among men worldwide. However, there is limited data on costs associated with prostate cancer in low- and middle-income countries particularly in the sub-Saharan region. From a societal perspective, this study aims to estimate the cost of prostate cancer in Eswatini.
Methods
This prevalence–based cost-of-illness study used diagnosis specific data from national registries to estimate costs associated to prostate cancer during 2018. The prevalence-based approach was used employing both top down and bottom up costing approaches. Costs data included health care utilization, transport, sick leave days and premature death.
Results
The total annual cost of prostate cancer was $6.2 million (ranging between $ 4.7 million and 7.8 million estimated with lower and upper bounds). Average cost-per patient for radiotherapy, chemotherapy and other non-medical direct costs (transport and lodging) were the highest cost drivers recording $16,648, $7,498 and $5,959 respectively whilst indirect costs including productive loss due to sick leave and pre-mature mortality was estimated at $58,320 and $113,760 respectively. Cost of managing prostate cancer increased with advanced disease and costs were highest for prostate cancer stages III and IV recording $1.1million, $1.9million respectively.
Conclusions
Prostate cancer is a public health concern in Eswatini, and it imposes significant economic burden to the society. This finding point areas for policy makers to perform cost containment regarding therapeutic procedures for prostate cancer and the need for strategies to increase efficiencies in the health care systems for increased value for health care services.
Similar content being viewed by others
Background
Among cancers, prostate cancer is the third commonest cancer after breast and lung cancer and the fifth cause of cancer mortality among men [1, 2]. In 2018, the number of new cases increased from 1.1 million in 2012 to 1.3 million in 2018 accounting for about 7.1% of the total cancer cases globally and 15% among men [2]. The causes of prostate cancer is attributable to genetic and environmental factors [2]. However, the incidence and mortality rate vary substantially within and across regions. Notably, high-income countries (HICs) reports high incidence rate compared to low- and -middle income countries (LMICs) [2]. In contrast, mortality rate is higher in developing countries particularly in sub-Saharan Africa regions [3]. The inequalities observed across regions with respect to prostate cancer incidence and mortality are in part linked to availability of effective screening and improved treatment modalities which are directly linked to resources availability [3, 4]. In Eswatini, compared to other common cancers, prostate cancer is ranked third accounting for 7.6% of total new cases 1074 in 2018 [5].
Prostate cancer causes clinical and economic burden to patients and governments. Screening tests include prostate-specific antigen (PSA) and digital rectal examination (DRG) [6, 7]. A positive screening tests results indicate further investigation [6]. Whilst PSA is the frequent screening test, it has been argued that PSA could potentially cause harm by over diagnosing low risk cancers that otherwise would have remained without clinical consequences for life time if left untreated [8]. In turn, this increases costs for prostate cancer [9]. In Sweden, annual costs associated with prostate cancer (screening, diagnosis and treatment) was estimated at €281 million [9]. In Ontario, the mean per patient cost for prostate cancer–related medication was $1211 [10]. In Iran, the total annual cost of prostate cancer was estimated at $2900 million [11]. Other studies estimated the economic burden of prostate cancer along with other cancer type. A study focusing on European countries, ranked prostate cancer the fourth cancer disease to cause health care costs compared to lung (€18.8billion), breast cancer (€15 billion), colorectal cancer (€13.1 billion) [12]. Similarly, in Korea, prostate cancer was among the top four cancers attributing to economic burden of disease [13].
There is limited evidence on the economic burden of prostate cancer from LMICs. Estimation of the economic burden of disease provide insight on treatment modalities and associated costs. The study aims to investigate the societal cost of prostate cancer in Eswatini during 2018.
Materials and methods
Study area
Eswatini formerly known as Swaziland is a country in Southern African bordering South Africa and Mozambique with an estimated population of 1.2 million [14]. The country’s economy is tied to South Africa and Eswatini’s domestic currency (Lilangeni=SZL) is pegged at parity with South African currency (Rand=ZAR) such that Eswatini cannot conduct its own monetary policy [15]. Eswatini fiscal revenue largely depend on Southern African Customs Union (SACU) revenues and remittance flowing mainly from South Africa [16, 17]. SACU receipts account for about a third of Eswatini’s total revenue and grants. However, over the past decades, SACU revenues have consistently declined leaving Eswatini’s economy constrained. The country records high national level poverty rate and income inequality which does not commensurate with its middle-income status. The national poverty rate is 58.9% percent at the international $1.90 poverty line and Gini index- a measure of inequality is 49.3 [17]. Eswatini ranks near the bottom of the World Bank’s Human Capital Index, with a score of 0.37 in 2020. Eswatini health spending as a share of the total budget is estimated at 10.1% and health per capita is estimated at $ 248 per annum [16]. Whilst Eswatini’s health expenditure is comparatively higher to some other countries in the Southern African region, the country’s health outcomes do not reflect its spending levels on health and its middle-income status. The health care service delivery is made up of public and private health care. Compared to the public, the private health care systems is better equipped both infrastructural and human resources however, at high health care costs. As such, private health care is accessed by less than 10% of the population, mainly those who owns health insurance [18].
Diagnostic and treatment capacity of conditions including cancer remains limited in the country mostly in the public health system. Through a government funded scheme namely Phalala, the Eswatini citizens are supported to access specialized health care services from neighboring countries mainly South Africa.
Methods of costing
This is a Cost of Illness (CoI) study investigating costs of prostate cancer from the societal perspective [19]. CoI studies estimate disease specific costs [20]. The prevalence based approach, was used employing both top down and bottom up costing approaches [19, 21]. The cost estimation involved identification, quantification and valuation of resources used. The total costs for prostate cancer was calculated by multiplying identified resources quantities and the respective unit costs. All costs were presented in US$ adjusted for 2018 ($1= SZL14.5).
Study population
Data on prostate cancer prevalence and mortality in 2018 was obtained from the National cancer registry [14]. The National Cancer Control Unit is led by the Ministry of Health. To estimate direct non-medical costs and annual gross earnings, estimates were obtained from a previous study that collected data using a direct non-medical costs patient questionnaire from a previous study on women diagnosed with breast cancer and receiving follow-up care at Mbabane Government chemotherapy unit (outpatient) in 2018 [22].
Management of prostate cancer in Eswatini
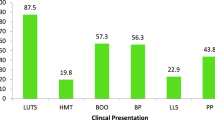
In Eswatini, routine prostate cancer screening is only recommended for men above age 50 every after two years [23]. The referral pathway shown in Fig. 1, simplifies the treatment pathway which begins by a man presenting with symptoms or eligible for screening at outpatient. Patient will be referred to urologist for screening tests including PSA and digital rectal examination (DRE) [6, 23]. These tests are not confirmatory however, they indicate changes in the prostate. Abnormal findings by either of the tests warrant further evaluation of patient and subsequent diagnostic test. These include biopsy (transrectal/perineal ultrasound guided biopsy (TRUS)). Patient with no cancer but presenting with symptom would receive management of lower urinary tract symptoms (LUTS). If cancer is confirmed further evaluation is conducted for cancer staging purposes in order to inform cancer management plan (metastasis screening). The evaluation includes radiology tests (bone scan, CT-scan and MRI pelvis). Staging is based on the tumor size (T) extent of lymph nodes involvement (N) and evidence of distant metastasis (M) [23, 24]. Depending on the risk score and prostate cancer stage, treatment include watchful waiting (cancer is monitored but not treated), surgery, radiation, chemotherapy and hormonal therapy (Androgen Deprivation Therapy) [23].
Most treatment modalities can be administered in various stages however for different intent [6, 23]. Radical prostatectomy, radiation and hormonal therapy can be applied for localised high risk prostate cancer (stage I and stage II) whilst for metastatic prostate cancer hormonal therapy will be first line in addition to radiotherapy, chemotherapy and hormonal therapy for palliation purposes. Radiation is not available in Eswatini and patient are referred to private hospitals in South Africa. Other surgical interventions for relieving symptoms such as transurethral resection of prostate (TURP) or bladder (TURB) can be conducted locally.
We used expert opinion from Mbabane Government Hospital - Chemotherapy Unit, Mbabane clinic -private hospital and information from Phalala Fund to establish patient referral pathway. Phalala Fund is a government funded scheme established to fund provision of specialized health care services to people of Eswatini that could not afford payment of specialized care that is not available in country [25]. The Eswatini standardized cancer care guidelines were used to establish screening, diagnosis and treatment variables. Costs were estimated based on market price. Radiotherapy is currently not available in Eswatini. As such, patients who require radiation are managed in South Africa through Phalala Fund. Chemotherapy is available locally through a government chemotherapy unit and local private clinic. However, it was established that most patients were still receiving chemotherapy from South Africa.
Costs
From a societal perspective, costs associated with prostate cancer were estimated to assess economic burden of prostate cancer in Eswatini. Direct medical costs were divided into recurrent and capital costs [19]. Recurrent costs included personnel, travel, consumables including medical supplies, administration, utilities and overheads. Capital costs consistent mainly of equipment, building, vehicle and everything that have a useful life of more than one year. Costs for prostate cancer were determined based on the data source presented in Table 1. All costs were presented in US Dollars using 2018 average exchange rate (1 USD ($) = 14.5 SZL).
Direct medical costs
Direct costs in this study include resource utilization for diagnosis, treatment (surgery, chemotherapy, radiotherapy and androgen deprivation therapy) and follow-up care. To estimate the directs costs, we estimated average cost of each intervention from screening, staging and treatment and multiplied by the number of corresponding patients who received the intervention. The number of men diagnosed with prostate cancer were obtained from the national cancer registry [26]. All diagnosed cases were assumed to have undergone screening test using PSA. Screening and diagnosis costs were obtained from private hospital and market pricing. Treatment costs mainly radiation, chemotherapy and androgen deprivation therapy were received from Phalala fund based on South African private hospitals fees. In Eswatini, a majority of the management costs are borne by the Eswatini Government through Phalala fund.
As per the standardized cancer care guidelines, we assumed that all the men with confirmed prostate cancer in 2018 underwent screening and diagnosis tests, treatment and incurred other direct costs including transport and accommodation. Follow-up care costs was estimated for one year for those reported alive in 2018.
Direct non-medical costs
Transport cost including return was estimated based on required patient follow-up visits based on the Eswatini Standardized Cancer Care Guidelines which state that follow-up visit should be every six months for the first two years and annually for up to five years following surgery [23]. Transport cost was estimated based on data from a previous study on breast cancer women receiving follow-up care at Mbabane Government Cancer Unit [22]. We assumed that all men completed treatment in 2018 had follow-up visits as per the Eswatini Standardized Cancer Guidelines. This study estimated one-year follow-up costs.
Indirect costs
We estimated the monitory value of prostate cancer related productivity loss due to morbidity (patient sick leave days incurred as a result of seeking health care) and pre-mature mortality).
The human capital method was used to estimate indirect costs related to productivity loss due to morbidity (sick leave as a result of seeking prostate cancer care) and pre-mature mortality [20]. We used average annual gross earnings computed from our previous study on breast cancer women receiving follow-up care in the chemotherapy unit, Mbabane Government hospital in Eswatini [22].
Morbidity costs
We estimated the number of sick leave days for men diagnosed with prostate cancer who are in the labor participation ages (18-60 years). Using findings from a previously published study [27], we assumed sick leave for an average of 54 days per person. The sick leave days included days for staging, treatment and follow-up care. Using findings from a previous study on breast cancer conducted in Eswatini [22], we assumed 20 working days per month and a full-time working day of 8 h with estimated costs per workday ($12) translating ($1.5) per work hour [22].
Mortality costs
To estimate the cost of lost productivity due to premature death related to prostate cancer, years of potential productive life lost (YPPLL) were calculated by subtracting age at death from the local retirement age of 60 years [28]. Prostate cancer age groups specific deaths were estimated assuming labor participation ages of Eswatini (18-60 years). We used full employment rate and annual average earnings obtained from a previous study. Average YPPLL was multiplied by average annual earnings. According to health economic recommendations, future costs were discounted at 3% and 5% [19, 29]. The number of prostate cancer related deaths was obtained from Eswatini Cancer Registry. In 2018, there were 31 prostate cancer related mortality with 4 that occurred within the labor participating ages of Eswatini (18-60) years [28].
Cancer mortality and years of potential productive life lost (YPLL)
The number of prostate cancer related deaths was obtained from Eswatini Cancer Registry from which the years of productive life lost was calculated. In 2018, there were 31 prostate cancer related mortality out of which 4 occurred within the labor participating ages of Eswatini (18-60) years [28].
Estimation of annual costs
We computed the aggregate total costs of screening, diagnosis and treatment of prostate cancer in 2018 as below:
Direct medical costs = Consisting of direct non-medical costs and direct medical costs.
Indirect costs = Consisting of morbidity costs and mortality costs (Patient time lost as a result of the condition and costs associated with premature mortality as a result).
All costs were reported in 2018 US dollars ($1=SZL14.5).
Sensitivity analysis
Sensitivity analysis was performed using ± 25% to account for the cost of follow-up prevalent cancer cases and to account for unrecorded cases by the facilities.
Results
Directs costs
In 2018, there were 90 prostate cancer cases of which 89% aged 60 years an above. The average age was 73 years. Table 2, shows unit average costs for treating prostate cancer cases including other direct costs such as transportation and accommodation.
Cost distribution by disease stage is shown in Table 3. Following the Eswatini Standard Cancer Care Guidelines we assumed that all confirmed cases underwent similar screening, diagnosis and treatment pathway shown in Table 2, and simplified referral pathway shown in Fig. 1. The average costs for the different pathway including treatment intervention differed with the prostate cancer stage. Radical prostatectomy was more frequent with early stages of prostate cancer whilst interventions like chemotherapy were common with prostate cancer stages III and IV. Table 3 shows the prostate cancer costs distribution by stage.
Radiation is not available in Eswatini and patients are referred to private hospitals in South Africa. On average, radiotherapy treatment is administered for a period of 5-weeks [25]. The estimated unit costs for radiotherapy was $16,648 whilst chemotherapy was $7,498. In addition to treatment costs, all patients referred for radiotherapy also incurred other direct costs including transport, lodging and allowance for accompanying staff (nurse and driver) at a unit costs $5,959, Table 3.
Direct non-medical costs
Using estimate from a previous study [22], the average transport cost per follow-up visit including return was $11 (inter quartile range (IQR)$4-46). On average, post treatment follow-up visits should be every 6 months resulting to four visits in a year including return. We assumed that all patients visited the hospital in the company of a relative. The total average transport costs including return was estimated at $5,029 (between $3,771 and 6,287 estimated with lower and upper bounds).
Indirect costs
Productive loss due to sick leave as a result of patient seeking health care for prostate cancer was estimated at $58,320, Table 4. Out of the 90 patients diagnosed with prostate cancer, there were 13 men within the labor participating ages which were assumed to be on average sick leave of 54 days per person excluding short term sick leave of 14 days that is usually covered by employers. A total of 31 men died of prostate cancer in 2018 out of which 4 were less than 60 years. Costs due to prostate cancer premature mortality was estimated at $113,760, Table 5.
Total annual costs
The total annual costs for prostate cancer was estimated at $ 6.2 million (between $4.7 million and 7.8 million estimated with lower and upper bounds), Table 6. Fourth 4% (40) of the cases were diagnoses with stage IV whilst only 11% (10) were diagnosed with stages I. Management of prostate cancer stages III and IV formed the greatest share of the costs for prostate cancer contributing about $1.2 and 2.1 million respectively. The total costs of stages I and II was estimated at $0.5 and $0.8 million. Transport and accommodation costs (cost incurred by those transferred to South Africa) were highest under other direct costs contributing about $0.5million. In 2018, there were 31 prostate cancer related deaths with only 4 occurred within the labor participating ages of Eswatini (18-60) years. The total year of productive life lost (YPPL) was 221 years. Indirect costs were estimated at $0.24 million and a majority (96%, $0.2 million) were productive loss from premature mortality, Table 6.
Discussion
The current study assessed the costs associated with prostate cancer in Eswatini, that is, screening, diagnosis, treatment and follow-up care. The study considered direct costs including follow-up care costs within one year of diagnosis. To our knowledge this is the first study to estimate the economic burden of prostate cancer in Eswatini. The estimated annual prostate cancer burden was $ 6.1 million in 2018. About 89% of the patient aged 60 years and above. Given the Eswatini Standardized Cancer Care and Guidelines [21], we assumed that all patients diagnosed in 2018 underwent the screening and diagnostic procedures. Treatment costs varied by cancer stage reflecting the utilization of treatment modalities per stage hence high costs observed in stages III ($1.2million) and IV (2.1million) versus Stage I and II with $0.5 and $0.8 million respectively. The findings indicate that managing advanced stages of the disease increases health care costs.
The study findings were in accordance with findings from other studies. A study assessing health care costs associated with prostate cancer in Canada reported increasing costs per stage I ($1,297), II ($3,289), III ($1,495), IV ($5,629) and V ($16,020) [30]. Similarly, a study conducted in Iran concluded that health care costs for metastatic stages were the highest compared to treatment costs for localized prostate cancer [11]. More studies had similar conclusions [31, 32]. Slightly different findings were from the United State of America who reported high treatment costs for initial diagnosis and metastatic phase with radical prostatectomy being the main cost driver [33]. Whilst in this study we found lesser cost with early stage cancer, however, both studies observed increasing costs with advanced cancer stages. Also, the differences could be partly explained by the men (20%) diagnosed with early stages of prostate cancer in our study. A systematic review of registry-based studies assessing economic burden of prostate cancer in Europe found that cost distribution across prostate cancer stages varied across countries [34]. This can be attributed to differences in prostate cancer detection and country specific management practice [34]. The authors also acknowledged the difference in methodologies applied in the studies as possible explanation to the varying outcome observed.
There seems to be lack of global consensus on prevention strategies particularly age of screening. The United State Preventive Service Task Force (USPSTF) recommend against routine screening for men 70 years and older for prostate cancer particularly using prostate specific antigen screening [35]. The Eswatini Standardized Cancer Care and Guidelines also discourages routine prostate cancer screening with an exception for men 50 years and above or symptomatic [23]. Other studies argue that increased screening lead to increased detection of low-grade cancers resulting to patient with indolent tumors receiving aggressive treatment [36].
In LICs such as Eswatini, the challenge is likely to be on a different direction than over diagnosing and consequently overt treatment. Lack of screening and comprehensive treatment remains the greatest challenge for most LMICs and LICs. Eswatini is not different from other low middle income countries from whom late diagnosis coupled with limited treatment options remains a challenge. In Eswatini, in 2018, more than 80% of the patients were diagnosed with advanced cancer (stages III and IV), yet major treatment is not available in country. These include radiotherapy and androgen deprivation therapy (ADT). Accessing care outside the country comes with additional costs, mainly accommodation, transportation and meals for patients referred to South Africa.
Lack of specialized and costly care have been reported in other countries particularly in Africa and mortality from prostate cancer is the highest in these countries and there is lack of cancer treatment guidelines [4, 37].
There is an urgent need to strengthen health systems enablers [38]. These include investments in the establishment of local cancer treatment centers, optimizing health workforce competencies throughout the continuum of care and ensuring availability of medical products and diagnostics technologies to facilitate local diagnosis, staging and management.
Despite the evidence that prostate cancer is a major public health challenge, literature on the economic burden of prostate cancer is however limited and severely so in low income countries particularly in the sub-Saharan region. Findings from a systematic review on the costs of prostate cancer studies indicated a need not only for harmonized methodologies but also to expand research in this field [39]. Similarly, another systematic literature review of registry-based studies reached similar conclusion on the need for further research in cost of illness studies focusing on prostate cancer [40].
In the study we assessed indirect costs by estimating the costs associated with unpaid sick leave days and productive loss due to premature mortality from prostate cancer. Of the total costs, indirect costs accounted for 4.2% ($0.24 million). Comparing these findings to previous cost analysis studies for prostate cancer, most of the studies did not consider assessing indirect costs, however a study from Sweden reported low proportion of productivity loss associated with prostater cancer [9]. Further comparison of the findings with studies from other cancer types conducted in Eswatini [22, 41], the indirect costs from this study accounted for a lesser share of the total cost. This could partly be explained by the fact that most participants (89%) were above the labor participating ages (18-60 years) and few deaths occurred below age 60 years. A similar pattern was observed in Sweden, again the finding were attributed to low number of prostate cancer cases and deaths among labor participation groups [9].
The key strength of our study was that this is the first study to estimate cost associated with prostate cancer in Eswatini. The study considered both direct and indirect costs of prostate cancer. Our study has notable findings that has implications on health care systems strengthening and resources allocation in Eswatini. Our study present description of resource utilization and associated health care costs in managing prostate cancer in Eswatini.
An important limitation is the absence of index cost in Eswatini. We considered private and market prices for best possible price estimates.
The estimates presented were based on available data however, estimates could be conservative due to several reasons, First, due to limited data availability we used information from literature and interview with experts for some treatment variables, as such, some information can be subject to context and preferences. Secondly, we only considered costs in the first year of diagnosis yet cost for follow-up care can be even beyond five years [6, 42]. Lastly, we employed human capital approach to estimate the costs related to productivity loss associated with prostate cancer. Whilst this is a commonly applied approach, it is mostly criticized for excluding individuals above the labor participation age group yet there is argument that some of those people can still be involved in labor activities that gives meaningful income. Another author argues that this has severe implication when valuing productivity loss for prostate cancer given that a majority of the patients are diagnosed after they have past the retirement age.
Conclusions
The findings of the study indicated that costs attributed to prostate cancer were substantial and they are a public health concern. The findings were consistent with those of other countries, a majority of which were conducted in developed countries. The study demonstrated the interventions and associated costs. Radiotherapy was the most expensive treatment intervention in Eswatini, yet other studies cited surgery related intervention as the major costs driver. This is a reasonable finding in the context of Eswatini given that radiotherapy treatment is not available locally, patients are referred to private hospitals outside the country. The findings point areas for policy makers to perform cost containment regarding therapeutic procedures for prostate cancer. Also, the study findings demonstrate that prostate cancer costs are likely to increase in future and there is a need for strengthening adherence to the Eswatini Standardized Cancer Care and Guidelines in order to ensure that resources are invested to diagnosing the most at risk groups.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. See study tables and figures.
Abbreviations
- ADT:
-
Androgen Deprivation Therapy
- DRE:
-
Digital Rectal Examination
- PSA:
-
Prostate Specific Antigen test
- TRUS:
-
Transrectal ultrasound
- LUTS:
-
Low Urinary Symptom
- HICs:
-
High Income Counties
- LICs:
-
Low Income Countries
- LMICs:
-
Low Middle Income Countries
- SDGs:
-
Sustainable Development Goals
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F: Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer 2015, 136(5):E359-E386
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 201810.3322/caac.21492.
Odedina FT, Ogunbiyi JO, Ukoli FAM: Roots of prostate cancer in African-American men. J Natl Med Assoc 2006, 98(4):539–543
Cassell A, Yunusa B, Jalloh M, Ndoye M, Mbodji MM, Diallo A, Kouka SC, Labou I, Niang L, Gueye SM. Management of Advanced and Metastatic Prostate Cancer: A Need for a Sub-Saharan Guideline. J Oncol. 2019;2019:1785428–1785428. https://doi.org/10.1155/2019/1785428.
Global Cancer Observatory: Cancer today https://gco.iarc.fr/
Chamberlain J, Melia J, Moss S, Brown J: The diagnosis, management, treatment and costs of prostate cancer in England and Wales. Health Technology Assessment (Winchester, England) 1997, 1(3):i
Eniu A, Carlson RW, Aziz Z, Bines J, Hortobágyi GN, Bese NS, Love RR, Vikram B, Kurkure A, Anderson BO: Breast cancer in limited-resource countries: treatment and allocation of resources. The breast journal 2006, 12 Suppl 1:S38-5310.1111/j.1075-122X.2006.00202.x.
Loeb S, Bjurlin MA, Nicholson J, Tammela TL, Penson DF, Carter HB, Carroll P, Etzioni R. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65(6):1046–55. https://doi.org/10.1016/j.eururo.2013.12.062.
Hao S, Östensson E, Eklund M, Grönberg H, Nordström T, Heintz E, Clements M. The economic burden of prostate cancer – a Swedish prevalence-based register study. BMC Health Services Research. 2020;20(1):448. https://doi.org/10.1186/s12913-020-05265-8.
Mittmann N, Liu N, Cheng SY, Seung SJ, Saxena FE, Hong NJL, Earle CC, Cheung MC, Leighl NB, Coburn NG: Health system costs for cancer medications and radiation treatment in Ontario for the 4 most common cancers: a retrospective cohort study. CMAJ open 2020, 8(1):E191
Mojahedian MM, Toroski M, Keshavarz K, Aghili M, Zeyghami S, Nikfar S: Estimating the Cost of Illness of Prostate Cancer in Iran. Clinical therapeutics 2019, 41(1):50–58
Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. The Lancet Oncology. 2013;14(12):1165–74. https://doi.org/10.1016/S1470-2045(13)70442-X.
Lee K-S, Chang H-S, Lee S-M, Park E-C: Economic burden of cancer in Korea during 2000-2010. Cancer research and treatment: official journal of Korean Cancer Association 2015, 47(3):387
Kingdom of Eswatini:The 2017 Population and Housing Census Preliminary Results 2017,http://www.gov.sz/images/planningministry/Volume-3-1.pdf.
WorldBank:The Kingdom of Eswatini Toward Equal Opportunity: Accelerating Inclusion and Poverty Reduction. Systematic Country Diagnostic. 2020.,https://www.hdl.handle.net/10986/34970
WorldBank: World Bank WDI and M acro Poverty Outlook. In.: World Bank; 2015.
UNICEF Eswatini: Health Budget Brief Report. 2018/19
Ngcamphalala C, Ataguba JE. An assessment of financial catastrophe and impoverishment from out-of-pocket health care payments in Swaziland. Global Health Action. 2018;11(1):1428473. https://doi.org/10.1080/16549716.2018.1428473.
Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW: Methods for the economic evaluation of health care programmes: Oxford university press; 2015
Hodgson TA, Meiners MR: Cost-of-Illness Methodology: A Guide to Current Practices and Procedures. The Milbank Memorial Fund Quarterly Health and Society 1982, 60(3):429-46210.2307/3349801.
Mogyorosy Z, Smith P:The main methodological issues in costing health care services: a literature review.2005
Cebisile NE, Ostensson; Themba, G, Ginindza.: The Societal costs of Breast Cancer In Eswatini. In.: University of KwaZulu Natal; 2020.
Ministry of Health:Eswatini Standardized Cancer Care and Guidelines.2020
Sobin LH, Gospodarowicz MK, Wittekind C: TNM classification of malignant tumours: John Wiley & Sons; 2011
Ministry of health:Phalala Fund Annual Report.2018
Ministry of Health: Swaziland National Cancer Registry Report on Cases of cancers in Swaziland In.; 2016.
Östensson E, Hellström A-C, Hellman K, Gustavsson I, Gyllensten U, Wilander E, Zethraeus N, Andersson S: Projected cost-effectiveness of repeat high-risk human papillomavirus testing using self-collected vaginal samples in the Swedish cervical cancer screening program. Acta Obstetricia et Gynecologica Scandinavica 2013, 92(7):830-84010.1111/aogs.12143.
Hanly P, Soerjomataram I, Sharp L. Measuring the societal burden of cancer: the cost of lost productivity due to premature cancer-related mortality in Europe. Int J Cancer. 2015;136(4):E134-145. https://doi.org/10.1002/ijc.29105.
Gold M: Panel on cost-effectiveness in health and medicine. Medical care 1996, 34(12):DS197-DS199
Krahn MD, Zagorski B, Laporte A, Alibhai SM, Bremner KE, Tomlinson G, Warde P, Naglie G: Healthcare costs associated with prostate cancer: estimates from a population-based study. BJU international 2010, 105(3):338–346
Penson DF, Schonfeld WH, Flanders SC, Henke CJ, Warolin KL, Carroll PR, Litwin MS: Relationship of first-year costs of treating localized prostate cancer to initial choice of therapy and stage at diagnosis: results from the CAPSURE database. Urology 2001, 57(3):499–503
Sangar VK, Ragavan N, Matanhelia SS, Watson MW, Blades RA: The economic consequences of prostate and bladder cancer in the UK. BJU international 2005, 95(1):59–63
Cronin P, Kirkbride B, Bang A, Parkinson B, Smith D, Haywood P: Long-term health care costs for patients with prostate cancer: a population‐wide longitudinal study in New South Wales, Australia. Asia‐Pacific Journal of Clinical Oncology 2017, 13(3):160–171
Roehrborn CG, Albertsen P, Stokes ME, Black L, Benedict A. First-year costs of treating prostate cancer: estimates from SEER-Medicare data. Prostate Cancer and Prostatic Diseases. 2009;12(4):355–60. https://doi.org/10.1038/pcan.2009.21.
Moyer VA: Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Annals of internal medicine 2012, 157(2):120–134
Moss SM, Melia J: Screening for prostate cancer: the current position. British Medical Bulletin 1998, 54(4):791–805 10.1093/oxfordjournals.bmb.a011730
Olapade-Olaopa EO, Obamuyide HA, Yisa GT: Management of advanced prostate cancer in Africa. The Canadian journal of urology 2008, 15(1):3890–3898
Abbas F, Scardino PT: The natural history of clinical prostate carcinoma. Cancer 1997, 80(5):827-833 10.1002/(sici)1097-0142(19970901)
Molinier L, Bauvin E, Combescure C, Castelli C, Rebillard X, Soulié M, Daurès J-P, Grosclaude P: Methodological Considerations in Cost of Prostate Cancer Studies: A Systematic Review. Value in Health 2008, 11(5):878–885https://doi.org/10.1111/j.1524-4733.2008.00327.x.
Rencz F, Brodszky V, Varga P, Gajdácsi J, Nyirády P, Gulácsi L: The economic burden of prostate cancer. A systematic literature overview of registry-based studies. Orvosi Hetilap 2014, 155(13):509–520
Cebisile NE, Östensson; Themba, G, Ginindza.: The Economic Burden of Cervical Cancer in Eswatini. In.: University of KwaZulu Natal; 2020.
Kirby RS, Fitzpatrick JM, Irani J: Prostate cancer diagnosis in the new millennium: strengths and weaknesses of prostate-specific antigen and the discovery and clinical evaluation of prostate cancer gene 3 (PCA3). BJU International 2009, 103(4):441-44510.1111/j.1464-410X.2008.08280.x.
Acknowledgements
We thank the Kingdom of Eswatini Ministry of Health for granting us permission to conduct the study. We are grateful for financial support for data collection received from University of KwaZulu Natal College of health sciences, Doctoral research Scholarship grant. We would like to acknowledge the Eswatini the National Cancer Control Unit, Monitoring and Information Systems. Much appreciation also goes to Phalala Fund office for providing costs information and staff at Mbabane Government Chemotherapy Unit.
Funding
The study was funded by the University of KwaZulu-Natal College of Health Sciences Doctoral Research Scholarship grant. The funder had no role in the study design, data collection and analysis, or decision to publish.
Author information
Authors and Affiliations
Contributions
Conceptualization: CN, Data curation:CN TG EO, Formal analysis: CN TG EO, Funding acquisition: CN, Investigation: CN, Methodology: CN TG EO, Project administration: CN, Supervision: TG EO, Validation: TG EO, Writing – original draft: CN, Writing – review & editing: CN TG EO. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the National Health Research Review Board (NHRRB) of Eswatini (FWA 00026661/IRB 00011253) and the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (BE 059/19). Ethics committees approved written informed consent, which was obtained from all the participants prior to participation to the study. The study was implemented in accordance with the approved protocol and ethics principles guiding human participation in research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Direct non-medical costs patient questionnaires.pdf
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ngcamphalala, C., Östensson, E. & Ginindza, T.G. The economic burden of prostate cancer in Eswatini. BMC Health Serv Res 22, 483 (2022). https://doi.org/10.1186/s12913-022-07817-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-022-07817-6