Abstract
Background
Osteoarthritis (OA) treatment aims to improve inflammation and delay cartilage degeneration. However, there is no effective strategy presently available. Ononin, a representative isoflavone glycoside component extracted from natural Chinese herbs, exerts anti-inflammatory and proliferative effects. However, the therapeutic effect of ononin on chondrocyte inflammation remains unclear.
Methods
In this study, we explored the therapeutic effect and potential mechanism of ononin in OA by establishing an interleukin-1 beta (IL-1β)-induced chondrocyte inflammation model.
Results
Our results verified that ononin alleviated the IL-1β-induced decrease in chondrocyte viability, attenuated the overexpression of the inflammatory factors tumour necrosis factor α (TNF-α) and interleukin 6 (IL-6), and simultaneously inhibited the expression of cartilage extracellular matrix (ECM)-degrading enzymes such as matrix metalloproteinase-13 (MMP-13). Furthermore, the decomposition of Collagen II protein could be alleviated in the OA model by ononin. Finally, ononin improved chondrocyte inflammation by downregulating the mitogen-activated protein kinase (MAPK) and nuclear factor kappa-B (NF-κB) signalling pathways.
Conclusion
Our findings suggested that ononin could inhibit the IL-1β-induced proinflammatory response and ECM degradation in chondrocytes by interfering with the abnormal activation of the MAPK and NF-κB pathways, indicating its protective effect against OA.
Similar content being viewed by others
Introduction
OA is a chronic degenerative joint disease characterized by ECM degeneration, subchondral bone sclerosis, syndesmophyte formation, and exacerbated joint destruction in the late stage of disease, resulting the loss of the patient’s ability to work [1]. Although ageing and wear have been considered the main causes of this degenerative disease, current research provides a new view of cartilage degeneration caused by low-grade inflammatory responses [2, 3]. Proinflammatory cytokines and chemokines are produced in OA patient cartilage, subchondral bone and synovium, leading to the release of matrix metalloproteinases (MMPs), particularly matrix metalloproteinase-13 (MMP-13), and ultimately cartilage degradation [4].
In recent years, with the in-depth study of signalling pathways in OA, it has been shown that the expression of proinflammatory factors and matrix-degrading enzymes is upregulated in OA chondrocytes. During the pathological reaction, the MAPK and NF-κB signalling pathways have been shown to play vital roles in the pathogenesis of OA through a series of changes in cartilage damage [5, 6]. MAPK includes extracellular signal regulated kinase (ERK), c-jun terminal kinase (JNK) and the p38 subfamilies, which are involved in regulating various cellular processes, including cell survival, apoptosis, proliferation and the inflammatory response [7]. NF-κB, which is composed of the p65 and p50 subunits, regulates the transcription of many genes and is a key factor in the transcription of many inflammatory genes [8]. Studies have shown that some chemical agents can protect against OA by inhibiting these two pathways [9, 10]. These drugs are superior to the current treatments for OA, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and temporary analgesics, easily resulting in side effects such as gastrointestinal complications [11, 12]. Currently, many antioxidants, especially natural isoflavones, have been reported to have anti-inflammatory and reactive oxygen species (ROS) scavenging functions in many diseases by inhibiting inflammatory signalling pathways [13, 14].
Ononin (Fig. 1) is a representative isoflavone component in traditional Chinese medicines, such as Astragalus membranaceus, Glycyrrhiza uralensis, Hedysarum and Pueraria lobata. Ononin has a wide range of biological activities, including antioxidant and anti-inflammatory activities, and can regulate cell proliferation and apoptosis [15, 16]. Ononin has a neuroprotective effect on acute cerebral ischaemia through inhibiting autophagy, apoptosis, and inflammation [17]. Meng et al. [18] confirmed that ononin could alleviate damage in rheumatoid arthritis (RA) by curbing the production of proinflammatory cytokines and inhibiting the NF-κB and MAPK inflammation pathways. These studies showed that ononin has therapeutic effects on inflammatory diseases. However, few studies have ascertained the role of ononin in OA.
In this study, we found that ononin could promote cell proliferation and reduce TNF-α and IL-6 production in an IL-1β-induced OA model in primary chondrocytes. ECM degradation was reversed by inhibiting the expression of MMP-13 and increasing the expression of Collagen II, further showing that ononin could hamper abnormal activation of the MAPK and NF-κB pathways. In conclusion, ononin may be an effective drug for treating OA.
Materials and methods
Reagents
Ononin was purchased from MedChemExpress (MCE) (New Jersey, USA). Dimethyl sulfoxide (DMSO), trypsin-EDTA, collagenase II and recombinant rat IL-1β were purchased from Sigma–Aldrich (St. Louis, Missouri, USA). DMSO was used to dissolve ononin, and the final concentration of DMSO in the medium was less than or equal to 1% (which was considered to cause no damage to cells). TNF-α and IL-6 ELISA kits were obtained from R&D Systems (Minneapolis, Minnesota, USA). Foetal bovine serum (FBS) and high glucose Dulbecco’s modified Eagle’s medium (high glucose DMEM) were obtained from HyClone (Grand Island, New York, USA). Rat cartilage-specific antibodies, such as MMP-13 and Collagen II, and the internal reference antibody GAPDH were purchased from Abcam (Cambridge, UK). Antibodies against MAPK and NF-κB pathway-related proteins, such as p-p65/p65, p-IκBα/IκBα, p-ERK/ERK, p-p38/p38 and p-JNK/JNK, and protease and phosphatase inhibitors, were all provided by Cell Signalling Technology (Beverly, Massachusetts, USA). Secondary antibodies, bovine serum albumin (BSA) and 1% penicillin–streptomycin were purchased from Boster (Wuhan, China).
Cell culture
Five-day-old male Sprague–Dawley (SD) suckling rats were provided by the Animal Laboratory Centre of Guangxi Medical University, Guangxi Zhuang Autonomous Region, China. Chondrocytes were isolated according to the method in a previous study [19]. In brief, cartilage tissues were cut into small pieces of approximately 1 mm2 on a superclean workbench, washed with phosphate buffered saline (PBS) several times, incubated with 0.25% trypsin-EDTA in a 37 °C incubator for 0.5 h, and treated with type II collagenase to digest them for another 8 h. Furthermore, the digested cell suspension was transferred to an EP tube and centrifuged at 1000 r/min for 5 min. The supernatant was removed and discarded, and chondrocytes were washed with high glucose DMEM containing 10% FBS 3 times. Finally, the chondrocytes were inoculated in a 25 cm2 culture flask containing high glucose DMEM (containing 10% FBS and 1% penicillin–streptomycin) and cultured in a 37 °C incubator with 5% CO2. Third-generation chondrocytes in the logarithmic growth stage were used for subsequent experiments.
Cell viability
We evaluated the effect of ononin on chondrocyte activity by a cell counting kit-8 (CCK-8 kit) assay from Dojindo (Kyushu Island, Japan) according to the experimental methods in a previous study [20]. In brief, third-generation chondrocytes with good growth conditions were selected and inoculated in a 96-well plate at a density of 5 × 103/well. After 1 d of culture, medium containing different concentrations of ononin (0 ~ 100 μM) and with or without IL-1β (10 ng/mL) was added to the cells. After 1 d of culture, 10 μL of CCK-8 reagent was added to each well, and the culture was terminated after being incubated at 37 °C with 5% CO2 for 1.5 h. Finally, the absorbance of each well at 450 nm was measured by enzyme-linked immunosorbent assays from Bio–Rad (Richmond, California, USA), and the absorbance value was used to indicate the activity of chondrocytes. Appropriate concentrations of ononin were selected as low, medium and high doses for further study.
Western blot analysis
Western blotting was used to examine related proteins in chondrocytes, as previously described [20]. In brief, after the experiment was completed, chondrocytes were washed twice with PBS and collected. Then, radioimmunoprecipitation assay (RIPA) lysis solution containing protease and phosphatase inhibitors was added to the chondrocytes and incubated on ice for 30 min. Next, 20 μg protein samples were used for electrophoresis. After that, the proteins in the SDS-polyacrylamide gels were transferred to the activated PVDF membrane by methanol. The edges of the membrane were cut to conform to the size of the antibody incubation boxes, and the membranes were then blocked with 5% BSA for 1 h and incubated with the corresponding diluted primary antibody overnight at 4 °C. After 5 washes with Tris-buffered saline with 0.1% Tween-20 (TBST), the diluted secondary antibody was added and incubated at room temperature for 1 h. After 5 washes with TBST, the enhanced chemiluminescence luminescence (ECL) solution was added. GAPDH was used as the internal reference, and the optical density of the target band was analysed by ImageJ software.
Immunofluorescence staining
Collagen II and MMP-13 protein levels in chondrocytes were examined by immunofluorescence. The detection method was described in a previous study [20]. In brief, after the cell intervention experiment, the culture medium was discarded, the cells were washed with PBS at room temperature 3 times, and then 4% paraformaldehyde was added and incubated at room temperature for 15 min. After that, the cells were washed 3 times with PBS and incubated in 0.2% Triton X-100 in PBS for 5 min. Then, the cells were blocked with 5% BSA for 30 min. The cells were incubated with Collagen II- or MMP-13-specific antibodies at 4 °C overnight. The cells were washed again 3 times with PBS, and the secondary antibody bound to Cy3 was added dropwise and incubated at 37 °C for 1 h in the dark. Then, the nuclei were stained with DAPI for 10 min. Finally, the protein expression of Collagen II and MMP-13 was observed and photographed by fluorescence microscopy (Evos Flauto, Life Technologies, USA) after the samples were sealed with a fluorescent sealing agent.
Enzyme-linked immunosorbent assay (ELISA)
The cell culture supernatant and chondrocytes in each group were collected together, and the expression levels of IL-1β and IL-6 were tested according to the instructions of the ELISA kits. In brief, the samples were incubated at 37 °C for 45 min after being added to the reaction well, the washing solution was used to wash each well, and the biotin-labelled antibody was added and reacted for 30 min at 37 °C. After that, streptavidin horseradish peroxidase (HRP) was added and mixed well at 37 °C for 30 min, and then the chromogenic agent was added to avoid discolouration for 15 min. Finally, the termination solution was added to terminate the reaction, and the absorbance of each well at 450 nm was measured by enzyme-linked immunosorbent assays.
Statistical analysis
GraphPad Prism 9 software was used for statistical analysis and mapping. The measurement data are expressed as the mean ± Sprague-Dawley (SD). Comparisons between multiple groups were performed by one-way analysis of variance (ANOVA), and pairwise comparison was performed with Tukey’s test. P < 0.05 indicates that the difference is statistically significant.
Results
Effects of Ononin on cell viability
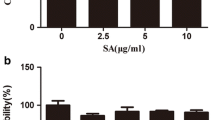
The toxicity of ononin on chondrocytes was assessed by a CCK-8 kit. Cells were cultured with ononin at concentrations ranging from 1 nM to 100 μM with or without IL-1β (10 ng/ml) for 24 h. As shown in Fig. 2A, compared with the control group, the DMSO group (≤ 1‰) exhibited no significant toxicity, as measured by cell proliferation (P > 0.05). Conversely, the activity of IL-1β-induced chondrocytes was decreased (P < 0.01), as shown in Fig. 2B. Ononin at concentrations of 10, 100, and 1000 nM for 24 h was not cytotoxic to chondrocytes in the presence or absence of IL-1β (10 ng/ml). However, cell viability was inhibited by high concentrations of ononin (≥ 10 μM) (P < 0.01). Interestingly, in IL-1β-induced chondrocytes treated with low doses of ononin (10 to 1000 nM), cell viability gradually increased compared with that in the control group (P < 0.01). Finally, we used phase-contrast microscopy and observed that the cells in the control group and the ononin alone group were highly dense, while the cell density in the IL-1β group was comparatively sparse, but the cell density gradually increased in response to ononin and recovered to approximately 75% in the 1000 nM ononin group compared with control group, suggesting that it has a strong promoting effect on cell activity, Fig. 2C, D. Therefore, ononin at 10, 100 and 1000 nM were selected for subsequent experiments.
Effects of Ononin on cell viability. (A) Rat chondrocytes were intervened on Ononin (1 nM to 100 μM) only or (B) with IL-1β (10 ng/ml) for 24 h and cell viability was detected by CCK-8 kit. (C) The cell density (% of Control) of different groups was calculated by Image-J software. (D) Chondrocytes were treated with Ononin with or without IL-1β (10 ng/ml) and photographed by phase-contrast microscopy (20×) (scale bar: 50 μm). **P < 0.01 compared with control group; ##P < 0.01 compared with IL-1β group
Effects of Ononin on IL-1β-induced production of the inflammatory cytokines TNF-α and IL-6 in rat chondrocytes
Next, we studied the effect of ononin on the expression of certain key cytokines in the pathogenesis of OA. To examine whether ononin could inhibit the upregulation of TNF-α and IL-6 induced by IL-1β, ELISA kits were used to evaluate the levels of these inflammatory factors. As shown in Fig. 3, IL-1β significantly induced the release of the inflammatory factors TNF-α and IL-6 by chondrocytes. In addition, 10, 100 and 1000 nM ononin markedly suppressed the production of TNF-α and IL-6 compared to that in the IL-1β group. In particular, the levels of TNF-α and IL-6 in the 1000 nM ononin group were reduced 2-fold and 3-fold, respectively, compared with those in the control group; moreover, this downregulation showed a decreasing trend with increasing ononin concentrations. In brief, these results suggested that ononin reduced the release of the inflammatory cytokines TNF-α and IL-6 in IL-1β-induced rat chondrocytes.
Effects of Ononin on IL-1β-induced production of TNF-α and IL-6 in rat chondrocytes. The cell culture supernatant and chondrocytes of each group were collected together. (A, B) expression levels of TNF-α and IL-6 levels were accessed by ELISA kits. All experiments were performed in 3 times and data were expressed by mean ± SD. **P < 0.01 compared with control group; ##P < 0.01 compared with IL-1β group
Effects of Ononin on IL-1β-induced cartilage degradation and overexpression of MMP-13 in chondrocytes
Collagen II is commonly used to assess cartilage tissue metabolism, and MMP-13 is the major cartilage degradation enzyme associated with the progression of OA [20]. The protein expression levels of Collagen II and MMP-13 were evaluated by Western blotting (Fig. 4A) and immunofluorescence staining (Fig. 4D, E). In our study, chondrocytes were treated with ononin with or without IL-1β for 24 h. As shown in Fig. 4B-C and F-G, IL-1β noticeably inhibited the expression of Collagen II and upregulated the expression of MMP-13. However, 10, 100 and 1000 nM ononin effectively reversed the degradation of Collagen II and the overexpression of MMP-13. Furthermore, ononin ameliorated IL-1β-induced adverse effects on Collagen II and MMP-13 protein expression in a concentration-dependent manner. The immunofluorescence staining results were consistent with the Western blot results, in the same light, in the 1000 nM ononin group, ononin decreased the expression of MMP-13 by approximately 2.9 times and Collagen II was restored to 90% compared with those in the IL-1β group, showing the ability to repair cartilage damage.
Effects of Ononin on IL-1β-induced ECM degradation in Rat Chondrocytes. (A) Expression levels of Collagen II and MMP-13 were detected by Western Blot. The samples derive from the same experiment and that different gels were processed in parallel. (B, C) The expression level of target proteins was expressed by the ratio of grey value of target band to GAPDH band by Image-J software. (D) Immunofluorescence staining of Collagen II protein (green) and nucleus (blue) was labelled with DAPI and photographed by fluorescence microscope (20×) (scale bar: 50 μm). (E) Immunofluorescence staining of MMP-13 protein (red) and nucleus (blue) was labelled with DAPI and photographed by fluorescence microscope (20×) (scale bar: 50 μm). (F, G) The summary data of fluorescence intensity of Collagen II and MMP-13 in situ. All experiments were performed in 3 times and data were expressed by mean ± SD. *P < 0.05 compared with control group; #P < 0.05 compared with IL-1β group; **P < 0.01 compared with control group; ##P < 0.01 compared with IL-1β group
Effects of Ononin on IL-1β-induced MAPK and NF-κB Signalling activation in rat chondrocytes
The MAPK and NF-κB signalling pathways play key roles in the occurrence and development of OA [21, 22]. To explore the effect of ononin on IL-1β-induced activation of the MAPK and NF-κB pathways in rat chondrocytes, first, the levels of phosphorylated ERK, JNK and p38 and their corresponding total protein levels were measured by Western blotting (Fig. 5A). As shown in Fig. 5B-D, the ratios of p-ERK/ERK, p-JNK/JNK and p-p38/p38 in the IL-1β group were significantly higher than those in the control group (P < 0.01). In contrast, ononin at concentrations of 10, 100 and 1000 nM effectively inhibited the phosphorylation of ERK, JNK and p-p38 (P < 0.05). In the 1000 nM ononin group, the ratios of p-ERK/ERK, p-JNK/JNK and p-p38/p38 decreased by 3.9 times, 2.1 times and 2 times, respectively, compared with those in the IL-1β group.
Effects of Ononin on MAPK and NF-κB signalling pathways. (A) Proteins of p-ERK/ERK, p-JNK/JNK, p-p38/ p38, p-IκBα/IκBα, and p-p65/p65 were detected by Western Blot. The samples derive from the same experiment and that different gels were processed in parallel. (B-F) The expression level of target proteins was expressed by the ratio of grey value of target band to the total of ERK, p38, JNK, IκBα and p65 were used as the internal control respectively by Image-J software. All experiments were performed in 3 times and data were expressed by mean ± SD. *P < 0.05 compared with control group; #P < 0.05 compared with IL-1β group; **P < 0.01 compared with control group; ##P < 0.01 compared with IL-1β group
Then, we used the same method to examine the related proteins in the NF-κB pathway, including p-IκBα/IκBα and p-p65/p65 (Fig. 5A). As shown in Fig. 5E-F, ononin at concentrations of 10, 100 and 1000 nM could also inhibit the IL-1β-induced upregulation of the ratios of p-IκBα/IκBα and p-p65/p65, which decreased 2.5 times and 3.5 times, respectively, with high concentrations of ononin (1000 nM ononin group). In summary, these two activated pathways can be partially restored by ononin in a dose-dependent manner.
Discussion
OA is a degenerative joint disease. The core pathological change in OA is the inflammatory reaction in the joints, which further leads to the loss of articular cartilage to different degrees [23, 24]. Therefore, inflammation is an important link in the pathogenesis of OA. There is still no effective treatment to prevent the progression of OA. Therefore, it is urgent to develop more effective OA drugs. Ononin, a natural isoflavone, is widely distributed in Astragalus membranaceus, Glycyrrhiza uralensis, Hedysarum and Pueraria lobata [25, 26]. Based on previous studies, ononin has anti-inflammatory and apoptotic effects [18, 27, 28]. In this study, we used IL-1β (10 ng/ml)-induced chondrocytes as an OA model in vitro and hypothesized that ononin mediated inflammation during OA through targets or signalling pathways. Here, we showed that ononin could inhibit the IL-1β-induced expression of the cytokines TNF-α and IL-6. Furthermore, ononin also alleviated the IL-1β-induced expression of MMP-13 and restored the degradation of collagen II in rat chondrocytes. Finally, ononin could play a protective role in chondrocytes by regulating the MAPK and NF-κB pathways. Overall, our results suggest that ononin may be a promising therapeutic agent for OA.
Many studies have elucidated that IL-1β, IL-6, TNF-α and IL-17 are the key proinflammatory cytokines leading to the pathogenesis of OA and are found at high levels in OA chondrocytes [29, 30]. Therefore, regulating the receptors of these cytokines may be favourable for the treatment of OA. Recent studies have widely reported that curcumin and resveratrol are potential therapeutic drugs for OA that inhibit the inflammatory response and oxidative stress in chondrocytes [31, 32]. Studies have also shown that ononin can protect LPS-induced RF inflammatory cells and RAW 264.7 cells from damage [18, 27]. In our study, ononin significantly inhibited the IL-1β-mediated enhancement of the inflammatory factors TNF-α and IL-6 in rat chondrocytes. Furthermore, various studies have confirmed that there is a high level of MMP-13 in damaged articular cartilage. Generally, MMP-13 is the main enzyme that targets cartilage catabolism and plays a crucial role in the process of articular cartilage degradation [33]. In general, proinflammatory factors such as TNF-α and IL-6 stimulate the production of MMPs and inhibit the synthesis of ECM [34,35,36]. In our study, we revealed that IL-1β-induced chondrocytes had increased expression of MMP-13 and inhibited expression of Collagen II, further demonstrating that ononin significantly ameliorated damage in a dose-dependent manner. Previous studies have shown that a growing number of Chinese herbal compounds also have anti-inflammatory effects against OA, which was consistent with our results. For instance, betulin can inhibit the degradation of extracellular matrix by downregulating the expression of MMP-13 and upregulating the expression of collagen II [37]. Another agent, xanthohumol, a natural prenylflavonoid with anti-inflammatory and antioxidant activities, has also been shown to effectively protect against ECM degradation by ameliorating MMP-13 expression in OA chondrocytes [38]. In conclusion, our data suggested that ononin could restore the imbalance in cartilage matrix anabolism.
In recent years, with the in-depth study of signalling pathways in the development of OA, the MAPK and NF-κB pathways have been increasingly shown to be important regulators of the inflammatory response, catabolism and ECM degradation during the OA process [39, 40]. MAPK is a serine/threonine protein kinase. Under inflammatory conditions, ERK, JNK and p38 are activated, and their phosphorylation levels are upregulated, thus stimulating downstream transcription factors and leading to the production of proinflammatory cytokines and the inflammatory response [41]. Akhtar et al. [42] confirmed that inflammatory factors such as IL-1β can induce the overexpression of miR-27b and promote the upregulation of MMP-13 by inducing JNK phosphorylation in melanoma. Sonder et al. [43] found that p38 MAPK, p44/42 and src tyrosine kinase inhibitors can inhibit the degradation of ECM by inhibiting MMPs, although p44/42 inhibitors are essential for delaying the degradation of proteoglycan. In addition, aucubin is a natural compound derived from plants that can inhibit IL-1β-mediated p65 phosphorylation and nuclear translocation, suggesting that aucubin may have anti-inflammatory and cartilage protective properties through the NF-κB signalling pathway [44]. Consistent with these studies, the present study showed that chondrocytes stimulated with IL-1β for 24 h had significantly increased phosphorylation of ERK, JNK and p38. However, when ononin was added to IL-1β-induced chondrocytes, the phosphorylation of these proteins was inhibited in a dose-dependent manner. This finding suggests that ononin may regulate the inflammatory response and ECM degradation by inhibiting the IL-1β-activated MAPK pathway.
The NF-κB signalling pathway is present in all kinds of cells, mainly in the form of an inactive p65 subunit. The upstream signal is transmitted to IκB kinase. Then, IκB kinase phosphorylates I-κB and exposes nuclear factor κB (p65), which is inhibited by I-κB, after which p65 is activated and phosphorylated. Finally, the phosphorylated protein is dissociated, and p65 is translocated into the nucleus, thus activating the inflammatory response and leading to the secretion of a series of proinflammatory cytokines [45]. Moreover, activation of the NF-κB pathway is necessary for chondrocytes to express MMPs, trigger the inflammatory response and exacerbate cartilage destruction [46]. Recently, resveratrol, a flavonoid with a stilbene structure, has been shown to block the NF-κB pathway to mitigate chondrocyte inflammation and ameliorate IL-1β-induced chondrocyte injury [32, 47]. In addition, the aqueous extract of Anthriscus sylvestris leaves, an agent similar to that in our study, can protect cartilage against OA by inhibiting IL-1β-induced MAPK phosphorylation and NF-κB p65 subunit translocation to the nucleus [48]. Moreover, Dai et al. [49] claimed that after anthocyanin treatment, the NF-κB signalling pathway was significantly inhibited, resulting in reductions in IL-1β and TNF-α and the expression of matrix metalloproteinase, which was shown to significantly alleviate the occurrence of pain and inflammation induced by OA. In our study, compared with that in the control group, the expression of p-IκBα in IL-1β-activated chondrocytes was increased, which led to the release of p65. Then, p65 translocated into the nucleus and activated inflammation, which upregulated the expression of secreted inflammatory factors and ECM decomposition. However, these results were significantly reversed in a dose-dependent manner after treatment with ononin.
Conclusion
The findings in this study were the first to reveal that ononin had clear anti-inflammatory therapeutic effects on OA. As we have discovered, inhibiting the inflammatory response and reducing ECM degradation may be the basis of the protective mechanism of ononin, which may further occur via the regulation of the MAPK and NF-κB signalling pathways. These results suggest that ononin could become a promising treatment against OA.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its important information files.
References
Sniekers YH, Weinans H, Bierma-Zeinstra SM, van Leeuwen JP, van Osch GJ. Animal models for osteoarthritis: the effect of ovariectomy and estrogen treatment - a systematic approach. Osteoarthr Cartil. 2008;16(5):533–41. https://doi.org/10.1016/j.joca.2008.01.002 Epub 2008/02/19. PubMed PMID: 18280756.
Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. https://doi.org/10.1155/2014/561459 Epub 2014/05/31. PubMed PMID: 24876674; PubMed Central PMCID: PMCPMC4021678.
Huang X, Xi Y, Pan Q, Mao Z, Zhang R, Ma X, et al. Caffeic acid protects against IL-1β-induced inflammatory responses and cartilage degradation in articular chondrocytes. Biomed Pharmacother. 2018;107:433–9. https://doi.org/10.1016/j.biopha.2018.07.161 Epub 2018/08/14. PubMed PMID: 30103115.
Park JS, Kim DK, Shin HD, Lee HJ, Jo HS, Jeong JH, et al. Apigenin Regulates Interleukin-1β-Induced Production of Matrix Metalloproteinase Both in the Knee Joint of Rat and in Primary Cultured Articular Chondrocytes. Biomol Ther (Seoul). 2016;24(2):163–70. https://doi.org/10.4062/biomolther.2015.217 Epub 2016/02/24. PubMed PMID: 26902085; PubMed Central PMCID: PMCPMC4774497.
Saklatvala J. Inflammatory signaling in cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr Drug Targets. 2007;8(2):305–13. https://doi.org/10.2174/138945007779940115 Epub 2007/02/20. PubMed PMID: 17305508.
Chow YY, Chin KY. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediators Inflamm. 2020;2020:8293921. https://doi.org/10.1155/2020/8293921 Epub 2020/03/20. PubMed PMID: 32189997; PubMed Central PMCID: PMCPMC7072120 of this paper.
Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–2. https://doi.org/10.1126/science.1072682 Epub 2002/12/10. PubMed PMID: 12471242.
Shih RH, Wang CY, Yang CM. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front Mol Neurosci. 2015;8:77. https://doi.org/10.3389/fnmol.2015.00077 Epub 2016/01/07. PubMed PMID: 26733801; PubMed Central PMCID: PMCPMC4683208.
Apostu D, Lucaciu O, Mester A, Oltean-Dan D, Baciut M, Baciut G, et al. Systemic drugs with impact on osteoarthritis. Drug Metab Rev. 2019;51(4):498–523. https://doi.org/10.1080/03602532.2019.1687511 Epub 2019/11/16. PubMed PMID: 31726891.
Nailwal NP, Doshi GM. Role of intracellular signaling pathways and their inhibitors in the treatment of inflammation. Inflammopharmacology. 2021;29(3):617–40. https://doi.org/10.1007/s10787-021-00813-y Epub 2021/05/19. PubMed PMID: 34002330.
Ghouri A, Conaghan PG. Update on novel pharmacological therapies for osteoarthritis. Ther Adv Musculoskelet Dis. 2019;11:1759720x19864492. https://doi.org/10.1177/1759720x19864492 Epub 2019/08/07. PubMed PMID: 31384314; PubMed Central PMCID: PMCPMC6651659.
van den Bosch MHJ. Osteoarthritis year in review 2020: biology. Osteoarthr Cartil. 2021;29(2):143–50. https://doi.org/10.1016/j.joca.2020.10.006 Epub 2020/11/27. PubMed PMID: 33242602.
Jain A, Singh R, Singh S, Singh S. Diacerein protects against iodoacetate-induced osteoarthritis in the femorotibial joints of rats. J Biomed Res. 2015;29(5):405–13. https://doi.org/10.7555/jbr.29.20130092 Epub 2015/10/08. PubMed PMID: 26442595; PubMed Central PMCID: PMCPMC4585436.
Jeong JH, Moon SJ, Jhun JY, Yang EJ, Cho ML, Min JK. Eupatilin Exerts Antinociceptive and Chondroprotective Properties in a Rat Model of Osteoarthritis by Downregulating Oxidative Damage and Catabolic Activity in Chondrocytes. PLoS One. 2015;10(6):e0130882. https://doi.org/10.1371/journal.pone.0130882 Epub 2015/06/18. PubMed PMID: 26083352; PubMed Central PMCID: PMCPMC4471346 regarding the publication of this paper.
Bernatoniene J, Kazlauskaite JA, Kopustinskiene DM. Pleiotropic Effects of Isoflavones in Inflammation and Chronic Degenerative Diseases. Int J Mol Sci. 2021;22(11). https://doi.org/10.3390/ijms22115656 Epub 2021/06/03. PubMed PMID: 34073381; PubMed Central PMCID: PMCPMC8197878.
Yu DH, Bao YM, An LJ, Yang M. Protection of PC12 cells against superoxide-induced damage by isoflavonoids from Astragalus mongholicus. Biomed Environ Sci. 2009;22(1):50–4. https://doi.org/10.1016/s0895-3988(09)60022-2 Epub 2009/05/26. PubMed PMID: 19462688.
Fu S, Gu Y, Jiang JQ, Chen X, Xu M, Chen X, et al. Calycosin-7-O-β-D-glucoside regulates nitric oxide /caveolin-1/matrix metalloproteinases pathway and protects blood-brain barrier integrity in experimental cerebral ischemia-reperfusion injury. J Ethnopharmacol. 2014;155(1):692–701. https://doi.org/10.1016/j.jep.2014.06.015 Epub 2014/06/17. PubMed PMID: 24930357.
Meng Y, Ji J, Xiao X, Li M, Niu S, He Y, et al. Ononin induces cell apoptosis and reduces inflammation in rheumatoid arthritis fibroblast-like synoviocytes by alleviating MAPK and NF-κB signaling pathways. Acta Biochim Pol. 2021;68(2):239–45. https://doi.org/10.18388/abp.2020_5528 Epub 2021/06/03. PubMed PMID: 34075738.
Oh JS, Cho IA, Kang KR, You JS, Yu SJ, Lee GJ, et al. Biochanin-a antagonizes the interleukin-1β-induced catabolic inflammation through the modulation of NFκB cellular signaling in primary rat chondrocytes. Biochem Biophys Res Commun. 2016;477(4):723–30. https://doi.org/10.1016/j.bbrc.2016.06.126 Epub 2016/07/02. PubMed PMID: 27363337.
Singh M, Valecha S, Khinda R, Kumar N, Singh S, Juneja PK, et al. Multifactorial Landscape Parses to Reveal a Predictive Model for Knee Osteoarthritis. Int J Environ Res Public Health. 2021;18(11). https://doi.org/10.3390/ijerph18115933 Epub 2021/06/03. PubMed PMID: 34073132; PubMed Central PMCID: PMCPMC8199148.
Yang G, Li S, Yuan L, Yang Y, Pan MH. Effect of nobiletin on the MAPK/NF-κB signaling pathway in the synovial membrane of rats with arthritis induced by collagen. Food Funct. 2017;8(12):4668–74. https://doi.org/10.1039/c7fo01311f Epub 2017/11/22. PubMed PMID: 29160881.
Shi JH, Sun SC. Tumor Necrosis Factor Receptor-Associated Factor Regulation of Nuclear Factor κB and Mitogen-Activated Protein Kinase Pathways. Front Immunol. 2018;9:1849. https://doi.org/10.3389/fimmu.2018.01849 Epub 2018/08/25. PubMed PMID: 30140268; PubMed Central PMCID: PMCPMC6094638.
Maldonado M, Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res Int. 2013;2013:284873. https://doi.org/10.1155/2013/284873 Epub 2013/09/27. PubMed PMID: 24069595; PubMed Central PMCID: PMCPMC3771246.
Mora JC, Przkora R, Cruz-Almeida Y. Knee osteoarthritis: pathophysiology and current treatment modalities. J Pain Res. 2018;11:2189–96. https://doi.org/10.2147/jpr Epub 2018/10/17. S154002. PubMed PMID: 30323653; PubMed Central PMCID: PMCPMC6179584.
Hoo RL, Wong JY, Qiao C, Xu A, Xu H, Lam KS. The effective fraction isolated from Radix Astragali alleviates glucose intolerance, insulin resistance and hypertriglyceridemia in db/db diabetic mice through its anti-inflammatory activity. Nutr Metab (Lond). 2010;7:67. https://doi.org/10.1186/1743-7075-7-67 Epub 2010/08/26. PubMed PMID: 20735814; PubMed Central PMCID: PMCPMC2936435.
Ko KP. Isoflavones: chemistry, analysis, functions and effects on health and cancer. Asian Pac J Cancer Prev. 2014;15(17):7001–10. https://doi.org/10.7314/apjcp.2014.15.17.7001 Epub 2014/09/18. PubMed PMID: 25227781.
Dong L, Yin L, Zhang Y, Fu X, Lu J. Anti-inflammatory effects of ononin on lipopolysaccharide-stimulated RAW 264.7 cells. Mol Immunol. 2017;83:46–51. https://doi.org/10.1016/j.molimm.2017.01.007 Epub 2017/01/18. PubMed PMID: 28095349.
Yu J, Bi X, Yu B, Chen D. Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients. 2016;8(6). https://doi.org/10.3390/nu8060361 Epub 2016/06/15. PubMed PMID: 27294954; PubMed Central PMCID: PMCPMC4924202.
Zhang J, Li Q, Chang S. The effects of particle density in moxa smoke on the ultrastructure of knee cartilage and expressions of TNF-α, IL-1b, BAX, and Bcl-2 mRNA in a rat model for osteoarthritis. J Cell Biochem. 2019;120(4):6589–95. https://doi.org/10.1002/jcb.27952 Epub 2018/11/16. PubMed PMID: 30430645.
Liu Y, Peng H, Meng Z, Wei M. Correlation of IL-17 Level in Synovia and Severity of Knee Osteoarthritis. Med Sci Monit. 2015;21:1732–6. https://doi.org/10.12659/msm.893771 Epub 2015/06/16. PubMed PMID: 26076201; PubMed Central PMCID: PMCPMC4480114.
Zhou Y, Ming J, Deng M, Li Y, Li B, Li J, et al. Chemically modified curcumin (CMC2.24) alleviates osteoarthritis progression by restoring cartilage homeostasis and inhibiting chondrocyte apoptosis via the NF-κB/HIF-2α axis. J Mol Med (Berl). 2020;98(10):1479–91. https://doi.org/10.1007/s00109-020-01972-1 Epub 2020/08/30. PubMed PMID: 32860098.
Liu S, Yang H, Hu B, Zhang M. Sirt1 regulates apoptosis and extracellular matrix degradation in resveratrol-treated osteoarthritis chondrocytes via the Wnt/β-catenin signaling pathways. Exp Ther Med. 2017;14(5):5057–62. https://doi.org/10.3892/etm.2017.5165 Epub 2017/12/05. PubMed PMID: 29201214; PubMed Central PMCID: PMCPMC5704318.
Takahashi A, de Andrés MC, Hashimoto K, Itoi E, Otero M, Goldring MB, et al. DNA methylation of the RUNX2 P1 promoter mediates MMP13 transcription in chondrocytes. Sci Rep. 2017;7(1):7771. https://doi.org/10.1038/s41598-017-08418-8 Epub 2017/08/12. PubMed PMID: 28798419; PubMed Central PMCID: PMCPMC5552713.
Nambi G. Does low level laser therapy has effects on inflammatory biomarkers IL-1β, IL-6, TNF-α, and MMP-13 in osteoarthritis of rat models-a systemic review and meta-analysis. Lasers Med Sci. 2021;36(3):475–84. https://doi.org/10.1007/s10103-020-03124-w Epub 2020/08/25. PubMed PMID: 32833088.
Limagne E, Lançon A, Delmas D, Cherkaoui-Malki M, Latruffe N. Resveratrol Interferes with IL1-β-Induced Pro-Inflammatory Paracrine Interaction between Primary Chondrocytes and Macrophages. Nutrients. 2016;8(5). https://doi.org/10.3390/nu8050280 Epub 2016/05/18. PubMed PMID: 27187448; PubMed Central PMCID: PMCPMC4882693.
Kubosch EJ, Heidt E, Bernstein A, Böttiger K, Schmal H. The trans-well coculture of human synovial mesenchymal stem cells with chondrocytes leads to self-organization, chondrogenic differentiation, and secretion of TGFβ. Stem Cell Res Ther. 2016;7(1):64. https://doi.org/10.1186/s13287-016-0322-3 Epub 2016/04/28. PubMed PMID: 27118471; PubMed Central PMCID: PMCPMC4845486.
Ren C, Jin J, Hu W, Chen Q, Yang J, Wu Y, et al. Betulin Alleviates the Inflammatory Response in Mouse Chondrocytes and Ameliorates Osteoarthritis via AKT/Nrf2/HO-1/NF-κB Axis. Front Pharmacol. 2021;12:754038. https://doi.org/10.3389/fphar.2021.754038 Epub 2021/11/02. PubMed PMID: 34721040; PubMed Central PMCID: PMCPMC8548689.
Zheng T, Zhou Q, Huang J, Lai J, Ji G, Kong D. Xanthohumol Inhibited Mechanical Stimulation-Induced Articular ECM Degradation by Mediating lncRNA GAS5/miR-27a Axis. Front Pharmacol. 2021;12:737552. https://doi.org/10.3389/fphar.2021.737552 Epub 2021/10/08. PubMed PMID: 34616299; PubMed Central PMCID: PMCPMC8489376.
Broome DT, Datta NS. Mitogen-activated protein kinase phosphatase-1: function and regulation in bone and related tissues. Connect Tissue Res. 2016;57(3):175–89. https://doi.org/10.3109/03008207.2015.1125480 Epub 2016/04/01. PubMed PMID: 27031422.
Jimi E, Fei H, Nakatomi C. NF-κB Signaling Regulates Physiological and Pathological Chondrogenesis. Int J Mol Sci. 2019;20(24). https://doi.org/10.3390/ijms20246275 Epub 2019/12/18. PubMed PMID: 31842396; PubMed Central PMCID: PMCPMC6941088.
Fecher LA, Amaravadi RK, Flaherty KT. The MAPK pathway in melanoma. Curr Opin Oncol. 2008;20(2):183–9. https://doi.org/10.1097/CCO.0b013e3282f5271c Epub 2008/02/28. PubMed PMID: 18300768.
Akhtar N, Rasheed Z, Ramamurthy S, Anbazhagan AN, Voss FR, Haqqi TM. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010;62(5):1361–71. https://doi.org/10.1002/art.27329 Epub 2010/02/05. PubMed PMID: 20131257; PubMed Central PMCID: PMCPMC3139404.
Sondergaard BC, Schultz N, Madsen SH, Bay-Jensen AC, Kassem M, Karsdal MA. MAPKs are essential upstream signaling pathways in proteolytic cartilage degradation--divergence in pathways leading to aggrecanase and MMP-mediated articular cartilage degradation. Osteoarthr Cartil. 2010;18(3):279–88. https://doi.org/10.1016/j.joca.2009.11.005 Epub 2009/11/26. PubMed PMID: 19932675.
Wang SN, Xie GP, Qin CH, Chen YR, Zhang KR, Li X, et al. Aucubin prevents interleukin-1 beta induced inflammation and cartilage matrix degradation via inhibition of NF-κB signaling pathway in rat articular chondrocytes. Int Immunopharmacol. 2015;24(2):408–15. https://doi.org/10.1016/j.intimp.2014.12.029 Epub 2015/01/13. PubMed PMID: 25576403.
Pomerantz JL, Baltimore D. Two pathways to NF-kappaB. Mol Cell. 2002;10(4):693–5. https://doi.org/10.1016/s1097-2765(02)00697-4 Epub 2002/11/07. PubMed PMID: 12419209.
Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471–8. https://doi.org/10.1097/BOR.0b013e328349c2b1 Epub 2011/07/27. PubMed PMID: 21788902; PubMed Central PMCID: PMCPMC3937875.
Chen J, Xie JJ, Shi KS, Gu YT, Wu CC, Xuan J, et al. Glucagon-like peptide-1 receptor regulates endoplasmic reticulum stress-induced apoptosis and the associated inflammatory response in chondrocytes and the progression of osteoarthritis in rat. Cell Death Dis. 2018;9(2):212. https://doi.org/10.1038/s41419-017-0217-y Epub 2018/02/13. PubMed PMID: 29434185; PubMed Central PMCID: PMCPMC5833344.
Lee SA, Moon SM, Han SH, Hwang EJ, Park BR, Kim JS, et al. Chondroprotective effects of aqueous extract of Anthriscus sylvestris leaves on osteoarthritis in vitro and in vivo through MAPKs and NF-κB signaling inhibition. Biomed Pharmacother. 2018;103:1202–11. https://doi.org/10.1016/j.biopha.2018.04.183 Epub 2018/06/06. PubMed PMID: 29864899.
Dai T, Shi K, Chen G, Shen Y, Pan T. Malvidin attenuates pain and inflammation in rats with osteoarthritis by suppressing NF-κB signaling pathway. Inflamm Res. 2017;66(12):1075–84. https://doi.org/10.1007/s00011-017-1087-6 Epub 2017/08/31. PubMed PMID: 28852776.
Acknowledgements
Thanks for the experimental platform provided by Guangxi Colleges and Universities Key Laboratory of Prevention and Control of Highly Prevalent Diseases.
Funding
This research was supported by the Guangxi Key R&D Program (Grant No. GuiKeAB17292073). There is no actual or potential conflict of interest including financial, personal or other relationships with other people or organizations.
Author information
Authors and Affiliations
Contributions
FX and TL carried out experiment design and feasibility analysis for this study. FX and LZ completed the whole process of cell experiment. ZL, RJ, LW and PL performed image analysis and data processing. FX wrote the manuscript, and QW revised the manuscript content constructively. All authors have repeatedly read and established the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted according to the purpose of Animal Care & Welfare Committee for Teaching and Research, Guangxi Medical University. The content of this experiment was approved by the Animal Care & Welfare Committee of Guangxi Medical University (NO: 202006017). The whole experimental process ensured the welfare of experimental animals and reduced their pain.
Consent for publication
Not applicable.
Competing interests
All authors declare that there are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, F., Zhao, LJ., Liao, T. et al. Ononin ameliorates inflammation and cartilage degradation in rat chondrocytes with IL-1β-induced osteoarthritis by downregulating the MAPK and NF-κB pathways. BMC Complement Med Ther 22, 25 (2022). https://doi.org/10.1186/s12906-022-03504-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-022-03504-5