Abstract
Background
The present evidence is deficient for the trade-offs between the pros and cons of single blastocyst transfer (SBT) versus double blastocyst transfer (DBT) in frozen-thawed embryo transfer cycles for women in advanced reproductive age, especially in the second cycle. The current study aimed to investigate the impact of transferred blastocyst numbers on pregnancy outcomes in the first and second embryo transfer for women ≥ 35 years.
Methods
This was a retrospective cohort study including 1284 frozen-thawed blastocyst transfer (FBT) cycles from two reproductive centers. We analyzed the pregnancy outcomes after SBT and DBT in the first and second FBT cycles. Moreover, stratified analysis was conducted by maternal age.
Results
In the first FBT cycle, the LBR was higher in the DBT group than that in the SBT group [52.3% vs. 33.9%; adjusted odds ratio (aOR), 1.65; 95% confidence interval (CI), 1.26–2.15, P < 0.001]. However, the LBR of the DBT group showed no remarkable difference compared with that of the SBT group in the second cycle of FBT (44.3% vs. 33.3%; aOR, 1.30; 95% CI, 0.81–2.08; P = 0.271). Furthermore, stratified analysis by age showed a higher LBR for the DBT group than the SBT group in patients aged 38–42 years (43.1% vs. 33.9%; aOR, 2.27; 95% CI, 1.05–4.90; P = 0.036).
Conclusions
The present study demonstrated that the SBT regimen is a better choice for both, the first and second frozen-thawed embryo transfer cycles, for women aged 35–37 years. Additionally, the DBT regimen is still recommended to achieve a high LBR in women aged 38–42 years in the second FBT cycle. These findings may be beneficial for deciding the embryo transfer regimens in women of advanced reproductive age.
Similar content being viewed by others
Background
Owing to the accumulation of clinical experience and advancement in cryopreservation techniques in the last two decades, the objective of assisted reproductive technology (ART) has switched from achieving better pregnancy rates to attaining comfortable and safe pregnancies [1]. Furthermore, there has been a growing number of frozen-thawed embryo transfer (FET) cycles. FET has been widely used in in-vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) as it can effectively improve the cumulative pregnancy rate, avoid successive oocyte retrieval procedures, and allow embryos to be transferred to a more physiological uterine environment. Although the transfer of two or more embryos has a high chance of achieving pregnancy, it also increases the chance of multiple pregnancies, which can cause serious health risks for both mother and offspring [2, 3]. Reducing the number of transferred embryos, particularly through single blastocyst transfer (SBT), has been demonstrated to be the most effective method for decreasing the incidence of multiple pregnancies without compromising pregnancy outcomes [4, 5].
After 35 years of age, the overall euploid rate of blastocysts decreases significantly and it continues to decrease with increasing age [6]. The Practice Committee of the American Society for Reproductive Medicine (ASRM) suggests the first FET cycle as a favorable factor for determining the number of embryos transferred [7]. For older patients, to achieve pregnancy within a short period and to reduce the cost of multiple ART cycles, most clinicians and patients are unwilling to adopt the SBT technique. However, multiple embryo transfers increase pregnancy complications. The researchers are still trying to elucidate if there is a better embryo transfer regimen for women of advanced age. Although this is an intriguing topic, it demands further specific investigations.
Therefore, we retrospectively investigated the appropriate the FET strategy for women aged ≥ 35 years to provide more clarity to clinicians and patients on this issue.
Methods
Study population
A retrospective cohort study was carried out in two reproductive centers in China, namely, Women’s Hospital of Nanjing Medical University, and Changzhou Maternal and Child Health Care Hospital. We included all frozen-thawed blastocyst transfer (FBT) cycles in women aged ≥ 35 years (N = 1813) between January 2017 and January 2021. All procedures and protocols performed in this study were approved by the ethics committee of Women’s Hospital of Nanjing Medical University (NJFY-2020-KY-070) and the Ethics Committee of Changzhou Maternal and Child Health Care Hospital (2020 No.75) and were conducted based on the principles of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
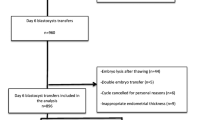
Eligible patients were 35–42 years old and underwent frozen-thawed autologous blastocyst transfer. The exclusion criteria were as follows: endometrium < 7 mm; evidence of a uterine malformation; cycles of preimplantation genetic testing (PGT); and ≥ 3 FET cycles. It is worth mentioning that all women included in this study that underwent cycle 2 had failed in cycle 1. Finally, a sum of 1,284 FBT cycles were included to conduct the investigation (Fig. 1).
Ovarian stimulation
Patients received either a mild stimulation regimen or a flexible gonadotrophin-releasing hormone (GnRH) antagonist regimen. For old patients who underwent the mild stimulation regimen, 5 mg of letrozole (Letrozole tablets, Hengrui, China) or 50 mg of clomiphene (Fertilan, Medovhrmie Ltd., Republic of Cyprus) was administrated from day-3 to day-7 combined with 75 or 150 IU of recombinant follicle-stimulating hormone (rFSH) once a day. In the flexible GnRH antagonist regimen, gonadotropin (recombinant follicle-stimulating hormone, Gonal-F, Merck Serono, Switzerland) at 150–300 IU/day was started on the third day of menstruation and the GnRH antagonist (Cetrorelix 0.25 mg, Pierre Fabre Medicament Production, Aquitaine Pharm International, France) was added when a leading follicle was ≥ 14 mm in mean diameter. Human chorionic gonadotrophin at 10,000 IU (hCG, Lizhu Co., China) was injected to induce final oocyte maturation when at least 3 follicles were > 17 mm in mean diameter. After 35–36 h, the oocyte was retrieved. Oocytes were inseminated by regular in vitro fertilization (IVF) or intracytoplasmic sperm injection based on sperm quality. The embryos were cultured under our previously published protocol [8]. If there were at least three cleavage-stage embryos with good morphology (8 cells and < 20% fragmentation) on day 3, embryos were cultured to the blastocyst stage.
Blastocyst grading
The blastocysts were graded based on Gardner’s scoring system [9], which involves blastocyst expansion, inner cell mass (ICM), and trophectoderm (TE). Blastocysts were evaluated on the expansion of the blastocyst cavity on a scale of 1–6. When the blastocyst arrived at a cavity expansion level 3 or over, ICM and TE were evaluated based on the size and density of the cells (A/B/C), respectively. A blastocyst evaluated 3 and above with an A or B for either ICM or TE was considered a good-quality embryo; if not, it was defined as a low-quality embryo (grades 3–6 AC/BC/CA/CB). Poor-quality blastocysts (grades 3–6 CC) were discarded due to their low developmental potential.
Endometrial preparation for FET cycles and luteal phase support
The endometrial preparation regimen for FET cycles was selected by the physicians at their discretion. The modified natural cycle (NC) regimen was used for participants with regular menstrual cycles. In the modified NC regimen, ovulation was determined by using ultrasound to detect the leading follicle and measuring the progesterone levels. When the dominant follicle had reached at least 18 mm, endometrial thickness was 6 mm or thicker, and meanwhile, progesterone level was ≤ 1.5 ng/mL, 10,000 IU of hCG was administered as the ovulatory trigger. After ultrasound confirmation of ovulation, luteal phase support (LPS) was started by administering 10 mg thrice a day of progesterone (dydrogesterone, Abbott Biologicals B.V., The Netherlands). In artificial cycles, oral estrogen treatment was started from the second to the fourth day of the menstruation for one week, at a dose of 4–6 mg/day (estradiol valerate tablets, Progynova, Bayer, France), which was adjusted to 6–8 mg depending on the serum estradiol (E2) levels and endometrial thickness. After approximately ten days later, when endometrial thickness ≥ 7 mm and serum E2 ≥ 200 pg/mL, the patients were given 90 mg of vaginal progesterone (Crinone, Merck Serono, UK) once a day and 10 mg of dydrogesterone thrice a day (P + 1). Seven days after the administration of hCG or 5 days after the addition of progesterone, FET was performed. In all cases, LPS with progesterone was continued after embryo transfer. Once gestational sac and embryonic heartbeat were detected by transvaginal ultrasound, the LPS was maintained until 10–12 weeks of pregnancy.
Clinical outcomes
The primary clinical outcome was the live birth rate (LBR) and the secondary.
clinical endpoints included clinical pregnancy rate (CPR), clinical miscarriage rate, and twin delivery rate (TDR). The delivery of a live infant after 28 weeks of pregnancy was considered a live birth. The presence of a gestational sac confirmed by transvaginal ultrasound 4 weeks after the transfer was considered a sign of clinical pregnancy. Miscarriage was defined as a loss of pregnancy before 20 weeks of gestation. TDR was calculated from the number of twin deliveries per FET cycle.
Statistical analysis
The SPSS 26.0 software (IBM Corp., Armonk, NY, USA) was used to perform all statistical analyses. For continuous variables, the normality was assessed using QQ-plots and the Kolmogorov-Smirnov test. The data were analysed using student’s t-tests and presented as (for normally distributed data) or Mann-Whitney U tests (for non-normally distributed data). Continuous variables were expressed as mean ± standard deviation if they were normally distributed, while the median and interquartile range (IOR) were provided for non-normally distributed data. Pearson’s χ2 test or Fisher’s exact test was used to compare categorical variables, whose data are presented as proportions. A multivariate logistic regression analysis was used to assess the independent effect of the transferred blastocyst number on the LBR. The covariates included were: age, basal FSH, and the number of good-quality blastocysts transferred (1 or 2 vs. 0). The odds ratios (OR) or adjusted odds ratios (aOR) and 95% confidence intervals (CI) are used to display the results. Statistical significance was accepted at a P-value < 0.05. Hosmer-Lemeshow goodness-of-fit test was used to assess the goodness of fit of the model.
Results
Demographic and clinical characteristics of women
In total, 1284 FBT cycles were enrolled during the study period. The demographic and basal characteristics of women with live births and non-live births are presented in Table 1. Overall, the LBR per transfer was 44.7%. We observed that patients with a live birth were younger, had a lower basal serum FSH level (P = 0.003), higher proportion of first FET cycle, greater number of blastocysts transferred, and greater number of good-quality blastocysts transferred than those without a live birth (Table 1). Moreover, body mass index, cause of infertility, the type and duration of infertility, basal serum luteinizing hormone and E2 levels, endometrial thickness, and the type of endometrial preparation were not significantly different between those with and without a live birth. Women with SBT were more likely to have secondary infertility. There were no differences in the other baseline characteristics between those with SBT and DBT group (Table 2).
Clinical outcomes
The clinical outcomes of the first and second FET cycles are presented in Table 3. In cycle 1, the LBR (52.3% vs. 39.9%; OR, 1.65; 95% CI, 1.28–2.14) and CPR (64.2% vs. 50.2%; OR, 1.78; 95% CI, 1.37–2.31) were significantly higher in the double blastocyst transfer (DBT) group than in the SBT group. The differences were statistically significant after multiple regression analysis (LBR: aOR, 1.65; 95% CI, 1.26–2.15; CPR: aOR, 1.78; 95% CI, 1.36–2.32). The confounding factors considered in the regression model were maternal age, basal FSH level, and quantity of good-quality blastocysts transferred. There were no statistical differences between the two groups in terms of the miscarriage rate. In cycle 2, LBR did not differ significantly between the two groups (44.3% vs. 33.3%; aOR, 1.30; 95% CI, 0.81–2.08) after adjusting for confounding factors (Table 3).
Analysis stratified by maternal age
We analyzed the association between transferred blastocyst number (2 vs. 1) and LBR in two subgroups stratified by age, i.e., 35–37 years and 38–42 years (Table 4). For patients aged 35–37 years, the LBR of the DBT group was considerably higher than that of the SBT group in the first cycle (aOR, 1.75; 95% CI, 1.27–2.43). However, the LBR of DBT did not increase in the second cycle (aOR, 0.94; 95% CI, 0.52–1.70). For patients aged 38–42 years, the LBR did not improve significantly in the DBT group compared with the SBT group in the first cycle (aOR, 1.45; 95% CI, 0.91–2.31). Meanwhile, we found the DBT group had significantly higher LBR than the SBT group in the second cycle (aOR, 2.27; 95% CI, 1.05–4.90). The confounding factors considered in the regression model were basal FSH level and the number of good-quality blastocysts transferred.
We then calculated the TDR for each age. We found that the TDR was higher in the DBT group (9.1–20.7%) compared with the SBT group (0.0–2.0%) for women aged 35–39 years (Fig. 2). The TDR was almost 0% for patients aged 40–42 years in both, cycle 1 and cycle 2.
Discussion
To the best of the author’s knowledge, our study is the first study to focus on the association between the rank of FET cycles and the number of blastocysts transferred in women of advanced reproductive age. Our findings revealed that DBT was associated with higher odds of live births than SBT in women aged 35–37 years in the first FET cycle and women aged 38–42 years with previously failed FET cycles. The TDR was almost zero in patients aged 40–42 years, irrespective of the cycle rank and the number of blastocysts transferred.
Previous studies have reported that single embryo transfer (SET) is not suitable for all women. Therefore, the SET regimen is not recommended for women older > 40 years [10]. Fujimoto et al. reported that the CPR and LBR were significantly lower after the SET regimen than the double embryo transfer (DET) regimen in women aged 37–40 years [11]. The latest guidelines by ASRM on the limit of the number of embryos transferred recommend less than three cleavage-stage embryos or blastocysts with unknown euploidy for women > 35 years [7], without specifying the exact number of embryos. However, multiple pregnancies could increase maternal and perinatal morbidity and mortality and further increase medical costs [12,13,14]. Moreover, there is a lack of evidence on the pros and cons of SBT and DBT regimens for older patients, and clinicians wonder whether the DBT regimen should be avoided to reduce the risk of multiple pregnancies regardless of patients’ desire to achieve pregnancy. The SBT regimen is longer than the DBT regimen for similar cumulative pregnancy outcomes [11], which is a remarkable disadvantage for women of advanced age. Currently, there is no consensus on how older women should choose the number of blastocysts to be transferred. Therefore, our study evaluated whether women between the age of 35 and 42 should receive single or double blastocysts.
We assumed that the first choice of all women of advanced age in cycle 1 is SBT. The TDR was significantly high in the DBT group in patients aged 35–37 years, and the LBR with the DBT regimen was comparable to SBT in women aged ≥ 38 years. Furthermore, when the first FET cycle fails, DBT can be considered for women aged 38–42 since the LBR was notably higher in the DBT group than in the SBT group. Additionally, although the TDR was still high after DBT in women aged 38–39, this group could obtain live births faster or restart their next oocyte retrieval cycle. Advanced maternal age is relevant to a decline in fertility and lower FET success rates, but one problem that cannot be ignored is cost-effectiveness (in terms of time and money). The extra time needed for multiple frozen-thawed SET may lead to the additional loss of fertility potential [15]. An earlier study reported that one fresh SET, followed by an extra frozen-thawed SET, is cost-effective in women less than 32 years of age compared with the DET regimen. In patients > 33 years, the DET regimen is more effective but it is costlier than the SET regimen [16].
Some studies have explored the effect of transferred blastocyst numbers on pregnancy outcomes in women stratified by age. An earlier study reported a similar pregnancy rate and a lower multiple pregnancy rate in the elective SBT (eSBT) regimen compared with the DBT regimen in women aged 35–39 years [17]. Some studies analyzed the clinical outcomes following eSBT and DBT regimens and the findings revealed a similar LBR in both cohorts [18, 19], but the eSBT regimen had a lower risk of multiple pregnancies in populations of all ages for both, fresh and vitrified-thawed cycles. However, the blastocyst quality was not analyzed in these studies. In 2020, Chen et al. [4] evaluated the pregnancy outcomes related to different amounts and qualities of transferred blastocysts in age-stratified women (≥ 35 years and < 35 years), and concluded that the eSBT regimen is an ideal alternative for patients of any age when good-quality blastocysts exist. Nonetheless, the aforementioned studies on advanced maternal age did not discuss the effect of FET cycle rank (first or second) on the choice of embryo transfer regimen. Our results were partly consistent with previous studies. In the first cycle of FET, DBT regimen has a higher LBR, but a higher TDR makes SBT a better choice. When the first cycle of FET fails, for women aged 35–37, DBT has a similar LBR as SBT. However, for women aged 38–42 years, a different embryo transfer strategy was needed for the first and second FET cycles. When the first cycle of FET fails, the DBT regimen should be decisively selected to achieve higher LBR.
A randomized trial reported similar LBR between eSBT and DBT regimens if PGT was used [20]. A previous study has reported euploid blastocyst rates in females aged 35–37 years, 38–40 years, and 41–42 years as 49.7%, 42.8%, and 28.2%, respectively [6]. Moreover, embryo aneuploidy and abortion rates are known to increase with maternal age [21]. Our study excluded the PGT cycle; however, the question arises, when the euploidy of the blastocyst is unknown, can the DBT regimen be more reasonable for older patients? Indeed, the DBT regimen led to significantly improved LBR for women aged 38–42 in cycle 2 in stratified analysis. Moreover, the TDR of women aged ≥ 40 years is low. Clinicians in some countries have voluntarily embraced the SBT regimen as the standard practice for IVF since it is often covered in the national healthcare insurance for IVF treatments [22, 23]. However, ART and PGT treatments are not covered by medical insurance in China. Since all costs are borne by patients, they financially burden them and thereby lead to the reduced willingness of selecting PGT as an option to achieve pregnancy.
The study has several advantages. A major strength is the population-based design and relatively large sample size of elderly infertile women. In addition, this is the first study to assess the relationship between cycle rank and the number of blastocysts transferred in advanced maternal age. Inevitably, there are some limitations to this study. (1) The retrospective nature of the study suggests that selection bias could not be avoided. (2) There was insufficient sample size to draw sufficiently compelling conclusions. (3) Owing to the limited number of cycles, our findings require verification in additional studies that involve more older women, including those who have had previously unsuccessful FET cycles and those who have a shortage of good-quality blastocysts.
Conclusions
In conclusion, our findings indicate that the number of blastocysts transferred to women aged 35–42 years should be determined by age as well as the FET cycle rank. We believe that our data will help clinicians and patients arrive at the best decisions to achieve pregnancy. Furthermore, when the blastocyst euploidy is unknown, only one blastocyst should be transferred in women aged 35–37 years, and the DBT regimen should be followed for women > 37 years in the second FET cycle.
Data Availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Abbreviations
- SBT:
-
Single blastocyst transfer
- DBT:
-
Double blastocyst transfer
- FBT:
-
Frozen-thawed blastocyst transfer
- ART:
-
Assisted reproductive technology
- ASRM:
-
American Society for Reproductive Medicine
- FET:
-
Frozen-thawed embryo transfer
- FBT:
-
Frozen-thawed blastocyst transfer
- PGT:
-
Preimplantation genetic testing
- GnRH:
-
Gonadotrophin-releasing hormone
- hCG:
-
Human chorionic gonadotrophin
- IVF:
-
In vitro fertilization
- ICM:
-
Inner cell mass
- TE:
-
Trophectoderm
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- E2 :
-
Estradiol
- OR:
-
Odds ratio
- CI:
-
Confidence intervals
- NC:
-
Natural cycle
- LPS:
-
Luteal phase support
- LBR:
-
Live birth rate
- CPR:
-
Clinical pregnancy rate
- TDR:
-
Twin delivery rate
- SET:
-
Single embryo transfer
- DET:
-
Double embryo transfer
- eSBT:
-
Elective single blastocyst transfer
References
Practice Committee of Society for Assisted Reproductive Technology. Practice Committee of American Society for Reproductive Medicine. Elective single-embryo transfer. Fertil Steril. 2012;97:835–42.
Practice Committee Of American Society For Reproductive Medicine. Multiple gestation associated with infertility therapy: an American Society for Reproductive Medicine Practice Committee opinion. Fertil Steril. 2012;97:825–34.
Kamath MS, Mascarenhas M, Kirubakaran R, Bhattacharya S. Number of embryos for transfer following in vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database Syst Rev. 2020;8:CD3416.
Chen S, Du H, Liu J, Liu H, Li L, He Y. Live birth rate and neonatal outcomes of different quantities and qualities of frozen transferred blastocyst in patients requiring whole embryo freezing stratified by age. BMC Pregnancy Childbirth. 2020;20:655.
Devine K, Connell MT, Richter KS, Ramirez CI, Levens ED, DeCherney AH, et al. Single vitrified blastocyst transfer maximizes liveborn children per embryo while minimizing preterm birth. Fertil Steril. 2015;103:1454–60.
Kaing A, Kroener LL, Tassin R, Li M, Liu L, Buyalos R, et al. Earlier day of blastocyst development is predictive of embryonic euploidy across all ages: essential data for physician decision-making and counseling patients. J Assist Reprod Genet. 2018;35:119–25.
Practice Committee of the American Society for Reproductive Medicine and the Practice Committee for the Society for Assisted Reproductive Technologies. Electronic address: ASRM@asrm.org. Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril. 2021;116:651–4.
Chen X, Zhang J, Wu X, Cao S, Zhou L, Wang Y, et al. Trophectoderm morphology predicts outcomes of pregnancy in vitrified-warmed single-blastocyst transfer cycle in a Chinese population. J Assist Reprod Genet. 2014;31:1475–81.
Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307–11.
Niinimaki M, Suikkari AM, Makinen S, Soderstrom-Anttila V, Martikainen H. Elective single-embryo transfer in women aged 40–44 years. Hum Reprod. 2013;28:331–5.
Fujimoto A, Morishima K, Harada M, Hirata T, Osuga Y, Fujii T. Elective single-embryo transfer improves cumulative pregnancy outcome in young patients but not in women of advanced reproductive age. J Assist Reprod Genet. 2015;32:1773–9.
McLernon DJ, Harrild K, Bergh C, Davies MJ, de Neubourg D, Dumoulin JCM, et al. Clinical effectiveness of elective single versus double embryo transfer: meta-analysis of individual patient data from randomised trials. BMJ. 2010;341:c6945.
Sazonova A, Källen K, Thurin-Kjellberg A, Wennerholm U, Bergh C. Neonatal and maternal outcomes comparing women undergoing two in vitro fertilization (IVF) singleton pregnancies and women undergoing one IVF twin pregnancy. Fertil Steril. 2013;99:731–7.
Bergh C. Single embryo transfer: a mini-review. Hum Reprod. 2005;20:323–7.
van Loendersloot LL, van Wely M, PM JL, van der Veen BSR. Predictive factors in in vitro fertilization (IVF): a systematic review and meta-analysis. Hum Reprod Update. 2010;16:577–89.
van Loendersloot LL, Moolenaar LM, van Wely M, Repping S, Bossuyt PM, Hompes PGA, et al. Cost-effectiveness of single versus double embryo transfer in IVF in relation to female age. Eur J Obstet Gynecol Reprod Biol. 2017;214:25–30.
Mullin CM, Fino ME, Talebian S, Krey LC, Licciardi F, Grifo JA. Comparison of pregnancy outcomes in elective single blastocyst transfer versus double blastocyst transfer stratified by age. Fertil Steril. 2010;93:1837–43.
Tannus S, Son W, Dahan MH. Elective single blastocyst transfer in advanced maternal age. J Assist Reprod Genet. 2017;34:741–8.
Eum JH, Park JK, Kim SY, Paek SK, Seok HH, Chang EM, et al. Clinical outcomes of single versus double blastocyst transfer in fresh and vitrified-warmed cycles. Clin Exp Reprod Med. 2016;43:164.
Forman EJ, Hong KH, Franasiak JM, Scott RT. Obstetrical and neonatal outcomes from the BEST trial: single embryo transfer with aneuploidy screening improves outcomes after in vitro fertilization without compromising delivery rates. Am J Obstet Gynecol. 2014;210:151–7.
Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Fetal Diagn Ther. 2011;29:263–73.
Maheshwari A, Griffiths S, Bhattacharya S. Global variations in the uptake of single embryo transfer. Hum Reprod Update. 2010;17:107–20.
Chambers GM, Illingworth PJ, Sullivan EA. Assisted reproductive technology: public funding and the voluntary shift to single embryo transfer in Australia. Med J Aust. 2011;195:594–8.
Acknowledgements
The authors gratefully acknowledge all the medical staff in the Women’s Hospital of Nanjing Medical University and Changzhou Maternal and Child Health Care Hospital for their support. The authors also thank Hong Lv and Xin Chen for statistical guidance.
Funding
Supported by the National Natural Science Foundation of China under Grant 81971386 and 81871210.
Author information
Authors and Affiliations
Contributions
YXZ and HJ contributed to patient selection, data collection and interpretation, and drafting of the publication. MQZ, JJZ, and XL contributed to the study data interpretation. JQZ and XFL contributed to the study design and data interpretation. CZ and LC critically corrected and reviewed the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was performed in compliance with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Women’s Hospital of Nanjing Medical University (NJFY-2020-KY-070) and the Ethics Committee of Changzhou Maternal and Child Health Care Hospital (2020 No.75). All methods were carried out following the relevant guidelines and regulations. The need for written informed consent was exempted by the ethics committee of Women’s Hospital of Nanjing Medical University and Changzhou Maternal and Child Health Care Hospital due to the study’s retrospective design.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Y., Ji, H., Zhang, M. et al. Single versus double blastocyst transfer in first and second frozen-thawed embryo transfer cycle in advance-aged women: a two-center retrospective cohort study. BMC Women's Health 24, 51 (2024). https://doi.org/10.1186/s12905-023-02753-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-023-02753-x