Abstract
Background
Endophytic fungi have recently been recognized as an impressive source of natural biomolecules. The primary objective of the research was to isolate fungal endophytes from Thysanolaena maxima Roxb., Dracaena spicata Roxb. and Aglaonema hookerianum Schott. of Bangladesh and assess their pharmacological potentialities focusing on antimicrobial, antioxidant, and cytotoxic properties.
Methods
The fungal isolates were identified up to the genus level by analyzing their macroscopic and microscopic characteristics. Ethyl acetate extracts of all the fungal isolates were screened for different bioactivities, including antimicrobial (disc diffusion method), antioxidant (DPPH scavenging assay), and cytotoxic (brine shrimp lethality bioassay) activities.
Results
Among the thirteen isolates, Fusarium sp. was the most recognized genus, while the others belonged to Colletotrichum sp. and Pestalotia sp. Comparing the bioactivity of all the extracts, Fusarium sp. was shown to be the most effective endophyte, followed by Colletotrichum sp. and Pestalotia sp. In the antimicrobial study, two isolates of Fusarium sp. (internal strain nos. DSLE-1 and AHPE-4) showed inhibitory activity against all the tested bacteria and the highest zone of inhibition (15.5 ± 0.4 mm) was exerted by AHPE-4 against Bacillus subtillis. All the fungal isolates produced mild to moderate free radical scavenging activity, where the highest antioxidant activity was revealed by one isolate of Fusarium sp. (internal strain no. AHPE-3) with an IC50 value of 84.94 ± 0.41 µg/mL. The majority of Fusarium sp. isolates exhibited notable cytotoxic activity, where AHPE-4 exhibited the highest cytotoxicity, having the LC50 value of 14.33 ± 4.5 µg/mL.
Conclusion
The findings of the study endorsed that the fungal endophytes isolated from T. maxima, D. spicata, and A. hookerianum hold potential as valuable origins of bioactive substances. Nevertheless, more comprehensive research is warranted, which could develop novel natural compounds from these endophytes to treat various infectious and cancerous diseases.
Similar content being viewed by others
Background
Endophytes are often asymptomatic microorganisms, including bacteria or fungi, that inhabit the internal plant tissues [1]. This coevolutionary process of the endophytic fungi and its symbiotic plants creates excellent attraction to the researcher for their prime involvement in novel drug discovery. Fungal endophytes have tremendous biosynthetic capability, allowing them to synthesize bioactive secondary metabolites with unique characteristics [2]. They are mostly reported to contain valuable bioactive compounds such as quinones, coumarins, isocoumarins, alkaloids, anthraquinones, naphthoquinones, terpenoids, steroids, lignans, and lactones [3, 4]. These bioactive substances have been reported to display a variety of biological activities, including antiparasitic, antimicrobial, antiviral, anti-inflammatory, anticancer, antioxidant and immunosuppressive activities [5, 6]. Endophytic fungi possess diverse taxonomic groups prevalently distributed within plants, playing numerous roles in plant health and productivity [7, 8]. The fungal taxonomy is mainly based on morphological characteristics, from which primary recognition of species or genera can be predicted.
Chittagong Hill Tracts (CHT) are large hilly areas in Bangladesh consisting of rich forest composition. The indigenous domestic people in this hilly region are invariably relying on wild medicinal plants to resolve their therapeutic purposes [9]. Thysanolaena maxima Roxb., Dracaena spicata Roxb., and Aglaonema hookerianum Schott. are renowned medicinal mountainous plants of CHT, Bangladesh. T. maxima has been used in treating of eye infections, tonsillitis, boils, and skin diseases by the tribal population of Bangladesh and India [10,11,12]. Traditional healers of different tribes of CHT use leaf juice of D. spicata in the treatment of fever, cold, coughs, and measles [13, 14]. A. hookerianum has been used for the treatment of hemorrhoids, arthritis, gout, conjunctivitis, constipation, and hysteria [15]. These ethnopharmacologically important plants and their secondary metabolites are also reported to have potential pharmacological properties, including antioxidant, antimicrobial, cytotoxic, analgesic, and CNS depressant activities [10,11,12, 15].
Endophytes can produce bioactive metabolites similar to their host plants and are capable of showing similar bioactivity [16]. As the aforementioned plants have intriguing ethnomedicinal properties, it is anticipated that the associated endophytic fungi may exhibit potential bioactivity in addition to their ability to produce a wide range of bioactive chemicals. Therefore, it is necessary to identify and investigate the potential endophytic fungal diversity of the selected medicinal plants. Moreover, the identification of prospective fungi using morphological analysis provides an opportunity to look for further analysis regarding novel compound investigation [17]. The present study describes the morphological characterization and bioactivity of endophytic fungi isolated from three well-known ethnomedicinal plants in hilly areas of Bangladesh.
Methods
Materials
DPPH (2,2-Diphenyl-1-picrylhydrazyl) was purchased from Sigma-Aldrich Co., USA. Potato dextrose agar media, water agar media, nutrient agar media, standard disc of kanamycin and ketoconazole were purchased from Hi media, India. All the chemicals and solvents used were of analytical grades.
Collection of plant samples
Fresh plant samples of T. maxima, D. spicata, and A. hookerianum were collected from Rangamati, CHT, Bangladesh, with proper permission from the local authority. Plant samples of T. maxima, D. spicata and A. hookerianum were identified by a taxonomist, Sarder Nasir Uddin, Principal Scientific Officer, Bangladesh National Herbarium, Dhaka, Bangladesh, with accession nos.: DACB 42267, DACB 40632 and DACB 40633, respectively. The voucher specimens of the plants have been deposited in the herbarium for further reference. The research methodology involving the plant materials was approved by the Research Committee of the Department of Pharmacy, Jahangirnagar University, Savar, Dhaka.
Isolation and extraction of endophytes
Endophytic fungi were isolated from fresh and healthy plant tissues (flower, stem, leaf, bark and petiole) of T. maxima, D. spicata and A. hookerianum using the surface sterilization method [18]. The respective plant parts were washed, cut into smaller pieces and surface-sterilized by immersing the plant parts into 70% ethanol, 1.3 M sodium hypochlorite, and 70% ethanol sequentially. The surface-sterilized plant parts were dried and placed on separate Petri dishes containing water agar (WA) medium. Streptomycin (100 mg/L) was mixed with the WA medium to inhibit the growth of endophytic bacteria. For the control study, unsterilized plant segments were also prepared simultaneously to isolate the surface-contaminating fungi. Petri dishes were incubated at 28 ± 2 °C for fungal growth for 4–6 weeks. The fungal hyphae grown on the plant segments after the incubation period were isolated and transferred onto potato dextrose agar (PDA) medium for subculture.
A total of 5 endophytic fungi were isolated from the plant T. maxima of which TMFE-1, TMFE-2 and TMFE-3 were isolated from flower stems and TMLE-2 and TMLE-3 were isolated from the leaves of the plant. Similarly, 4 endophytic fungi were isolated from D. spicata of which DSLE-1, DSLE-2 and DSLE-4 were isolated from leaves and DSBE-1 was isolated from the bark of the plant. On the other hand, 4 endophytic fungi were isolated from A. hookerianum of which AHLE-1 and AHLE-4 were isolated from leaves and AHPE-3 and AHPE-4 were isolated from petioles of the plant. All the isolated fungal endophytes were cultivated on PDA medium for 21 days at room temperature. The culture medium for each fungus was then extracted with ethyl acetate for 7 days to obtain the crude extracts. After filtration and solvent evaporation, the extracts yielded a crude mixture of microbial secondary metabolites [19].
Morphological identification of endophytes
Isolated endophytes were identified on the basis of morphological features following macroscopic and microscopic characteristics using standard identification manuals [20]. For macroscopic identification, the specific morphology of the fungal colonies (e.g., color, mycelia, hyphae, margin, texture, growth rate etc.) was observed. For microscopic identification, the Lactophenol Cotton Blue (LPCB) staining method was followed to prepare the slides from the cultures to observe the spore characteristics [21].
Antimicrobial activity
The extracts of endophytic fungi were tested for antimicrobial activity following the disc diffusion method [22]. All the fungal extracts were tested against four gram-positive bacteria including Bacillus cereus (ATCC 14579), Bacillus megaterium (ATCC 25918), Bacillus subtilis (ATCC 6059), and Staphylococcus aureus (ATCC 25923) and six gram-negative bacteria including Salmonella typhi (ATCC 13311), Escherichia coli (ATCC 28739), Vibrio mimicus (ATCC 33653), Shigella dysenteriae (ATCC 26131), Shigella boydii (ATCC 13147) and Pseudomonas aeruginosa (ATCC 27833). To determine the antifungal activity, two pathogenic fungi Aspergillus flavus and Aspergillus niger were used. The test microorganisms were inoculated on nutrient agar (for bacteria) and PDA medium (for fungi). The test samples (crude fungal extracts) were prepared (100 µg/disc) and kanamycin (30 µg/disc) and ketoconazole (30 µg/disc) standard discs were used as the reference. The inoculated strains were incubated at 37 ± 2 °C (for bacteria) and 25 ± 2 °C (for fungi) for 24 h for their optimum growth. The antimicrobial activity of the samples was determined by measuring the diameter of the zone of inhibition produced by the samples in millimeters (mm).
Antioxidant activity
The DPPH free radical scavenging activity of all the fungal extracts was tested to determine the antioxidant activity [23]. To measure the effectiveness, varying concentrations of test samples dissolved in methanol were prepared from 200.0 µg/mL to 12.5 µg/mL using the serial dilution method. For the control study, ascorbic acid and methanol were used following similar sample preparations. The absorbance of the samples was measured at 517 nm using methanol as a blank. Inhibition of free radical DPPH in percent (%) was calculated as follows:
where Acontrol is the absorbance of the control which contains all reagents excluding the samples. The concentration at which the sample provides 50% inhibition (IC50) indicating the scavenging activity, was calculated from the graph plotted against the concentration of the extracts.
Cytotoxic activity
All fungal extracts were examined for preliminary cytotoxicity following the brine shrimp lethality bioassay [24]. The brine shrimp eggs were allowed to hatch for 24 h in seawater to be matured as nauplii. Test tubes were prepared to contain 5 mL of seawater along with different sample solutions prepared from 400 µg/mL to 0.39062 µg/mL using dimethyl sulfoxide (DMSO) and 10 living nauplii were added to each of the test tubes. For the control group, samples of vincristine sulfate and DMSO were prepared in the same manner. After 24 h, the number of surviving nauplii was counted and the 50% lethal concentration (LC50) of each sample was calculated by linear correlation obtained from the graph of the logarithm of concentration against the percentage of mortality.
Results
Morphological identification of isolated fungi
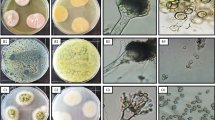
A total of thirteen endophytic fungi were isolated from T. maxima, D. spicata and A. hookerianum (Fig. 1). Among the isolates, seven isolates belonged to Fusarium sp. (TMLE-3, DSLE-1, DSLE-2, DSLE-4, DSBE-1, AHPE-3 and AHPE-4), five isolates belonged to Colletotrichum sp. (TMFE-2, TMFE-3, TMLE-2, AHLE-1 and AHLE-4) and only one isolate was identified as Pestalotia sp. (TMFE-1). Identification of the genus was based on macroscopic and microscopic characteristics broadly described in Tables 1 and 2 and confirmed according to the previous morphological investigation of the genus [25,26,27].
Antimicrobial activity
In the antimicrobial screening, the isolated fungal extracts showed mild to moderate activities (8–15.5 mm) against all the tested bacteria and fungi (Table 3). Among the isolates, DSLE-1 (Fusarium sp.) and AHPE-4 (Fusarium sp.) showed inhibitory activity against all the gram-positive and gram-negative bacteria while TMLE-2 (Colletotrichum sp.), TMLE-3 (Fusarium sp.), DSLE-1 (Fusarium sp.), DSBE-1 (Fusarium sp.) and AHPE-4 (Fusarium sp.) showed inhibitory activity against the gram-positive bacteria only. The extract of AHPE-4 (Fusarium sp.) showed the highest antibacterial activity (15.5 ± 0.4 mm) against Bacillus subtilis compared to the standard kanamycin. In determining antifungal activity, DSBE-1 (Fusarium sp.), AHPE-3 (Fusarium sp.) and AHPE-4 (Fusarium sp.) showed inhibition against Aspergillus niger where DSBE-1 showed the highest antifungal activity (13.3 ± 0.2 mm). The zone of inhibition < 7 mm produced by the fungal extracts was considered inactive against microorganisms.
Antioxidant activity
All the fungal extracts showed free radical scavenging activity in our present study (Table 4). However, AHPE-3 (Fusarium sp.) exhibited the lowest IC50 value of 84.94 ± 0.41 µg/mL indicating slightly potent antioxidant activity compared to the standard ascorbic acid (2.38 ± 0.75 µg/mL).
Cytotoxic activity
In the brine shrimp lethality bioassay, TMLE-3 (Fusarium sp.), DSLE-1 (Fusarium sp.), AHPE-3 (Fusarium sp.) and AHPE-4 (Fusarium sp.) showed potent cytotoxicity with LC50 values of 25.98 ± 5.2 µg/mL, 18.88 ± 3.84 µg/mL, 17.15 ± 2.4 µg/mL and 14.33 ± 4.5 µg/mL, respectively compared to the standard vincristine sulfate (1.63 ± 0.44 µg/mL). The rest of the fungal extracts showed mild to moderate cytotoxic activity (Fig. 2).
Chemical screening
Chemical screening of all the fungal isolates was conducted by thin-layer chromatography (TLC) to evaluate the presence of various secondary metabolites. The screening of all the extracts was executed by visual observation, under UV light (254 and 365 nm) and after spraying with vanillin-H2SO4 spray reagent (Table 5). Analysis of the TLC spots of the extracts showed the presence of diversified secondary metabolites such as coumarins, isocoumarins, or their derivatives, flavonoids, steroids, terpenoids, anthocyanins, anthraquinones and naphthoquinones [28, 29].
Discussion
This study aimed to determine the presence and explore the pharmacological activities of the endophytic fungi isolated from three different medicinal plants, T. maxima, D. spicata, and A. hookerianum, which are widely distributed in the hill tracts of Bangladesh. These plants are an integral part of folklore medicine with sufficient scientific proof of persistent pharmacological activities [30]. Our study led to the isolation of thirteen taxonomically recognized fungal endophytes belonging to Fusarium sp., Colletotrichum sp., and Pestalotia sp. The fungal isolates were characterized morphologically based on the data obtained from macroscopic and microscopic analyses and comparing those features with authentic identification manuals [20, 31].
All the crude fungal extracts were analyzed for their in vitro antimicrobial, antioxidant, and cytotoxic activities. Most of the extracts of Fusarium sp. displayed inhibition against both gram-positive and gram-negative bacteria and pathogenic fungi. One isolate of Fusarium sp. (AHPE-4) showed notable antibacterial activity against B. subtilis (15.5 ± 0.4 mm), S. typhi (15.1 ± 0.2 mm), S. aureus (14.6 ± 0.4 mm) and E. coli (14.3 ± 0.4 mm). Another isolate of Fusarium sp. (DSBE-1) produced antifungal activity against A. niger producing a zone of inhibition of 13.3 ± 0.2 mm. This trait supports the potential of the compounds of Fusarium sp. for the development of antimicrobial agents against several pathogenic bacteria and fungi. Fusarium sp. is one of the most potential fungal genera and has the ability to produce diversified bioactive secondary metabolites due to having many unique gene clusters [32]. Chemical screening of the extracts of Fusarium sp. also confirms the presence of coumarins, terpenoids, and quinones, which are reported to have antimicrobial activities [33]. Some previous studies [34, 35] reported that promising antimicrobial compounds such as fusaric acid, bikaverin, dehydrofusaric acid and beauvericin were isolated from different endophytic genera of Fusarium sp. Fusaric acid, a recognized mycotoxin, potentially displays antimicrobial effects by directly regulating the transcription of several genes associated with the pyocyanin pathway in Pseudomonas sp. On the other hand, bikaverin functions as an antimicrobial agent by hindering the DNA and protein synthesis processes within microorganisms. Hence, the existence of these phytochemicals might be accountable for the antimicrobial effects of Fusarium sp. However, further comprehensive investigations are necessary to achieve a complete understanding of this phenomenon.
In the present study, the crude extract of Fusarium sp. (AHPE-3) exhibited moderate DPPH free radical scavenging activity with IC50 values of 84.94 ± 0.41 µg/mL. Several previous studies also reported the antioxidant effects of endophytic Fusarium sp. [36, 37]. Natural antioxidants such as polyphenolic chemicals and flavonoids contain multiple hydroxyl groups which allow them to transfer an electron to unstable free radical DPPH and reduce oxidative stress [38]. Numerous coumarins were shown to have antioxidant activities by influencing the generation and scavenging of reactive oxygen species [39]. The presence of coumarins, flavonoids and/or their derivatives in the extracts might be responsible for exerting such antioxidant activity [40]. However, the rest of the fungal extracts exhibited mild radical scavenging activity in this study. Future studies should be conducted to establish the antioxidant potential of the fungal endophytes through other methods which could establish more specific antioxidative pathways for the specific biomolecules.
To evaluate the preliminary cytotoxicity of the samples, brine shrimp lethality bioassay has been serving as a popular tool as it is simple, affordable, and requires no specialized equipment or aseptic environment [41]. In the present study, most of the extracts of Fusarium sp. (AHPE-3, AHPE-4, DSBE-1, DSLE-4, and TMLE-3) demonstrated potent cytotoxicity producing LC50 values from 14.33 ± 4.5 µg/mL to 25.98 ± 5.2 µg/mL. Literature studies have shown that Fusarium sp. can produce numerous mycotoxins such as enniatins, fusaric acid, fumonisin, and moniliformin with strong cytotoxic activities [42]. Moreover, the presence of coumarins, isocoumarins, terpenoids, naphthoquinone, and anthraquinone was established by the TLC analysis of the extracts and these compounds were also reported to have potential cytotoxicity. However, a more specific investigation is required to establish the relation between the cytotoxicity and the reported secondary metabolites.
Our study has presented the endophytic fungal species of three ethnomedicinal plants of Bangladesh as the alternate ecological resources of bioactive molecules. To the best of our knowledge, this is the first study on the endophytic fungal flora associated with these plants in Bangladesh, which opens an unexplored area for further research.
Conclusion
This investigation presents remarkable data about the morphology and pharmacological activities of the fungal endophytes isolated from the mentioned three medicinal plants of Bangladesh. The findings of the study established Fusarium sp. as one of the prospective endophytes because of their significant cytotoxicity and antimicrobial activity, in addition to moderate antioxidant activity. TLC analysis revealed the existence of diverse secondary metabolites in all the crude fungal extracts. Further comprehensive research on the specific endophytes is needed to discover new bioactive compounds that could be effective in combating infections and cancers.
Data availability
Further raw data involved in this study are available on request from the corresponding authors.
References
Elghaffar RA, Amin BH, Hashem AH, Sehim AE. Promising endophytic Alternaria alternata from leaves of Ziziphus spina–christi: phytochemical analyses, antimicrobial and antioxidant activities. Appl Biochem Biotechnol. 2022;194:3984–4001. https://doi.org/10.1007/s12010-022-03959-9.
Abdelaziz AM, Kalaba MH, Hashem AH, Sharaf MH, Attia MS. Biostimulation of tomato growth and biocontrol of Fusarium wilt disease using certain endophytic fungi. Bot Stud. 2022;63:34. https://doi.org/10.1186/s40529-022-00364-7.
Ismail, Hamayun M, Hussain A, Iqbal A, Khan SA, Lee IJ. Endophytic fungus Aspergillus japonicus mediates host plant growth under normal and heat stress conditions. Biomed Res Int. 2018; 2018: 7696831. https://doi.org/10.1155/2018/7696831.
Attia MS, Hashem AH, Badawy AA, Abdelaziz AM. Biocontrol of early blight disease of eggplant using endophytic aspergillus terreus: improving plant immunological, physiological and antifungal activities. Bot Stud. 2022;63:26. https://doi.org/10.1186/s40529-022-00357-6.
You YH, Park JM, Park JH, Kim JG. Diversity of endophytic fungi associated with the roots of four aquatic plants inhabiting two wetlands in Korea. Mycobiology. 2015;43(3):231–8. https://doi.org/10.5941/MYCO.2015.43.3.231.
Khalil AMA, Abdelaziz AM, Khaleil MM, Hashem AH. Fungal endophytes from leaves of Avicennia marina growing in semi-arid environment as a promising source for bioactive compounds. Lett Appl Microbiol. 2021;72(3):263–74. https://doi.org/10.1111/lam.13414.
Zhang CL, Zheng BQ, Lao JP, Mao LJ, Chen SY, Kubicek CP, Lin FC. Clavatol and patulin formation as the antagonistic principle of aspergillus clavatonanicus, an endophytic fungus of Taxus mairei. Appl Microbiol Biotechnol. 2008;8(5):833–40. https://doi.org/10.1007/s00253-008-1371-z.
Hashem AH, Attia MS, Kandil EK, Fawzi MM, Abdelrahman AS, Khader MS, et al. Bioactive compounds and biomedical applications of endophytic fungi: a recent review. Microb Cell Factories. 2023;22:107. https://doi.org/10.1186/s12934-023-02118-x.
Baul TK, Peuly TA, Nandi R, Kar S, Mohiuddin M. Composition of homestead forests and their contribution to local livelihoods and environment: a study focused on Bandarban hill district, Bangladesh. Trees for People. 2021;5:100117. https://doi.org/10.1016/j.tfp.2021.100117.
Hoque N, Sohrab MH, Debnath T, Rana MS. Antioxidant, antibacterial and cytotoxic activities of various extracts of Thysanolaena maxima (Roxb.) Kuntze available in Chittagong hill tracts of Bangladesh. Int J Pharm Pharm Sci. 2016;8(7):168–72.
Hoque N, Sohrab MH, Afroz F, Rony SR, Sharmin S, Moni F, Hasan CM, Rana MS. Cytotoxic metabolites from Thysanolaena maxima Roxb. Available in Bangladesh. Clin Phytoscience. 2020;6:89. https://doi.org/10.1186/s40816-020-00226-4.
Tiwary BK, Bihani S, Kumar A, Chakraborty R, Ghosh R. The in vitro cytotoxic activity of ethno-pharmacological important plants of Darjeeling district of West Bengal against different human cancer cell lines. BMC Complement Altern Med. 2015;15:22. https://doi.org/10.1186/s12906-015-0543-5.
Rahmatullah M, Hossan MS, Hanif A, Roy P, Jahan R, Khan M, Chowdhury MH, Rahman T. Ethnomedicinal applications of plants by the traditional healers of the Marma tribe of Naikhongchhari, Bandarban district, Bangladesh. Adv Nat Appl Sci. 2009;3(3):392–401.
Khisha T, Karim R, Chowdhury SR, Banoo R. Ethnomedical studies of Chakma communities of Chittagong hill tracts, Bangladesh. Bangladesh Pharm J. 2012;15(1):59–67.
Goni O, Khan MF, Rahman MM, Hasan MZ, Kader FB, Sazzad N, et al. Pharmacological insights on the antidepressant, anxiolytic and aphrodisiac potentials of Aglaonema hookerianum Schott. J Ethnopharmacol. 2021;268:113664. https://doi.org/10.1016/j.jep.2020.113664.
Zhao J, Zhou L, Wang J, Shan T, Zhong L, Liu X, Gao X. Endophytic fungi for producing bioactive compounds originally from their host plants. Current research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. 1st ed. Formatex Research Centre, Badajoz, Spain; 2010. 567–76.
Santos IPD, Silva LCND, Silva MVD, Araújo JMD, Cavalcanti MDS, Lima VLDM. Antibacterial activity of endophytic fungi from leaves of Indigofera suffruticosa Miller (Fabaceae). Front Microbiol. 2015;6:350. https://doi.org/10.3389/fmicb.2015.00350.
Hoque N, Afroz F, Khatun F, Rony SR, Hasan CM, Rana MS, Sohrab MH. Physicochemical, pharmacokinetic and cytotoxicity of the compounds isolated from an endophyte Fusarium oxysporum: in vitro and in silico approaches. Toxins. 2022;14(3):159. https://doi.org/10.3390/toxins14030159.
Srivastava A, Anandrao RK. Antimicrobial potential of fungal endophytes isolated from leaves of Prosopis juliflora (SW.) DC. An important weed. Int J Pharm Pharm Sci. 2015;7(12):128–36.
Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. St. Paul, Minnesota: APS Press; 1998. ISBN: 0-89054-192-2.
Shamly V, Kali A, Srirangaraj S, Umadevi S. Comparison of microscopic morphology of fungi using lactophenol cotton blue (LPCB), iodine glycerol and congo red formaldehyde staining. J Clin Diagn Res. 2014;8(7):DL01–2. https://doi.org/10.7860/JCDR/2014/8521.4535.
Khatun MCS, Muhit MA, Hossain MJ, Al-Mansur MA, Rahman SA. Isolation of phytochemical constituents from Stevia rebaudiana (Bert.) And evaluation of their anticancer, antimicrobial and antioxidant properties via in vitro and in silico approaches. Heliyon. 2021;7(12):e08475. https://doi.org/10.1016/j.heliyon.2021.e08475.
Farzana M, Hossain MJ, El-Shehawi AM, Sikder MAA, Rahman MS, Al-Mansur MA, et al. Phenolic constituents from Wendlandia tinctoria var. grandis (Roxb.) DC. Stem deciphering pharmacological potentials against oxidation, hyperglycemia, and diarrhea: phyto-pharmacological and computational approaches. Molecules. 2022;27(18):5957. https://doi.org/10.3390/molecules27185957.
Jannat T, Hossain MJ, El-Shehawi AM, Kuddus MR, Rashid MA, Albogami S, et al. Chemical and pharmacological profiling of Wrightia coccinea (roxb. Ex hornem.) Sims focusing antioxidant, cytotoxic, antidiarrheal, hypoglycemic, and analgesic properties. Molecules. 2022;27(13):4024. https://doi.org/10.3390/molecules27134024.
de Silva DD, Groenewald JZ, Crous PW, Ades PK, Nasruddin A, Mongkolporn O, et al. Identification, prevalence and pathogenicity of Colletotrichum species causing anthracnose of Capsicum annuum in Asia. IMA Fungus. 2019;10:8. https://doi.org/10.1186/s43008-019-0001-y.
Maharachchikumbura SS, Guo LD, Chukeatirote E, Bahkali AH, Hyde KD. Pestalotiopsis—morphology, phylogeny, biochemistry and diversity. Fungal Divers. 2011;50:167–87. https://doi.org/10.1007/s13225-011-0125-x.
Trabelsi R, Sellami H, Gharbi Y, Krid S, Cheffi M, Kammoun S, et al. Morphological and molecular characterization of Fusarium spp. associated with olive trees dieback in Tunisia. 3 Biotech. 2017;7:28. https://doi.org/10.1007/s13205-016-0587-3.
Harborne AJ. Phytochemical methods: a guide to modern techniques of plant analysis. London: Chapman and Hall Ltd; 1998. pp. 21–72.
Mahmud SN, Sohrab MH, Begum MN, Rony SR, Sharmin S, Moni F, et al. Cytotoxicity, antioxidant, antimicrobial studies and phytochemical screening of endophytic fungi isolated from Justicia gendarussa. Ann Agri Sci. 2020;65(2):225–32.
Rahmatullah M, Pk SR, Al-Imran M, Jahan R. The Khasia tribe of Sylhet district, Bangladesh, and their fast-disappearing knowledge of medicinal plants. J Altern Complement Med. 2013;19(7):599–606. https://doi.org/10.1089/acm.2012.0254.
Leslie JF, Summerell BA. The Fusarium Laboratory Manual. John Wiley & Sons; 2006.
Shi S, Li Y, Ming Y, Li C, Li Z, Chen J, Lou M. Biological activity and chemical composition of the endophytic fungus fusarium sp. TP-G1 obtained from the root of Dendrobium officinale Kimura et Migo. Rec Nat Prod. 2018;12:549–56. https://doi.org/10.25135/rnp.62.17.12.201.
Li M, Yu R, Bai X, Wang H, Zhang H. Fusarium: a treasure trove of bioactive secondary metabolites. Nat Prod Rep. 2020;37(12):1568–88. https://doi.org/10.1039/D0NP00038H.
Wang QX, Li SF, Zhao F, Dai HQ, Bao L, Ding R, et al. Chemical constituents from endophytic fungus fusarium oxysporum. Fitoterapia. 2011;82(5):777–81. https://doi.org/10.1016/j.fitote.2011.04.002.
Son SW, Kim HY, Choi GJ, Lim HK, Jang KS, Lee SO, Lee S, Sung ND, Kim JC. Bikaverin and fusaric acid from Fusarium oxysporum show antioomycete activity against Phytophthora infestans. J Appl Microbiol. 2008;104(3):692–8. https://doi.org/10.1111/j.1365-2672.2007.03581.x.
Mani VM, Priya MS, Dhaylini S, Preethi K. Antioxidant and antimicrobial evaluation of bioactive pigment from Fusarium sp. isolated from stressed environment. Int J Curr Microbiol App Sci. 2015;4(6):1147–58.
Pan F, Hou K, Li DD, Su TJ, Wu W. Exopolysaccharides from the fungal endophytic Fusarium sp. A14 isolated from Fritillaria unibracteata Hsiao et KC Hsia and their antioxidant and antiproliferation effects. J Biosci Bioeng. 2019;127(2):231–40. https://doi.org/10.1016/j.jbiosc.2018.07.023.
Mitra S, Rauf A, Sutradhar H, Sadaf S, Hossain MJ, Soma MA, et al. Potential candidates from marine and terrestrial resources targeting mitochondrial inhibition: insights from the molecular approach. Comp Biochem Physiol Part - C: Toxicol Pharmacol. 2022;264:109509. https://doi.org/10.1016/j.cbpc.2022.109509.
Tsivileva OM, Koftin OV, Evseeva NV. Coumarins as fungal metabolites with potential medicinal properties. Antibiotics. 2022;11(9):1156. https://doi.org/10.3390/antibiotics11091156.
Mitra S, Lami MS, Uddin TM, Das R, Islam F, Anjum J, et al. Prospective multifunctional roles and pharmacological potential of dietary flavonoid narirutin. Biomed Pharmacother. 2022;150:112932. https://doi.org/10.1016/j.biopha.2022.112932.
Ajibola OO, Lihan S, Hussaini A, Saat R, Ahmed IA, Abideen W, Sinang FM, Adeyinka GC. Toxicity assessment of Lactococcus lactis IO-1 used in coconut beverages against Artemia salina using brine shrimp lethality test. Appl Food Biotechnol. 2020;7(3):127–34.
Hof H. The medical relevance of Fusarium spp. J Fungi. 2020;6(3):117. https://doi.org/10.3390/jof6030117.
Acknowledgements
Authors are grateful to the Pharmaceutical Sciences Research Division, BCSIR, Dhaka for allocating the necessary instrumental facilities and reagent supports for the research work.
Funding
The authors received no financial support for the research.
Author information
Authors and Affiliations
Contributions
The study was conceptualized and designed by NH and MHS. The research was carried out by NH, ZRK, PTR, MNB, SS, MJH, MSR, and MHS, who also gathered and organized the research materials. The data were analyzed and interpreted by NH, ZRK, PTR, MNB, SS, MJH and MHS. The article was written and edited by NH, ZRK, PTR, MNB, SS, MJH and MHS. All authors reviewed the manuscript and gave their approval for the final draft.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The research methodology involving the plant materials was approved by the Research Ethics Committee of the Department of Pharmacy, Jahangirnagar University, Savar, Dhaka, Bangladesh.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hoque, N., Khan, Z.R., Rashid, P.T. et al. Antimicrobial, antioxidant, and cytotoxic properties of endophytic fungi isolated from Thysanolaena maxima Roxb., Dracaena spicata Roxb. and Aglaonema hookerianum Schott.. BMC Complement Med Ther 23, 347 (2023). https://doi.org/10.1186/s12906-023-04185-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-023-04185-4