Abstract
Purpose
Hyperglycemia is affected by lifestyle and genetic factors. We investigated if dietary patterns associate with glycemia in individuals with high or low genetic risk for type 2 diabetes (T2D).
Methods
Men (n = 1577, 51–81 years) without T2D from the Metabolic Syndrome in Men (METSIM) cohort filled a food-frequency questionnaire and participated in a 2-hour oral glucose tolerance test. Polygenetic risk score (PRS) including 76 genetic variants was used to stratify participants into low or high T2D risk groups. We established two data-driven dietary patterns, termed healthy and unhealthy, and investigated their association with plasma glucose concentrations and hyperglycemia risk.
Results
Healthy dietary pattern was associated with lower fasting and 2-hour plasma glucose, glucose area under the curve, and better insulin sensitivity (Matsuda insulin sensitivity index) and insulin secretion (disposition index) in unadjusted and adjusted models, whereas the unhealthy pattern was not. No interaction was observed between the patterns and PRS on glycemic measures. Healthy dietary pattern was negatively associated with the risk for hyperglycemia in an adjusted model (OR 0.69, 95% CI 0.51–0.95, in the highest tertile), whereas unhealthy pattern was not (OR 1.08, 95% CI 0.79–1.47, in the highest tertile). No interaction was found between diet and PRS on the risk for hyperglycemia (p = 0.69 for healthy diet, p = 0.54 for unhealthy diet).
Conclusion
Our findings suggest that healthy diet is associated with lower glucose concentrations and lower risk for hyperglycemia in men with no interaction with the genetic risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes (T2D) is increasing globally and is one of the leading causes of morbidity and mortality in the world [1]. It is preceded by worsening glycemia i.e. prediabetes, which itself is a health hazard [2, 3]. Hyperglycemia (prediabetes or T2D) can be successfully prevented or delayed with lifestyle modification (diet and physical activity) [4, 5]. Genetic factors also worsen glycemia [6,7,8].
Diet is one of the key elements affecting the risk of T2D and hyperglycemia [9]. Dietary patterns describe a typical diet, with a focus on total diet, accounting all foods and beverages, instead of focusing on single foods or nutrients. Healthy dietary patterns, often characterized by high consumption of vegetables, fruits and berries, fish, vegetable oils, whole-grain products, and low-fat dairy, have been associated with lower plasma glucose concentrations, lower risk for hyperglycemia and lower incidence of T2D [10,11,12,13,14,15,16,17,18,19]. Accordingly, unhealthy dietary pattern has been associated with worsening of glycemia and the risk for T2D [13, 18, 20,21,22].
In addition to dietary causes, T2D has a strong genetic background. Whether the response to diet is similar between those with low and high genetic risk is currently limitedly known. There are studies that have investigated the role of single genetic variants or single foods and nutrients on glycemia. E.g. the relationship of dietary fats and carbohydrates on T2D risk may be dependent on glucose-dependent insulinotropic polypeptide receptor genotype [23], and the effect of dietary fibre and whole-grain on glycemia may depend on genetic variants, especially transcription factor-7-like 2 variant [24,25,26,27]. Benefits of fruit intake may be modified by genetic risk score [28]. Meat consumption and sugar-sweetened beverages have been linked to higher fasting glucose and insulin concentrations regardless of the polygenic risk score (PRS) [29, 30]. A meta-analysis modelled that replacement of 5 E% of carbohydrates from refined starch and sugars with polyunsaturated fatty acids lowered the risk for T2D without interaction with the PRS [31].
Previous studies investigating the association of glycemic effects of dietary patterns and genetic risk scores are inconclusive. In the Malmö Diet and Cancer Cohort study, the dietary choices were associated with T2D risk independently of the genetic risk score [32, 33]. Similarly, the EPIC-InterAct consortium found no significant interaction between diet and the PRS on T2D risk [34, 35]. A meta-analysis concluded that healthy diet is associated with decreased fasting glucose and insulin concentrations without interaction with fasting glucose and insulin-related loci [36]. However, some previous studies have indicated that hyperglycemic or protective effects of diet are influenced by the PRS. Health Professionals Follow-up Study found a significant interaction between the high genetic risk and unhealthy, western diet on T2D risk [37]. Similar results have been found in Korean population [38]. A large study from the UK Biobank showed that the protective effect of diet is modified by PRS; especially those in the highest risk benefited from healthy diet [39].
The aim of this study was to establish data-driven dietary patterns and investigate their effect on plasma glucose concentration and the risk for hyperglycemia in middle-aged to elderly Finnish men without prior T2D taking also account of the genetic risk for T2D.
Methods
Study participants
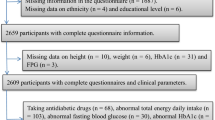
Study participants (n = 1577) were Finnish men, aged 51–81 years, attending to the Metabolic Syndrome in Men (METSIM) cohort [40]. A total of 2077 men had a 2-hour oral glucose tolerance test (OGTT), and they filled a food frequency questionnaire (FFQ) during 2016–2018 (Fig. 1). Exclusion criteria were previously diagnosed type 1 or type 2 diabetes, missing relevant dietary data or failed OGTT measurements.
Clinical assessment
Anthropometric measurements, OGTT procedure and laboratory methods used in this study have been previously described [41]. We defined hyperglycemia as either having prediabetes (fasting plasma glucose (FPG) 5.6–6.9 mmol/l, 2-hour plasma glucose (2-h PG) 7.8–11.0 mmol/l, or HbA1C 5.7–6.4%) or diabetes (FPG ≥ 7.0 mmol/l, 2-h PG ≥ 11.1 mmol/l), or HbA1C ≥ 6.5% (≥ 48 mmol/mol)), according to American Diabetes Association diagnostic criteria [42]. For insulin sensitivity and β-cell function assessment, we calculated Matsuda insulin sensitivity index (Matsuda ISI) and disposition index (DI) as described earlier [41]. Leisure time exercise was divided into two groups, physically active, meaning physical exercise regularly at least 30 min week, and physically inactive with little or no leisure time exercise or physical exercise in context of other hobbies or physical exercise occasionally. For current smoking status we included those who smoke cigarettes, cigars and/or pipes.
Assessment of diet
We assessed dietary habits using a self-administered qualitative food frequency questionnaire (Online Resource 1, Supplementary Information 1). The questionnaire is a slightly modified version from the FFQ used as a part of Finrisk 2007 National Health Survey basic questionnaire [43]. Consumption frequency of 40 foods were asked using an 8-scale question with reply choices: consumption less than once per month or never, 1–3 times per month, once per week, 2–4 times per week, 5–6 times per week, once per day, 2–3 times per day, or over 4 times per day. Bread consumption was asked for three different bread types using an 8-scale reply choices: less than 1 slice per day or none, 1 slice per week, 2–4 slices per week, 5–6 slices per week, 1 slice per day, 2–3 slices per day, 4–5 slices per day, or 6 or more slices per day. The filling of the questionnaire was instructed by a registered nurse or a clinical nutritionist, and the participants had the possibility to ask for help in filling the questionnaire while attending a study visit.
Genotyping and genetic risk groups
For genotyping, we used either HumanOmniExpress BeadChip-12v1 (Illumina, San Diego, CA, USA; 733,202 markers) or HumanExome-12v1.1 Beadchip (Illumina, 247,870 markers), as described earlier by the METSIM study group [44]. We calculated PRS based on the 76 genetic variants associated with T2D risk up until 2016 [45, 46]. In the analyses including PRS, we compared low and high risk groups (n = 736 and n = 699, respectively).
Statistical analysis
We used SPSS (version 27, IBM Corp., Armonk, NY, USA) in the statistical analysis, and R (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria) for creating figures. Outcome measurements were concentrations of FPG and 2-h PG, glucose area under the curve (AUC), Matsuda ISI, disposition index, and incidence hyperglycemia.
We used principal components analysis (PCA) to suppress the total of 43 dietary variables into fewer and to create dietary patterns. After assessing the applicability of PCA with Kaiser-Meyer-Olkin Measure of Sampling Adequacy (KMO) and Bartlett’s test of sphericity, with the KMO value of 0.791 and Bartlett’s test of p < 0.001, we used PCA with orthogonal (varimax) rotation, with small coefficients with absolute values below 0.3 suppressed. Using Eigenvalue above 1 and PCA scree plot to determine the fixed number of factors, we further extracted 2 dietary components, explaining 17.5% of total cumulative variance in food consumption (Online Resource 2, Supplementary Table 1). We classified the patterns as “healthy” and “unhealthy” (explaining 7.5% and 10% of the variance in food consumption, respectively). Each participant received a value for both dietary patterns.
We then tested how these two dietary components are associated with the glucose variables using linear regression. We performed the statistical analyses with an unadjusted model and adjusted models including body mass index (BMI), age, leisure time exercise (in two categories), current smoking, and total alcohol consumption (g/week). To assess whether the response to dietary pattern is affected by the genetic risk of T2D, we also stratified the participants into the low and high PRS groups. Interaction between the diet and PRS was analysed using a two-way between groups ANOVA.
We used Kolmogorov-Smirnov test to assess the normality. Glucose variables were logarithmically transformed for linear regression and ANOVA analyses. We used untransformed variables for ANOVA to calculate the effect sizes to retain clinically explicable values. We performed a binary logistic regression to assess how the consumption frequency of both healthy or unhealthy foods affect the risk of hyperglycemia (prediabetes or T2D diagnosed at the study visit). For this, we created the tertiles of both healthy and unhealthy food consumption patterns based on subject’s factor scores and used the lowest consumption tertile as the reference category. In addition to unadjusted model including food consumption pattern only, we used models including food consumption pattern, age, BMI, leisure time exercise, current smoking status, and total alcohol consumption. We repeated the analyses stratified with the PRS and looked at the dietary interaction between PRS and dietary pattern. P-values < 0.05 were considered statistically significant for all analyses.
Results
Characteristics of the participants
A total of 26.6% (n = 420) of participants were normoglycemic, and 73.4% (n = 1157) hyperglycemic (of which 898 participants with isolated impaired fasting glucose (IFG), 21 with isolated impaired glucose tolerance (IGT), 157 with both, and 81 had a new diabetes at study visit). Study participants were middle-aged to elderly, mean age was 63.8 years (SD 6.2) and mean BMI 27.2 kg/m2 (SD 3.7) (Table 1). The tertiles of dietary pattern for both healthy and unhealthy differed in age, weight, BMI, waist-hip-ratio, leisure time exercise and total alcohol consumption.
Participants in the highest healthy dietary pattern tertile were younger, leaner, exercised more and consumed less alcohol than participants in the lower tertiles. The opposite was true for unhealthy dietary pattern. In the healthy pattern, participants in dietary pattern tertiles also differed for concentrations of triglycerides and HDL cholesterol, diastolic blood pressure, and smoking. For unhealthy pattern, tertile differences were significant for a total and LDL cholesterol concentrations, and systolic blood pressure. Low (n = 736) and high (n = 699) genetic risk groups had statistically significant differences in BMI and waist-to-hip ratio.
Associations between dietary patterns and plasma glucose concentrations
The healthy dietary pattern was associated with lower concentrations of FPG (β=-0.053, p = 0.044), 2-h PG (β=-0.100, p < 0.001), lower plasma glucose AUC (β=-0.101, p < 0.001), higher Matsuda ISI (β = 0.098, p < 0.001) and higher DI (β = 0.065, p = 0.014) even after adjusting with BMI, age, leisure time exercise, smoking, and alcohol consumption (Table 2). The unhealthy dietary pattern showed no associations after adjustments.
After stratification into the low and high PRS groups and adjusting with BMI, age, leisure time exercise, smoking, and alcohol consumption, we still found an association of healthy dietary pattern with lower 2-h PG, lower glucose AUC, and higher Matsuda ISI in low PRS group, and with higher Matsuda ISI and DI in high PRS group (Table 3).
We found no interaction between the diet and PRS on the blood glucose variables on either healthy or unhealthy dietary pattern (Fig. 2). Both low and high PRS groups seem to behave in a similar manner across different tertiles of healthy dietary pattern.
The interaction between dietary patterns (consumption tertiles) and polygenetic risk score (PRS, low and high groups) on glucose variables (means), p-values for interaction from two-way between groups ANOVA. P-values < 0.05 are considered statistically significant. PRS polygenic risk score; Glucose AUC glucose area under the curve; Matsuda ISI Matsuda insulin sensitivity index. 1 High in fresh salad, fresh vegetables; fresh or frozen berries; boiled side vegetables; fruits; oil-based salad dressing or oil with vegetables; fish and fish dishes; chicken, turkey and chicken dishes; unsweetened or artificially sweetened yoghurt (including dairy-, oat-, soy- and rice-based products), quark, Nordic sour milk, or skyr (≤ 1% fat); vegetable dishes; whole grain porridges; whole grain pasta or rice; low-fat cheeses (fat ≤ 17%); boiled or mashed potatoes. 2 High in fried potatoes or French fries; sausage dishes, sausages; hamburgers; pizza; refined pasta or rice; other sweet pastries; sausage cutleries; other candy; savory pies and pastries; savory snacks; ice cream or puddings; French roll, baquette, or other white bread; sweet cookies, biscuits; meat dishes; ready-meals; other cheeses; sweetened yoghurt (including dairy-, oat-, soy- and rice-based products), quark, or Nordic sour milk (> 1% fat); sour cream based salad dressing
Risk for hyperglycemia
Highest tertile of healthy dietary pattern was associated with lower risk for hyperglycemia when all participants were considered (odds ratio (OR) 0.61, 95% CI 0.46–0.80, p < 0.001) as compared to the lowest tertile (Fig. 3). The lower risk remained statistically significant after adjusting with BMI, age, leisure time exercise, smoking, and alcohol consumption (OR 0.69, 95% CI 0.51–0.94, p = 0.021). For the middle tertile, there was no significant difference in the hyperglycemia risk in either unadjusted or adjusted models. When we compared the highest tertile of unhealthy dietary pattern to the lowest tertile, we saw no significant results in either unadjusted or fully adjusted models.
Dietary patterns and the risk of hyperglycemia (= prediabetes or newly diagnosed type 2 diabetes) in all participants. Model 1 = unadjusted values, Model 2 = adjusted with BMI, Model 3 = adjusted with BMI, age, leisure time exercise, smoking, and total alcohol consumption. P-values < 0.05 are considered statistically significant. 1 High in fresh salad, fresh vegetables; fresh or frozen berries; boiled side vegetables; fruits; oil-based salad dressing or oil with vegetables; fish and fish dishes; chicken, turkey and chicken dishes; unsweetened or artificially sweetened yoghurt (including dairy-, oat-, soy- and rice-based products), quark, Nordic sour milk, or skyr (≤ 1% fat); vegetable dishes; whole grain porridges; whole grain pasta or rice; low-fat cheeses (fat ≤ 17%); boiled or mashed potatoes. 2 High in fried potatoes or French fries; sausage dishes, sausages; hamburgers; pizza; refined pasta or rice; other sweet pastries; sausage cutleries; other candy; savory pies and pastries; savory snacks; ice cream or puddings; French roll, baquette, or other white bread; sweet cookies, biscuits; meat dishes; ready-meals; other cheeses; sweetened yoghurt (including dairy-, oat-, soy- and rice-based products), quark, or Nordic sour milk (> 1% fat); sour cream based salad dressing
After stratification by PRS, we saw that the healthy dietary pattern was associated with lower risk of hyperglycemia in both PRS groups in an unadjusted model (OR 0.62, 95% CI 0.42–0.92, p = 0.018, for low GRS, and OR 0.60, 95% CI 0.40–0.91, p = 0.017, for high GRS) (Fig. 4). After adjusting with BMI alone, or with BMI, age, leisure time exercise, smoking, and alcohol consumption, the association was no longer significant. Unhealthy diet was not associated with hyperglycemia risk in either PRS group. PRS did not seem to modify the effect of dietary patterns on the hyperglycemia risk (P interaction 0.69 for healthy diet, and P interaction 0.54 for unhealthy diet).
Risk for hyperglycemia in low and high polygenetic risk score (PRS) group in the highest dietary pattern tertiles. Model 1 = unadjusted values, Model 2 = adjusted with BMI, Model 3 = adjusted with BMI, age, leisure time exercise, smoking, and total alcohol consumption. P-values < 0.05 are considered statistically significant. PRS polygenic risk score. 1 High in fresh salad, fresh vegetables; fresh or frozen berries; boiled side vegetables; fruits; oil-based salad dressing or oil with vegetables; fish and fish dishes; chicken, turkey and chicken dishes; unsweetened or artificially sweetened yoghurt (including dairy-, oat-, soy- and rice-based products), quark, Nordic sour milk, or skyr (≤ 1% fat); vegetable dishes; whole grain porridges; whole grain pasta or rice; low-fat cheeses (fat ≤ 17%); boiled or mashed potatoes. 2 High in fried potatoes or French fries; sausage dishes, sausages; hamburgers; pizza; refined pasta or rice; other sweet pastries; sausage cutleries; other candy; savory pies and pastries; savory snacks; ice cream or puddings; French roll, baquette, or other white bread; sweet cookies, biscuits; meat dishes; ready-meals; other cheeses; sweetened yoghurt (including dairy-, oat-, soy- and rice-based products), quark, or Nordic sour milk (> 1% fat); sour cream based salad dressing
Discussion
Our cross-sectional study suggests that healthy dietary pattern is associated with lower plasma glucose concentrations and lower risk for hyperglycemia in middle-aged and elderly Finnish men. These results remained statistically significant after adjusting for confounding factors.
When the participants were stratified by their polygenetic risk score, healthy diet was still associated with lower plasma glucose concentrations in both low and high risk groups. The significance remained even after adjusting with BMI for all variables except for FPG in the low PRS group. After adjusting with age and other lifestyle factors (exercise, smoking and alcohol consumption), the healthy dietary pattern was associated with lower 2-h PG, gluc AUC, and higher Matsuda in the low PRS group, and with higher Matsuda and DI in the high PRS group. Both PRS groups seem to benefit from a healthy diet. There was no association between the unhealthy pattern and plasma glucose concentrations.
Healthy dietary pattern was associated with lower risk of hyperglycemia in both low and high genetic risk groups in an unadjusted model. A significant association was, however, lost after the adjustment for BMI. This may imply that the effects of a healthy dietary pattern in the hyperglycemia risk are mediated by BMI. Since adjusting with lifestyle factors beyond diet, such as smoking, exercise, and alcohol consumption, erases the statistical significance, it may be that the benefits of a healthy diet are confounded by an overall healthier lifestyle. The lack of statistical significance after the adjustments can also be due to lack of power when the study population has been divided into smaller groups. Our results suggest that focusing on adding healthy food choices is more essential than avoidance of unhealthy choices.
We found no interaction between the dietary patterns and the PRS in plasma glucose concentrations or in the risk of hyperglycemia. This suggests that the genetic risk does not modify the possible effects of diet.
Similarly to our data-driven pattern, foods such as fruits and berries, vegetables, non-tropical vegetable oils, whole grain products, fish, and low-fat dairy products, load into many established patterns such as Mediterranean Diet Score (MDS), Dietary Approaches to Stop Hypertension (DASH), Healthy Eating Index (HEI), Healthy Nordic Food Index (HNFI) and Baltic Sea Diet Score (BSDS) [47,48,49,50,51]. The two dietary patterns derived with PCA explained 17.5% of the food consumption variance. This level of explanatory value is in line with some similar data-driven dietary pattern studies [16, 37]. Since the principal component analysis aims to distinguish differences in dietary habits, there can be dietary components that are either beneficial or harmful for glucose metabolism that are not shown in the patterns. The dietary patterns identified by principal component analysis only explain a portion of the variance in food consumption. There may be individual dietary components with potential benefits or harmful effects on glucose metabolism that the PCA did not capture.
Our results are in line with previous studies showing beneficial effects of healthy dietary patterns and improved plasma glucose concentration [10], decreased risk for prediabetes [14, 16, 18], and decreased risk for T2D [13,14,15, 17, 52,53,54,55]. There are also some studies showing that unhealthy dietary pattern is associated with inferior plasma glucose concentration [22] and greater risk of prediabetes [18] and T2D [13, 21]. Our study implies similar results, but the results were not statistically significant.
Some previous studies support our findings showing that PRS does not affect the impact of diet on plasma glucose levels [29, 30, 36] or risk of T2D [31,32,33,34]. A few studies, however, report that the dietary impact on T2D risk may have interactions with the genetics [23, 28, 35, 37, 38], and that especially those in the highest genetic risk might benefit from healthy diet [39].
The strengths of the study include a rather large study population with oral glucose tolerance test enabling plasma glucose measurements beyond fasting levels, polygenetic risk score, and many variables measured that we were able to use to adjust for confounders.
The limitation of this study is its cross-sectional design. This allows us to make conclusions on associations without the possibility for interpreting causal relationships. Since our study was conducted in middle-aged Finnish men of Caucasian origin, the findings may not be extrapolated to other populations due to e.g. cultural, genetic, and lifestyle differences. Similar studies need to be conducted in females and participants of different ages and ethnicities. Our PRS stratified sample sizes may be inadequate to detect statistical differences across genetic groups. A retrospective food frequency questionnaire is based on the recall of the study participants, and self-reported dietary assessment methods are prone to misreporting [56]. Apart from bread consumption where the consumption was asked with quantitative measures, the qualitative questionnaire captures consumption frequencies of certain foods instead of measuring total food, energy, or nutrient intakes. We have no data on the amounts of foods consumed and there can be aspects of healthy diet that are not captured in this paper due to this limitation. The questionnaire covers a range of typical foods consumed in Finland and can be used to rank participants’ dietary habits. It also measures the usual diet from the past 12 months, which is a rather long period covering seasonal changes in the diet. The questionnaire is, however, a non-exhaustive list of foods consumed, which limits the components included in the dietary patterns. Even with adjustment with covariates such as BMI, age, exercise, smoking, and alcohol consumption, we cannot rule out possible residual confounders that may mediate the results found. We acknowledge the potential risk of multiple testing error, which is always present when more than one endpoint is being tested.
Our results support the existing literature on the protective effect of healthy dietary pattern against hyperglycemia. In addition to reduced risk for T2D, healthy dietary patterns have been shown to reduce risk for cardiovascular diseases, cancer, bone health, mental health, obesity, and premature death [57].
Conclusion
Our study suggests that a healthy dietary pattern, consisting of foods such as vegetables, fruits and berries, fish, whole-grain products, non-tropical vegetable oils, low-fat yoghurt, poultry, and potatoes, is associated with better plasma glucose concentrations and decreased risk for hyperglycemia.There was no interaction between diet and genetic risk score; it seems that the genetic risk for T2D does not modify the effects of a healthy diet. This highlights the importance of a healthy diet for everyone in maintaining healthy glycemia and preventing T2D. These findings can be used for lifestyle counseling and healthcare planning in diabetes prevention. Further studies with prospective and intervention designs, and with different ethnicities, genders, and ages, are still needed.
Data availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve the confidentiality of the participants. The corresponding author will, on request, detail the restrictions and any conditions under which access to some data may be provided.
Abbreviations
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- E%:
-
Percentage of total energy
- FFQ:
-
Food frequency questionnaire
- FPG:
-
Fasting plasma glucose
- HbA1C :
-
Hemoglobin A1C
- HDL:
-
High-density lipoprotein
- IFG:
-
Impaired fasting glucose
- IGT:
-
Impaired glucose tolerance
- KMO:
-
Kaiser-Meyer-Olkin measure of sampling adequacy
- LDL:
-
Low-density lipoprotein
- Matsuda ISI:
-
Matsuda insulin sensitivity index
- METSIM:
-
Metabolic syndrome in men
- OGTT:
-
Oral glucose tolerance test
- OR:
-
Odds ratio
- PCA:
-
Principal component analysis
- PG:
-
Plasma glucose
- PRS:
-
Polygenetic risk score
- SD:
-
Standard deviation
- T2D:
-
Type 2 diabetes
References
IDF Diabetes Atlas (2022) 10th edition. https://diabetesatlas.org/atlas/tenth-edition/. Accessed 22
Edwards CM, Cusi K (2016) Prediabetes: a Worldwide Epidemic. Endocrinol Metab Clin North Am 45:751–764. https://doi.org/10.1016/j.ecl.2016.06.007
Huang Y, Cai X, Mai W et al (2016) Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ 355:i5953. https://doi.org/10.1136/bmj.i5953
Knowler WC, Barrett-Connor E, Fowler SE et al (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403. https://doi.org/10.1056/NEJMoa012512
Tuomilehto J, Lindström J, Eriksson JG et al (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350. https://doi.org/10.1056/NEJM200105033441801
Fuchsberger C, Flannick J, Teslovich TM et al (2016) The genetic architecture of type 2 diabetes. Nature 536:41–47. https://doi.org/10.1038/nature18642
Laakso M (2019) Biomarkers for type 2 diabetes. Mol Metab 27:S139–S146. https://doi.org/10.1016/j.molmet.2019.06.016
Vujkovic M, Keaton JM, Lynch JA et al (2020) Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet 52:680–691. https://doi.org/10.1038/s41588-020-0637-y
O’Hearn M, Lara-Castor L, Cudhea F et al (2023) Incident type 2 diabetes attributable to suboptimal diet in 184 countries. Nat Med 29:982–995. https://doi.org/10.1038/s41591-023-02278-8
Williams DEM, Prevost AT, Whichelow MJ et al (2000) A cross-sectional study of dietary patterns with glucose intolerance and other features of the metabolic syndrome. Br J Nutr 83:257–266. https://doi.org/10.1017/S0007114500000337
Fung TT, Schulze M, Manson JE et al (2004) Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med 164:2235–2240. https://doi.org/10.1001/archinte.164.20.2235
Montonen J (2005) Dietary patterns and the incidence of type 2 diabetes. Am J Epidemiol 161:219–227. https://doi.org/10.1093/aje/kwi039
Odegaard AO, Koh W-P, Butler LM et al (2011) Dietary patterns and incident type 2 diabetes in Chinese men and women: the Singapore Chinese Health Study. Diabetes Care 34:880–885. https://doi.org/10.2337/dc10-2350
Chiu THT, Huang H-Y, Chiu Y-F et al (2014) Taiwanese vegetarians and omnivores: Dietary Composition, Prevalence of Diabetes and IFG. PLoS ONE 9:e88547. https://doi.org/10.1371/journal.pone.0088547
Ericson U, Brunkwall L, Alves Dias J et al (2019) Food patterns in relation to weight change and incidence of type 2 diabetes, coronary events and stroke in the Malmö Diet and Cancer cohort. Eur J Nutr 58:1801–1814. https://doi.org/10.1007/s00394-018-1727-9
Ericson U, Brunkwall L, Hellstrand S et al (2020) A Health-Conscious Food Pattern is Associated with prediabetes and Gut Microbiota in the Malmö offspring study. J Nutr 150:861–872. https://doi.org/10.1093/jn/nxz293
den Braver NR, Rutters F, van der Spek ALJK et al (2020) Adherence to a food group-based dietary guideline and incidence of prediabetes and type 2 diabetes. Eur J Nutr 59:2159–2169. https://doi.org/10.1007/s00394-019-02064-8
Cao Y, Chen C, Cui L et al (2020) A population-based survey for dietary patterns and prediabetes among 7555 Chinese adults in urban and rural areas in Jiangsu Province. Sci Rep 10:10488. https://doi.org/10.1038/s41598-020-67028-z
Aas A-M, Axelsen M, Churuangsuk C et al (2023) Evidence-based European recommendations for the dietary management of diabetes. Diabetologia 66:965–985. https://doi.org/10.1007/s00125-023-05894-8
van Dam RM, Rimm EB, Willett WC et al (2002) Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med 136:201–209. https://doi.org/10.7326/0003-4819-136-3-200202050-00008
Schulze MB, Hoffmann K, Manson JE et al (2005) Dietary pattern, inflammation, and incidence of type 2 diabetes in women2. Am J Clin Nutr 82:675–684. https://doi.org/10.1093/ajcn.82.3.675
Doostvandi T, Bahadoran Z, Mozaffari-Khosravi H et al (2016) Food intake patterns are associated with the risk of impaired glucose and insulin homeostasis: a prospective approach in the Tehran lipid and glucose study. Public Health Nutr 19:2467–2474. https://doi.org/10.1017/S1368980016000616
Sonestedt E, Lyssenko V, Ericson U et al (2012) Genetic variation in the glucose-dependent insulinotropic polypeptide receptor modifies the association between carbohydrate and fat intake and risk of type 2 diabetes in the Malmo Diet and Cancer cohort. J Clin Endocrinol Metab 97:E810–818. https://doi.org/10.1210/jc.2011-2444
Fisher E, Boeing H, Fritsche A et al (2008) Whole-grain consumption and transcription factor-7-like 2 (TCF7L2) rs7903146: gene–diet interaction in modulating type 2 diabetes risk. Br J Nutr 101:478–481. https://doi.org/10.1017/S0007114508020369
Hindy G, Sonestedt E, Ericson U et al (2012) Role of TCF7L2 risk variant and dietary fibre intake on incident type 2 diabetes. Diabetologia 55:2646–2654. https://doi.org/10.1007/s00125-012-2634-x
Hindy G, Mollet IG, Rukh G et al (2016) Several type 2 diabetes-associated variants in genes annotated to WNT signaling interact with dietary fiber in relation to incidence of type 2 diabetes. Genes Nutr 11:6. https://doi.org/10.1186/s12263-016-0524-4
Eriksen R, Gibson R, Aresu M, Endocrinology et al (2019) Diabetes Metab 2:e00074. https://doi.org/10.1002/edm2.74
Jia X, Xuan L, Dai H et al (2021) Fruit intake, genetic risk and type 2 diabetes: a population-based gene–diet interaction analysis. Eur J Nutr 60:2769–2779. https://doi.org/10.1007/s00394-020-02449-0
Fretts AM, Follis JL, Nettleton JA et al (2015) Consumption of meat is associated with higher fasting glucose and insulin concentrations regardless of glucose and insulin genetic risk scores: a meta-analysis of 50,345 caucasians. Am J Clin Nutr 102:1266–1278. https://doi.org/10.3945/ajcn.114.101238
McKeown NM, Dashti HS, Ma J et al (2018) Sugar-sweetened beverage intake associations with fasting glucose and insulin concentrations are not modified by selected genetic variants in a ChREBP-FGF21 pathway: a meta-analysis. Diabetologia 61:317–330. https://doi.org/10.1007/s00125-017-4475-0
Merino J, Guasch-Ferré M, Ellervik C et al (2019) Quality of dietary fat and genetic risk of type 2 diabetes: individual participant data meta-analysis. BMJ 366:l4292. https://doi.org/10.1136/bmj.l4292
Ericson U, Hindy G, Drake I et al (2018) Dietary and genetic risk scores and incidence of type 2 diabetes. Genes Nutr 13:13. https://doi.org/10.1186/s12263-018-0599-1
Zhang S, Stubbendorff A, Olsson K et al (2023) Adherence to the EAT-Lancet diet, genetic susceptibility, and risk of type 2 diabetes in Swedish adults. Metabolism 141:155401. https://doi.org/10.1016/j.metabol.2023.155401
Langenberg C, Sharp SJ, Franks PW et al (2014) Gene-Lifestyle Interaction and Type 2 diabetes: the EPIC InterAct Case-Cohort Study. PLoS Med 11:e1001647. https://doi.org/10.1371/journal.pmed.1001647
Li SX, Imamura F, Schulze MB et al (2018) Interplay between genetic predisposition, macronutrient intake and type 2 diabetes incidence: analysis within EPIC-InterAct across eight European countries. Diabetologia 61:1325–1332. https://doi.org/10.1007/s00125-018-4586-2
Nettleton JA, Hivert M-F, Lemaitre RN et al (2013) Meta-analysis investigating associations between healthy diet and fasting glucose and insulin levels and modification by loci associated with glucose homeostasis in data from 15 cohorts. Am J Epidemiol 177:103–115. https://doi.org/10.1093/aje/kws297
Qi L, Cornelis MC, Zhang C et al (2009) Genetic predisposition, western dietary pattern, and the risk of type 2 diabetes in men. Am J Clin Nutr 89:1453–1458. https://doi.org/10.3945/ajcn.2008.27249
Kim DS, Kim BC, Daily JW, Park S (2018) High genetic risk scores for impaired insulin secretory capacity doubles the risk for type 2 diabetes in asians and is exacerbated by western-type diets. Diab/Metab Res Rev 34:e2944. https://doi.org/10.1002/dmrr.2944
Zhuang P, Liu X, Li Y et al (2021) Effect of Diet Quality and genetic predisposition on Hemoglobin A1c and type 2 diabetes risk: Gene-Diet Interaction Analysis of 357,419 individuals. Diabetes Care 44:2470–2479. https://doi.org/10.2337/dc21-1051
Laakso M, Kuusisto J, Stančáková A et al (2017) The metabolic syndrome in men study: a resource for studies of metabolic and cardiovascular diseases. J Lipid Res 58:481–493. https://doi.org/10.1194/jlr.O072629
Stančáková A, Javorský M, Kuulasmaa T et al (2009) Changes in insulin sensitivity and insulin release in relation to Glycemia and glucose tolerance in 6,414 Finnish men. Diabetes 58:1212–1221. https://doi.org/10.2337/db08-1607
American Diabetes Association (2013) Diagnosis and classification of diabetes Mellitus. Diabetes Care 37:S81–S90. https://doi.org/10.2337/dc14-S081
Finnish Institute for Health and Welfare The National FINRISK study. Questionnaires. https://thl.fi/documents/189940/4850942/finriski2007questionnaire.pdf/ee4e02c0-1cde-4474-9d4d-9e55697007b5. Accessed 25 Sep 2023
Stančáková A, Kuulasmaa T, Kuusisto J et al (2017) Genetic risk scores in the prediction of plasma glucose, impaired insulin secretion, insulin resistance and incident type 2 diabetes in the METSIM study. Diabetologia 60:1722–1730. https://doi.org/10.1007/s00125-017-4313-4
Mohlke KL, Boehnke M (2015) Recent advances in understanding the genetic architecture of type 2 diabetes. Hum Mol Genet 24:R85–R92. https://doi.org/10.1093/hmg/ddv264
Zeggini E, Scott LJ, Saxena R et al (2008) Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 40:638–645. https://doi.org/10.1038/ng.120
Trichopoulou A, Kouris-Blazos A, Wahlqvist ML et al (1995) Diet and overall survival in elderly people. BMJ 311:1457–1460
Appel LJ, Moore TJ, Obarzanek E et al (1997) A clinical trial of the effects of Dietary patterns on blood pressure. N Engl J Med 336:1117–1124. https://doi.org/10.1056/NEJM199704173361601
Shams-White MM, Pannucci TE, Lerman JL et al (2023) Healthy eating Index-2020: review and update process to reflect the Dietary guidelines for americans, 2020–2025. J Acad Nutr Dietetics 123:1280–1288. https://doi.org/10.1016/j.jand.2023.05.015
Olsen A, Egeberg R, Halkjær J et al (2011) Healthy aspects of the nordic Diet are related to lower total Mortality1,2. J Nutr 141:639–644. https://doi.org/10.3945/jn.110.131375
Kanerva N, Kaartinen NE, Schwab U et al (2014) The Baltic Sea Diet score: a tool for assessing healthy eating in nordic countries. Public Health Nutr 17:1697–1705. https://doi.org/10.1017/S1368980013002395
Liese AD, Nichols M, Sun X et al (2009) Adherence to the DASH Diet is inversely Associated with incidence of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care 32:1434–1436. https://doi.org/10.2337/dc09-0228
Tertsunen H-M, Hantunen S, Tuomainen T-P, Virtanen JK (2021) Adherence to a healthy Nordic diet and risk of type 2 diabetes among men: the Kuopio Ischaemic Heart Disease risk factor study. Eur J Nutr 60:3927–3934. https://doi.org/10.1007/s00394-021-02569-1
Khalili-Moghadam S, Mirmiran P, Bahadoran Z, Azizi F (2019) The Mediterranean diet and risk of type 2 diabetes in Iranian population. Eur J Clin Nutr 73:72–78. https://doi.org/10.1038/s41430-018-0336-2
Cespedes EM, Hu FB, Tinker L et al (2016) Multiple healthful dietary patterns and type 2 diabetes in the women’s Health Initiative. Am J Epidemiol 183:622–633. https://doi.org/10.1093/aje/kwv241
Poslusna K, Ruprich J, De Vries JHM et al (2009) Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr 101:S73–S85. https://doi.org/10.1017/S0007114509990602
Nordic Council of Ministers (2023) Nordic Nutrition recommendations 2023 - integrating environmental aspects. https://doi.org/10.6027/nord2023-003
Acknowledgements
We thank the METSIM study participants and personnel, especially Teemu Kuulasmaa, for their contributions to the data acquisition of this paper. We thank Petrus Nuotio for his help in creating figures.
Funding
METSIM study was supported by grants from the Academy of Finland (321428), Sigrid Juselius Foundation, Finnish Foundation for Cardiovascular Research, Kuopio University Hospital, and Centre of Excellence of Cardiovascular and Metabolic Diseases, supported by the Academy of Finland (to Markku Laakso). The contribution by Ulla Tolonen was supported by Finnish Cultural Foundation (North Savo Regional Fund, Kerttu and Oiva Turunen trust), State Research funding (to Ursula Schwab), and the Doctoral School of Health Sciences (Faculty of Health Sciences, University of Eastern Finland).
Open access funding provided by University of Eastern Finland (including Kuopio University Hospital).
Author information
Authors and Affiliations
Contributions
Conceptualization: Ulla Tolonen, Maria Lankinen, Markku Laakso, Ursula Schwab. Formal analysis: Ulla Tolonen. Investigation: Ulla Tolonen, Maria Lankinen, Markku Laakso, Ursula Schwab. Resources: Markku Laakso. Writing – original draft: Ulla Tolonen. Writing – editing & reviewing: Maria Lankinen, Markku Laakso, Ursula Schwab. Visualization: Ulla Tolonen. Project administration: Markku Laakso. Funding acquisition: Ulla Tolonen, Markku Laakso, Ursula Schwab. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
METSIM Study was approved by the Ethics Committee of the Kuopio University Hospital (number: 174/2004; approval: 29 November 2004) and is conducted in accordance with the Helsinki Declaration.
Informed consent
All study participants gave written informed consent.
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tolonen, U., Lankinen, M., Laakso, M. et al. Healthy dietary pattern is associated with lower glycemia independently of the genetic risk of type 2 diabetes: a cross-sectional study in Finnish men. Eur J Nutr (2024). https://doi.org/10.1007/s00394-024-03444-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00394-024-03444-5