Abstract
Background
Genitourinary syndrome of menopause (GSM) comprises genital symptoms (dryness, burning, itching, irritation, bleeding), sexual symptoms (dyspareunia and other sexual dysfunctions) and urinary symptoms (dysuria, frequency, urgency, recurrent urinary infections) associated with menopause. To avoid invasive testing and painful physical examinations, validated questionaries, which can assess the prevalence and risk factors associated with symptoms of GSM. We aimed to investigate the prevalence and risk factors associated with GSM in middle-aged and older women in the communities of Beijing, China.
Methods
A cross-sectional, questionnaire study was performed among 35–70 years old Chinese woman. Vaginal health index score and urinary distress inventory (UDI-6) was used to evaluate vulvovaginal atrophy (VVA) and urinary incontinence (UI). Stages of pelvic organ prolapse (POP) was measured during gynecological examination with POP-Q system. Mean ± standard deviation (SD) and proportion/percentages were used to summarize continuous and categorial variables respectively. The Bonferroni method was used to adjust for multiple comparisons.
Results
A total of 2702/3000 participants completed the questionnaire survey. The mean ± SD age of participants was 53.7 ± 7.0 years and prevalence of VVA among participants was 34.8% (941/2702). In UDI-6 questionnaires total 47.5% (1284/2702) participants reported experiencing urinary incontinence (UI). Further, POP was highly prevalent in anterior vaginal wall 38.9% (1050/2702) followed by posterior vaginal wall 25.3% (683/2702) and uterine 22.2% (599/2702). Besides, multiple logistic regression analysis inferred older age (45–54 years [OR (95% CI): 3.38 (2.03, 5.64)]; 55–64 years [OR (95% CI): 8.63 (5.09, 14.64)]), menopause [OR (95% CI): 2.20 (1.71, 2.85)] and Faecal Inconsistence (FI) [OR (95% CI): 1.31(1.00, 1.72)] as independent risk factors for VVA.
Conclusions
Our study evidenced that GSM is prevalent in old age Chinese women. GSM is related with UI, POP and VVA. Further older age, menopause and FI were risk factors associated with VVA. Our findings could help health care personnel to get a comprehensive overview of factors associated with VVA and urinal distress, which may facilitate early detection and prevention of GSM.
Similar content being viewed by others

Background
Menopause is a condition resulting from estrogen deficiency [1]. The average age of natural menopause is 48.9 years in Chinese woman and it evidences that one-third of women's life is spent in menopause and post menopause [2]. General menopausal symptoms include physical, genitourinary and psychological symptoms, such as mood swings, hot flashes, bone and joint symptoms, and urogenital discomfort [3]. Genitourinary syndrome of menopause (GSM) is a relatively new term, first introduced in 2014 by the international society for the study of women’s sexual health and the North American menopause society and describes various menopausal symptoms [4]. The new definition of GSM comprises genital symptoms (dryness, burning, itching, irritation, bleeding), sexual symptoms (dyspareunia and other sexual dysfunctions) and urinary symptoms (dysuria, frequency, urgency, recurrent urinary infections) associated with menopause [5]. It describes the constellation of lower urogenital tract signs and symptoms associated with a low-estrogen state, such as vulvovaginal atrophy (VVA) and urinary incontinence (UI). GSM have great impact on both premenopausal & postmenopausal women health but it mostly occurs during the menopausal transition [6, 7].
The prevalence of symptoms of GSM has been reported in about 30% of Chinese mid-life women [8]. Further, diagnosis of GSM is challenging because, 50% of postmenopausal women with mild or moderate GSM are asymptomatic [9]. It has been documented that UI and VVA are directly associated with GSM [10, 11]. Moreover, GSM increases the likelihood of pelvic organ prolapse (POP) (ureteral and vaginal prolapse) [12]. GSM is still an under-reported problem because of limitations of previous studies like limited sample size, specific ethnical homogeneity of participants, biased selection criteria (general women were not examined only gynaecological OPD patients were examined), and hard to recruit women having an interest in study participation [7, 13]. Besides, diagnosis of GSM includes physical pelvic examination, vaginal pH and invasive testing such as vulval, vaginal and urethral anatomical biopsies [14]. To avoid invasive testing and painful physical examinations, validated questionaries, which can assess the prevalence and risk factors associated with symptoms of GSM are much warranted [7]. Early detection of GSM can avoid the long-term risks and complications that may severely compromise quality of life and sexuality of a women [15]. There are few large scale sample epidemiological studies on GSM in Chinese population [14, 16]. Therefore, the current study was aimed to investigate the prevalence and risk factors associated with GSM in middle-aged and older women in the communities of Beijing, China.
Methods
Study design and participants
In this cross-sectional study, Chinese woman (35–70 years of age) who voluntarily participated in cervical cancer and breast cancer screening tests within the administrative division of Beijing No. 2 Hospital and the Yuetan Community Health Service Center under the Fuxing Hospital of Capital Medical University were invited for a questionnaire survey from October to December 2016. After informing the content and significance of the study, a written informed consent according to the Declaration of Helsinki was obtained from the participants. The study was approved by the medical ethics committee of the Peking University People's Hospital (Code number: 2015PHB087-01).
Study measures
Socio-demographic survey questionnaire
The study questionnaires collected the information about socio-demographic characteristics of participants including age, educational level, menstrual status/condition, menopausal hormone replacement therapy (HRT), physical disease (high blood pressure, heart disease, hyperthyroidism, hypothyroidism, Diabetes Mellitus, arthritis, dyslipidemia, tumor), gynaecological surgery, body mass index (BMI), parturition mode and income level by certified medical staff.
Assessment of GSM-questionnaire
A validated and simple questionnaire was used to asses GSM. The following associated symptoms were assessed.
Menopause/perimenopause We defined ‘menopause’ as 12 months of amenorrhea (without any other explanations). Perimenopause is considered as persistent difference of 7 or more days in the length of consecutive cycles (persistence meant 10-cycle- recurrence from the first variable length cycle) [17, 18].
Vaginal health index Vaginal health index score (VHIS) was evaluated based on following parameters: elasticity, fluid volume, pH, epithelial integrity and moisture. The score of each parameter varied between 1 (worst condition) and 5 (best condition), the maximum possible score was 25 and the minimum possible score was 0. If the total score is < 15, it is considered that there is vulvovaginal atrophy (VVA) [19]. Participants were also inquired about the presence of VVA symptoms: vaginal pain, dyspareunia, vaginal pruritus and vaginal burning [1].
Urinary distress continence Urinary distress was evaluated by UDI-6 [20, 21] UDI-6 consists of 6 questions: 1 – Frequent urination, 2 – Leakage related to feeling of urgency, 3 – Leakage related to activity, 4 –Coughing, or sneezing small amounts of leakage (drops), 5 – Difficulty emptying the bladder, and 6 – Pain or discomfort in the lower abdominal or genital area. Based on above questions, Urinary incontinence was classified into three categories: Stress urinary incontinence (SUI) - (questions 3 and 4), Urgency urinary incontinence (UUI) (questions 1 and 2) and Mixed Urinary Inconsistence (MUI)- (questions 1,3 and 5,6).
Faecal Inconsistence (FI) Fecal incontinence was assessed by following questions: (1) Do you usually lose stool beyond your control if your stool is well formed? (2) Do you usually lose stool beyond your control if your stool is loose? (3) Do you usually lose gas from the rectum beyond your control? Subjects responded with either “none (0), slight (1), moderate (2), or greatly (3)” [22].
Pelvic organ prolapses (POP) POP refers to descent of one or more of anterior vaginal wall, posterior vaginal wall, the uterus (cervix), or the apex of the vagina. The POP stage of the participants was evaluated during gynecological examinations via lithotomy position by Pelvic Organ Prolapse Quantification system (POP-Q [23, 24]. We defined POP as Stage I–IV, which meant the most distal portion of the prolapse was > 1 cm above the level of the hymen (i.e., its quantitation value was < − 1 cm). The investigators were trained to form a professional team familiar with the contents of questionnaire and POP-Q system.
The questionnaires took approximately 20 min to complete, and the investigators checked the questionnaires after collection to guarantee the quality of the responses.
Statistical analysis
Mean ± standard deviation (SD) and proportion/percentages were used to summarize continuous and categorial variables respectively. Comparison of two or more groups of categorial variables was performed by chi-square. p < 0.05 was considered as statistically significant. The Bonferroni method was used to adjust for multiple comparisons. When two of the three groups were compared, the Bonferroni method was used, and p < 0.001 was considered statistically significant. Multiple logistic regression analysis was used to analyze the risk factors of urinary distress and POP. SPSS 19.0 software was used for statistical analysis of data.
Results
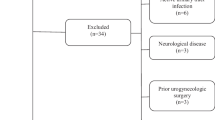
A total of 3000 women participated in this survey out of which 2702 completed the questionnaire (response rate 90.1%), 107(3.6%) copies of questionnaire were missing due to human or system errors, 11(0.4%) copies were excluded because participants were not ready to disclose the GSM results, 91 (3.0%) participants were rejected because they had undergone hysterectomy and 89 (2.9%) participants were excluded because they had undergone both modes of parturition (vaginal and caesarean section).
Demographic characteristics
The demographic characteristics of the participants are presented in Table 1. The mean age of participants was 53.7 ± 7.0 years. More than half of the participants were menopausal (58.5%) and the mean age of menopausal women was 50.0 ± 3.1 years. All the participants had not undergone any surgery or treatment for POP.
Prevalence and risk factors associated with symptoms of genitourinary GSM
The mean VHIS score of the participants was 15.1 ± 3.4 (elasticity 2.97 ± 0.77, moisture 2.98 ± 0.76, pH 3.02 ± 0.73, epithelial integrity 3.11 ± 0.68, fluid volume 3.00 ± 0.79). Overall, 34.8% (941/2,702) of the participants enrolled in the study suffered from VVA and the most prevalent symptoms of VVA includes vaginal pain 8.2% (222/2702), dyspareunia 15.5% (418/2702), vaginal pruritus 19.7% (533/2702) and vaginal local burning sensation 8.0% (215/2702). Further comparison of the VVA symptoms based on menopausal stage (Table 2) shown that prevalence of dyspareunia (17.8%) as most significant VVA symptom in menopausal women when compared to pre-menopausal (11.0%) or peri menopausal women (16.1%), p < 0.001. Moreover, multiple logistic regression analysis (Table 3) inferred older age (45–54 years [OR (95% CI): 3.38 (2.03, 5.64)], p < 0.001; 55–64 years [OR (95% CI): 8.63 (5.09, 14.64)], p < 0.001); menopause [OR (95% CI): 2.20 (1.71, 2.85)], p < 0.001; gynecological diseases [OR (95% CI): 1.61(1.28, 2.03)], p < 0.001 and higher education level (College or bachelor degree[OR (95% CI): 1.70 (1.30, 2.23)], p < 0.001) as significant risk factors for VVA.
Furthermore, among the 2702 subjects, 47.5% (1284/2702) experienced UI, among whom 24.8% (671/2702) experienced UUI, 40.1% (1084/2702) experienced SUI and 18.8% (508/2702) reported MUI. Among the urinary distress symptoms, the top three were urine leakage related to coughing, sneezing, or laughing (40.1%; 1084/2702), frequent urination (27.4%; 741/2702), urine leakage associated with a feeling of urgency (24.8%; 671/2702). In addition, 12.3% (333/2702) reported experiencing FI. Besides, multiple logistic regression analysis (Table 4) demonstrated that physical disease [OR (95% CI) 1.538 (1.295, 1.8280], p < 0.001; menopause drugs application [OR (95% CI): 1.79 (1.44, 2.22)], p < 0.001 and FI [OR (95% CI): 8.303(5.55, 12.40)], p < 0.001 were significant risk factors for urinary distress.
Additionally, we investigated the prevalence of different types of POP among participants and the results obtained shown higher prevalence of anterior vaginal wall prolapse 38.9% (1050/2702) followed by posterior vaginal wall prolapse 25.3% (683/2702) and uterine prolapse 22.2% (599/2702). Further, comparison based on parturition modes (Table 5) shown that the prevalence of POP is higher in cesarean section group 47.7% (327/686) as compared to vaginal delivery group or nullipara group.
Discussion
To the best of our knowledge, this is the largest cross- sectional study to evaluate the prevalence and risk factors associated with symptoms of GSM in Chinese women of Beijing community. Current study evidenced that 34.8% of the enrolled participants suffered from VVA. Further, comparison of the VVA symptoms based on menopausal status revealed dyspareunia (17.8%) as most significant VVA symptom in menopausal women when compared to pre-menopausal (11.0%) or peri menopausal women (16.1%) p < 0.001. With an exception of study population from different regions these findings were in agreement with earlier studies reporting the prevalence of VAA. Earlier, Nappi et al., conducted a structured online questionnaire study by including around 3520 postmenopausal women aged 55–65 years and reported 45% prevalence of VAA [25]. Later, Lin et.al conducted a retrospective study involving 3450 perimenopausal women reported a prevalence of VVA at 12.4% [26]. The prevalence of VVA in the current survey was higher than the Lin’s study, which might be attributed to the participants “older age’. Besides, this finding could be attributed to those women unable to discuss about VAA because of personal embarrassment and cultural reasons. Further, the expression of "GSM" is more implicit than the word "VVA", which is more conducive to patients seeking for treatment. Further, in the current study we have found postmenopausal women were more likely to develop VVA, which may be due to the different composition of the vaginal microbiota in the vagina and estrogenic effect on vaginal environment.
Also, UDI-6 can accurately and credibly assess the severity of urinary distress and their impacts on the quality of life of pelvic floor disorder women, as well as assess the general women [27, 28]. In the current study, around 47.5% experienced UI and the results obtained were lower than the previous reported studies where, Gyhagen et al. conducted a registry-based national cohort study by including 5236 participants reported that 63% women had UI [29]. These differences might be due to sample size or due to inclusion of study population from different regions. Moreover, in the present study among all symptoms, SUI (40.1%), occurred more frequently. However, earlier studies reported the SUI occurs at a frequency of 22–25% [30, 31]. The reason for this disparity is that in our study, we were aiming at middle-aged and older women, where as other studies has included women aged over 20 years. In addition, aging and age-related changes in the bladder might play a significant role in the development of UI.
Similarly, delivery mode had a significant impact on the UI, which might be attributed to more damage of pelvic floor tissue caused by vaginal delivery. Our study evidenced that cesarean section as protective factor for urinary distress [7]. These findings are in agreement with earlier reports where, Rortveit’s et al. [32] reported that the risk of UI was higher among women who had vaginal deliveries than among women who have had cesarean sections. Moreover, multivariate logistic regression analysis revealed that physical disease, gynecological diseases, menopause drugs application, POP and FI as independent risk factors for urinary distress. These findings are in consistent with previous reports where, Legendre et.al conducted a review of literature dealing with the epidemiology of UI in women and reported that oral HRT had a detrimental effect on SUI and had less effect on UI [33]. In addition, Zhu et al. [34], conducted a cross-sectional survey with approximately, 20,000 Chinese women reported that perimenopause and post menopause status were potential risk factors for SUI. Moreover, recent studies reported that the presence urinary incontinence increases the risk for fecal incontinence ranging between 2-and 4-fold [35].
Despite these noteworthy observations, the current study has certain limitations. Firstly, the study lacks temporality due to cross-sectional design which limits causal inference. Therefore, longitudinal studies are needed for clarification. Secondly, we selected only two communities in Beijing, hence limited sample size could not completely represent the situation of all women in Beijing and limits the generalizability of results.
To conclude the current study provided an insight on the factors associated with GSM. Similar studies are needed to further investigate the factors associated with prevalence of GSM in women from all provinces of China.
Conclusion
VVA was found to be more prevalent (34.8%) among middle-aged and older Chinese women. POP was highly prevalent in interior vaginal wall followed by posterior vaginal wall. Older age, menopause, higher education level and gynecological diseases were found to be significant risk factors and dyspareunia was most significant symptom associated with VVA. Our findings would help health care personnel to communicate easily with patients and improve their understanding about GSM. Clinicians can enquire about the GSM risk factors during routine gynecological examination, which would help in early diagnosis and treatment of GSM.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sarmento ACA, Costa APF, Vieira-Baptista P, Giraldo PC, Eleutério J, Gonçalves AK. Genitourinary syndrome of menopause: epidemiology, physiopathology, clinical manifestation and diagnostic. Front Reprod Health. 2021;3:779398.
Age at natural menopause and associated factors in adult women: Findings from the China Kadoorie Biobank study in Zhejiang rural area - PubMed. https://pubmed.ncbi.nlm.nih.gov/29668705/. Accessed 19 Sep 2022.
Chuni N, Sreeramareddy CT. Frequency of symptoms, determinants of severe symptoms, validity of and cut-off score for menopause rating scale (MRS) as a screening tool: a cross-sectional survey among midlife Nepalese women. BMC Womens Health. 2011;11:30.
Portman DJ, Gass MLS. Vulvovaginal atrophy terminology consensus conference panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International society for the study of women’s sexual health and the North American menopause society. Menopause N Y N. 2014;21:1063–8.
Addressing Vulvovaginal Atrophy (VVA)/Genitourinary Syndrome of Menopause (GSM) for Healthy Aging in Women - PubMed. https://pubmed.ncbi.nlm.nih.gov/31496993/. Accessed 19 Sep 2022.
Angelou K, Grigoriadis T, Diakosavvas M, Zacharakis D, Athanasiou S. The genitourinary syndrome of menopause: an overview of the recent data. Cureus. 2020;12: e7586.
Ruan X, Zhang L, Cui Y, Gu M, Mueck AO. Genitourinary syndrome of menopause in Chinese perimenopausal and postmenopausal women. Climact J Int Menopause Soc. 2021;24:297–304.
The prevalence and determinants of genitourinary syndrome of menopause in Chinese mid-life women: a single-center study - PubMed. https://pubmed.ncbi.nlm.nih.gov/29734845/. Accessed 19 Sep 2022.
Cureus|The Genitourinary Syndrome of Menopause: An Overview of the Recent Data.
Kołodyńska G, Zalewski M, Rożek-Piechura K. Urinary incontinence in postmenopausal women causes symptoms treatment. Przegla̜d Menopauzalny Menopause Rev. 2019;2019(18):46–50.
Nappi RE, Seracchioli R, Salvatore S, Cagnacci A, Di Paolantonio T, Busacca M, et al. Impact of vulvovaginal atrophy of menopause: prevalence and symptoms in Italian women according to the EVES study. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2019;35:453–9.
Hillery S. The impact of genitourinary syndrome of menopause on continence. Br J Nurs Mark Allen Publ. 2020;29:342–4.
Moral E, Delgado JL, Carmona F, Caballero B, Guillán C, González PM, et al. Genitourinary syndrome of menopause. Prevalence and quality of life in Spanish postmenopausal women. The GENISSE study. Climact J Int Menopause Soc. 2018;21:167–73.
Geng L, Zheng Y, Zhou Y, Li C, Tao M. The prevalence and determinants of genitourinary syndrome of menopause in Chinese mid-life women: a single-center study. Climact J Int Menopause Soc. 2018;21:478–82.
Gandhi J, Chen A, Dagur G, Suh Y, Smith N, Cali B, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;215:704–11.
Srisukho S, Pantasri T, Piyamongkol W, Phongnarisorn C, Morakote N. The experience of genitourinary syndrome of menopause (GSM) among Thai postmenopausal women: the non-reporting issue. Int Urogynecol J. 2019;30:1843–7.
Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause N Y N. 2012;19:387–95.
Delamater L, Santoro N. Management of the perimenopause. Clin Obstet Gynecol. 2018;61:419–32.
Alvisi S, Gava G, Orsili I, Giacomelli G, Baldassarre M, Seracchioli R, et al. Vaginal health in menopausal women. Medicina (Mex). 2019;55:615.
Mikuš M, Ćorić M, Matak L, Škegro B, Vujić G, Banović V. Validation of the UDI-6 and the ICIQ-UI SF - Croatian version. Int Urogynecol J. 2020;31:2625–30.
Wang X, Jin Y, Xu P, Feng S. Urinary incontinence in pregnant women and its impact on health-related quality of life. Health Qual Life Outcomes. 2022;20:13.
Lee JT, Madoff RD, Rockwood TH. Quality-of-life measures in fecal incontinence: is validation valid? Dis Colon Rectum. 2015;58:352–7.
Persu C, Chapple C, Cauni V, Gutue S, Geavlete P. Pelvic organ prolapse quantification system (POP–Q)—a new era in pelvic prolapse staging. J Med Life. 2011;4:75–81.
Ai F, Deng M, Mao M, Xu T, Zhu L. Depressive symptoms screening in postmenopausal women with symptomatic pelvic organ prolapse. Menopause N Y N. 2018;25:314–9.
Nappi RE, Kokot-Kierepa M. Vaginal health: insights, views & attitudes (VIVA)—results from an international survey. Climact J Int Menopause Soc. 2012;15:36–44.
Lin Y. Analysis of disease survey results in 3450 postmenopausal women. China J Ctrl Endem Dis. 2014;29:84.
Gafni-Kane A, Goldberg RP, Sand PK, Botros SM. Enhanced interpretability of the PFDI-20 with establishment of reference scores among women in the general population. Neurourol Urodyn. 2012;31:1252–7.
de Villiers TJ, Pines A, Panay N, Gambacciani M, Archer DF, Baber RJ, et al. Updated 2013 international menopause society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climact J Int Menopause Soc. 2013;16:316–37.
Gyhagen M, Åkervall S, Milsom I. Clustering of pelvic floor disorders 20 years after one vaginal or one cesarean birth. Int Urogynecol J. 2015;26:1115–21.
Hannestad YS, Rortveit G, Sandvik H, Hunskaar S, Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. J Clin Epidemiol. 2000;53:1150–7.
Zhu L, Lang J, Wang H, Han S, Liu C. The study on the prevalence and associated risk factors of female urinary incontinence in Beijing women. Zhonghua Yi Xue Za Zhi. 2006;86:728–31.
Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S, Norwegian EPINCONT Study. Urinary incontinence after vaginal delivery or cesarean section. N Engl J Med. 2003;348:900–7.
Legndre G, Ringa V, Fauconnier A, Fritel X. Menopause, Hormone Treatment and Urinary Incontinence at Midlife. Maturitas. 2013;74(1):26-30.
Zhu L, Lang J, Liu C, Han S, Huang J, Li X. The epidemiological study of women with urinary incontinence and risk factors for stress urinary incontinence in China. Menopause N Y N. 2009;16:831–6.
Varma MG, Brown JS, Creasman JM, Thom DH, Van Den Eeden SK, Beattie MS, et al. Fecal incontinence in females older than aged 40 years: who is at risk? Dis Colon Rectum. 2006;49:841–51.
Acknowledgements
The authors would like to acknowledge Dr. Shanky Garg and Dr. Amit Bhat of Indegene Pvt Ltd, India for their medical writing and editorial support.
Funding
The study was supported by the National Natural Science Foundation of China (No. U1903124).
Author information
Authors and Affiliations
Contributions
YZ has collected the data and wrote the manuscript. JW has collected and analyzed the data. XY have revised the manuscript. WZ has offered funding support. WZ has collected the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the medical ethics committee of the Peking University People's Hospital (Code number: 2015PHB087-01). The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Competing interests
The authors declare that they do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhu, Y., Wei, J., Yang, X. et al. Investigation on prevalence and risk factors associated with genitourinary syndrome of menopause in middle-aged and older women in Beijing community: a cross sectional study. BMC Women's Health 22, 558 (2022). https://doi.org/10.1186/s12905-022-02099-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-022-02099-w