Abstract
Background
Cervical adenosquamous carcinoma (ASC) was previously thought to be a subtype of cervical adenocarcinoma, but recent studies have found that the clinical features of the two diseases are different. Moreover, the pathological characteristics, survival, prognosis, and optimal ASC therapy remain unknown. This study aims to retrospectively analyze the postoperative survival of patients with early-stage ASC and to evaluate their condition after treatment with postoperative concurrent chemoradiotherapy (CCRT) and prophylactic irradiation of the para-aortic lymphatic drainage area.
Methods
This study enrolled 131 patients with pathologically confirmed ASC screened from 3502 patients with confirmed stage I–II cervical cancer diagnosis who had completed surgical treatments in our hospital. Among the 131 enrolled patients, 75 patients received CCRT, 33 patients received chemotherapy (CT), and 23 patients did not receive adjuvant treatment (named surgery alone (S alone). Of the 75 patients CCRT, 43 patients received prophylactic irradiation of the para-aortic lymphatic drainage area. The efficacy of the postoperative treatments of patients among groups (CCRT, CT, and S alone) was compared.
Results
The median follow-up time, age, and overall survival (OS) were 76 months, 43 years, and 74 months, respectively. The 3- and 5-year survival rates were 82% and 71.4%, respectively. The median disease-free survival (DFS) was 64 months. Cox regression analysis showed that postoperative adjuvant treatment modalities and positive lymph node metastases were associated with OS and DFS. Patients who received CCRT treatment had higher OS and DFS than those with CT and S alone. Prophylactic irradiation of the para-aortic lymphatic drainage area did not improve the OS and DFS of patients with CCRT treatment. However, further subgroup analysis suggested that it might improve survival rates in patients who had positive pelvic lymph nodes as confirmed by postoperative pathology.
Conclusion
Postoperative CCRT improved the survival rates in patients with early-stage ASC. The value of prophylactic irradiation of the para-aortic lymphatic drainage area remains debatable, but it may benefit patients with pelvic lymph node involvement.
Similar content being viewed by others
Background
Cervical cancer is a common gynecological malignancy, and contributes to 270,000 deaths annually [1]. Cervical cancer can be affected by multiple factors, such as human papillomavirus (HPV) infection, marriage at an early age, multiple childbirths, multiple sexual partners, and genetic factors. With cervical screening and the use of vaccines, cervical cancer can be prevented and controlled in a timely manner. The management of early-stage cancer is vital for improving patient prognosis and survival.
Cervical adenosquamous carcinoma (ASC) is a special histological type of cervical cancer with an extremely low incidence rate, accounting for only 5–10% of cervical cancer cases [2, 3]. The prognosis of ASC was first reported in 1956, and its formation was suggested to result from the concurrent differentiation and development of reserve cells into glandular and squamous cells, resulting in tumoral tissues with components of both adenocarcinoma and squamous cell carcinoma [4, 5]. Previous studies have suggested that ASC is a subtype of cervical adenocarcinoma. Since they have always been studied together as a whole [6, 7], no difference in outcomes between the two carcinomas has been described [8,9,10,11]. However, recent studies have demonstrated that the clinical characteristics of the two carcinomas are not the same. ASC exhibits a higher invasiveness and probability of lymph node metastasis, resulting in a worse prognosis than cervical adenocarcinoma [12,13,14]. One study has suggested that para-aortic lymph node metastasis is a major cause of cervical cancer relapse and that the uncontrolled rate of para-aortic lymph node involvement after routine concurrent chemoradiotherapy (CCRT) is 10–25% [15]. Therefore, it is believed that relapse in the para-aortic lymph node area may be a crucial factor affecting the prognosis of patients with cervical cancer. Although there are no standard principles for the treatment of this special pathological type of cervical cancer, the standard guidelines and criteria for the diagnosis and therapy of cervical cancer are often followed. Thus, the pathological characteristics, survival, prognosis, and optimal therapy for ASC remain unknown.
The present study aims to retrospectively analyze the postoperative survival of patients with early-stage ASC, investigate the appropriate postoperative adjuvant treatment modalities, and explore whether prophylactic irradiation of the para-aortic lymphatic drainage area affects survival.
Methods
Participants and data collection
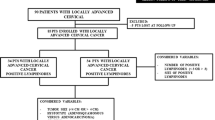
This retrospective study screened 3502 patients with early-stage cervical cancer (International Federation of Gynecology and Obstetrics [FIGO]2009 stage IB1-IIA2) who were admitted to Jiangxi Maternal and Child Health Hospital between January 2005 and September 2016. Patients with ASC, as confirmed by postoperative pathology, were enrolled in this study. Exclusions included other histological types, failure to undergo surgery, cervical conization, preoperative neoadjuvant chemotherapy (CT), postoperative adjuvant therapy in other medical centers, brachytherapy, a second primary cancer, and incomplete data. Finally, 131 patients were enrolled. The clinicopathologic characteristics, treatment modalities, and relapse patterns of the participants were then recorded. The protocols and contents of this research were approved by the ethical board of the Institute of the Jiangxi Maternal and Child Health Hospital. Written informed consent to participate in the study was obtained from all the patients.
Treatment methods
Of the 131 patients enrolled in the study, 120 patients had undergone the radical hysterectomy, 6 patients had the radical trachelectomy, 4 patients received total laparoscopic hysterectomy, and 1 had the patient received lesion resection. Based on surgical exploration and postoperative pathology, the high-risk factors were identified as positive parametrium, surgical margin, and lymph nodes, and the intermediate-risk factors as tumor diameter ≥ 4 cm, stromal invasion ≥ 1/2, and presence of lymphovascular space invasion. Patients with a high-risk factor or more than two intermediate-risk factors were treated with CCRT. Patients with the intermediate-risk factor vessel carcinoma embolus received chemotherapy alone or observation, and those with the intermediate-risk factor interstitial infiltration > 1/2 received pelvic radiotherapy or observation (no patients in this study chose radiotherapy). For patients with an intermediate risk factor, treatment was chosen according to the individual wishes of each patient. Patients who received radiotherapy met the Sedlis criteria as revealed by their postoperative pathology.
We used a Sweden Elekta linear accelerator for 6-MV X-rays with two-field irradiation or intensity modulated radiation therapy. The target volume included the vaginal stump, parametrium, and pelvic lymph node drainage area (common iliac, internal and external iliac, presacral, and obturator lymph nodes). For patients who have undergone radical resection, the status of para-aortic lymph nodes was determined according to the pathological results of intraoperative abdominal para-aortic lymph node dissection or biopsy and imaging features of initial diagnosis. If one of the results indicated that the para-aortic lymph node was positive, that lymph nodes was considered positive. Patients with positive common iliac or para-aortic lymph nodes received para-aortic extended-field irradiation. In this study, prophylactic para-aortic irradiation was performed in 43 patients without metastasis in the common iliac and para-aortic lymph nodes as shown by postoperative pathology and imaging (upper boundary: lower border of the left renal vein). The radiotherapy dose was 45 Gy, 1.8 Gy/session, for a total of 25 sessions. One patient with bulky pelvic lymph node metastases underwent a laparoscopic hysterectomy. Since the surgeons were not confident that the lesion was completely removed, they placed a titanium clip and suggested that the patient undergo a postoperative radiation boost. We administered a dose of radiotherapy of up to 60 Gy in the marked area, and postoperative magnetic resonance imaging did not indicate any obvious residual disease.
A total of 75 patients received concurrent CT. Among them, 32 received paclitaxel liposome (90 mg) and nedaplatin (30–35 mg/m2), 36 patients received docetaxel (25–30 mg/m2) + carboplatin (area under the plasma concentration time curve [AUC] = 2), 3 patients received a single dose of nedaplatin (35 mg/m2), and 4 patients received carboplatin (AUC = 3) on a weekly cycle. A total of 33 patients received adjuvant CT alone. Among them, 23 received docetaxel (75 mg/m2) + carboplatin (AUC = 5) and 10 patients received paclitaxel liposome (2 with 135 mg/m2 and 8 with 175 mg/m2) + nedaplatin (80 mg/m2), on a 3-week cycle for a total of 4 cycles. See Table 1.
Follow-up
The patients were followed up once every 3 months in post-treatment years 1–2, once every 6 months in years 3–5, and once every year thereafter. Medical records were reviewed for regular follow-ups, and telephone calls were made to patients for non-scheduled follow-ups. The data of patients lost to follow-up were censored from the date of loss to follow-up.
Statistical methods
Statistical analysis was performed using the SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA). The χ2 test, Kaplan–Meier method, and log-rank test were used for counting data, calculating the survival rates, and performing the intergroup comparisons. The statistical significance level was set at p < 0.05.
Results
Participant characteristics
A total of 3502 patients with early-stage cervical cancer (FIGO2009 stage IB1–IIA2) were included in this study. Among them, 141 patients with ASC were selected. Ten of the selected patients were excluded due to incomplete data or not receiving postoperative treatments in our hospital. Finally, 131 patients were enrolled. The median age of the participants was 43 years (range: 22–76 years). Of the enrolled patients, 98, 18, 13, and 2 participants had stage IB1, IB2, IIA1, and IIA2 cancer, respectively (baseline data are shown in Table 2).
Survival time and relapse
The median follow-up period and overall survival (OS) were 76 months (range: 4–156 months) and 74 months (95% CI: 44.79–103.21 months), respectively, while the 3-year and 5-year survival rates were 82% and 71.4%, respectively. The disease-free survival (DFS) was 64 months (95% CI: 40.88–87.13 months) and the 3-year and 5-year DFS rates were 69.8% and 51%, respectively. There were 26 cases of relapse (8 of local recurrence and 18 cases of distant metastasis). The cases of distant metastasis included 9 cases of lung metastasis, 4 of bone metastasis, 1 liver metastasis, 2 distant metastasis and 2 local recurrence, and distant metastasis at unknown sites (Fig. 1A, B).
Analysis of survival-related factors
The clinicopathological factors, such as FIGO staging, postoperative adjuvant therapy methods, lymphovascular space invasion, depth of cervical stromal invasion; tumor diameter, lymph node metastasis, and prophylactic irradiation of the para-aortic lymphatic drainage area were grouped, and their impacts on survival were analyzed. The Cox regression analysis results suggested that lymphovascular space invasion was only associated with DFS and did not appear to be related to OS (Table 3). Moreover, the results also indicated that postoperative adjuvant treatment modalities (95% CI: 1.530–2.592, p < 0.001 and 95% CI: 1.825–3.430, p < 0.001, respectively) and lymph node metastasis (95% CI: 0.150–0.584, p < 0.001 and 95% CI: 0.182–0.737, p = 0.050 respectively) were related to OS and DFS, respectively. (Table 3).
Survival following postoperative adjuvant therapy
We compared the postoperative adjuvant therapy methods (CCRT, CT and surgery[S] alone). The results demonstrated that, in terms of OS, CCRT (n = 75) was significantly superior to S alone (n = 23) (χ2 = 17.719, p < 0.001) and to CT (n = 33) (χ2 = 23.584, p < 0.001), but there was no difference between the patients who received CT or underwent S alone (χ2 = 0.012, p = 0.913). Similarly, for DFS, CCRT was significantly better than S alone (χ2 = 12.388, p < 0.001) and CT χ2 = 24.438, p < 0.001) on DFS, but there was no difference between the patients who received CT or underwent S alone (χ2 = 0.373, p = 0.541) (Fig. 2A, B).
A Overall survival curves of patients who received the three postoperative adjuvant therapy methods. B Disease-free survival curves of patients who received the three postoperative adjuvant therapy methods. Group 1, received concurrent chemoradiotherapy (CCRT); Group 2, only received chemotherapy (CT); and Group 3, received no therapy after surgery (S alone)
Survival following prophylactic para-aortic irradiation
In this study, 75 patients received CCRT. Among these, 43 patients received prophylactic para-aortic irradiation, and 32 patients did not receive prophylactic para-aortic irradiation. There was no statistical difference in improvement of OS between patients who did and did not receive prophylactic para-aortic irradiation (χ2 = 3.483; p = 0.062). Additionally, there was no significant difference in DFS between the patients with and without prophylactic para-aortic irradiation (χ2 = 1.845; p = 0.174) (Fig. 3A, B).
A Overall survival curves of patients who received prophylactic para-aortic irradiation. B Disease-free survival curves of patients who received prophylactic para-aortic irradiation. C Results of subgroup analysis of the positive pelvic lymph nodes. Overall survival rate curves of patients with positive lymph nodes with or without prophylactic irradiation of the para-aortic lymphatic drainage area. D Results of subgroup analysis of the positive pelvic lymph nodes. Disease-free survival rate curves of patients with positive lymph nodes with or without prophylactic irradiation of the para-aortic lymphatic drainage area
Results of the subgroup analysis
Overall, 57 patients were found to have positive nodes after undergoing a radical hysterectomy. Among these, 42, 10, and 5 patients received CCRT, CT and S alone respectively. The pelvic lymph node positivity was used as a stratification factor for subgroup analysis. The results indicated that the survival rate was significantly higher in patients who received prophylactic irradiation of the para-aortic lymphatic drainage area (n = 26) than in those patients who had not received prophylactic irradiation (n = 16) (χ2 = 4.350, p = 0.037). But there was no no significant difference in disease-free survival between these two patient groups (χ2 = 3.843, p = 0.050) (Fig. 3C, D).
Discussion
Cervical cancer is one of the most common malignancies among women worldwide. While this disease’s morbidity and mortality has significantly decreased due to the popularity of cervical screening [16], because screening is not widely available worldwide, cervical cancer continues to be a threat to patient health, especially in low-income and middle-income territories [1]. ASC, a rare subtype of cervical cancer, has been found to have a poor prognosis due to its high degrees of invasiveness and malignancy; therefore, the reasonable management of ASC is vital. The present study retrospectively analyzed the effects of postoperative management and adjuvant therapy on the outcomes of ASC patients.
With the development of diagnosis and treatment technology, the management of early cervical cancer is becoming increasingly effective. For example, screening procedures, especially when combined with HPV DNA tests, provide an opportunity to identify pre-cancerous lesions and increases the timely diagnosis of early cervical cancer [17]. Moreover, the detection accuracy of lymph node metastasis in early cervical cancer has been greatly improved [18]. However, no large-scale prospective study has explored this special pathological type of cervical cancer to date, because of the low incidence of cervical adenosquamous carcinoma. Most retrospective studies have conducted investigations that included cervical adenocarcinoma. Many studies have investigated the survival and prognostic significance of patients with early-stage ASC and cervical adenocarcinoma. We summarized some of these in Table 4, showing 65.8% to 100% of 5-year OS rates and 55.1% to 92.3% of 5-year DFS rates [2, 11, 19,20,21]. Our study showed that the median OS was 74 months, and the 3-and 5-year OS rates were 82% and 71.4%, respectively. The median DFS was 64 months, and the 3- and 5-year DFS rates were 69.8% and 51%, respectively. The survival rates of the patients in our study were lower than those in previous studies, which may be explained by the following: first, most of the previous studies had few cases of ASC, and second, the short follow-up time in our study may have led to statistical bias.
The treatment of cervical cancer is dictated the by FIGO staging system; in this system, the appropriate surgical and postoperative radiotherapy and chemotherapy approaches are recommended for patients with early-stage diseases. For patients with early-stage cervical cancer, the primary treatment choices are radical hysterectomy and pelvic lymphadenectomy, which are performed using minimally invasive surgical techniques (laparoscopy or robotics) [22, 23]. However, for patients of childbearing age, preservation of reproductive function is desirable [23]. Of the 131 patients with cervical ASC in our study, 124 patients (94.7%) underwent radical hysterectomy, 6 patients (4.6%) underwent fertility-preserving cervical canal resection, and 1 patient (0.7%) underwent resection of the lesion only.
Results of Cox regression analysis in our study indicated that postoperative CCRT is associated with survival, although, the therapeutic effectiveness of primary chemoradiation for cervical adenocarcinoma cancer and ASC is still unclear. Peter et al. reported that adenocarcinoma and ASC of the cervix are associated with worse OS when treated with radiation alone, but have similar progression free and overall survival compared to squamous cell carcinomas of the cervix when treated with cisplatin-based chemoradiation [24]. Lee et al. concluded that intermediate/high-risk patients with ASC may be successfully treated with postoperative CCRT [25]. In both studies, ASC was compared to squamous and adenocarcinoma rather than to itself, which may lead to bias.
Moreover, our study showed that 43 patients who received prophylactic para-aortic irradiation did not have improved survival rates. Although several studies have investigated the efficacy of prophylactic para-aortic irradiation for advanced stage cervical cancer [26,27,28,29,30,31], the value of prophylactic para-aortic irradiation remains controversial. Some studies claimed that prophylactic para-aortic irradiation improves survival and reduces the risk of recurrence [26,27,28], whereas the results from other studies supported contrary claims: prophylactic para-aortic irradiation does not reduce the risk of recurrence for patients with advanced-stage cervical carcinoma [29,30,31]. No randomized controlled study has explored the value of prophylactic para-aortic irradiation for treating ASC. In fact, our study is the first and largest sample to analyze the effects of different irradiation treatment modalities on survival.
Previous studies have found that FIGO stage, tumor diameter, lymphovascular space invasion and lymph node metastasis are independent prognostic factors for poor survival in patients with early-stage cervical cancer [11, 20, 32,33,34]. In the results of our study, lymph node metastasis rather than tumor diameter or depth of cervical stromal invasion was the independent prognostic factor for ASC. It is worth noting that in our study, pelvic lymph node metastasis status was used as a stratification factor for analysis, and the results demonstrated that the prophylactic irradiation of the para-aortic lymphatic drainage area improved survival rates in patients with positive pelvic lymph nodes. Interestingly, this is also the first study that specifically analyzed the impact of this radiotherapy modality on survival, which can serve as a reference in the clinical setting.
Overall, our study only included patients with early-stage ASC who had undergone surgery. Specifically, 130 of the 131 included patients received the standard surgery for cervical cancer. Moreover, the postoperative adjuvant therapy method was relatively standardized, as the chemotherapy regimen, irradiation, and radiotherapy were completed in the same unit. Thus, we ensured the therapy regimens’ consistency and excluded the interference of subsequent therapies. Nevertheless, our study has several limitations. First, we did not directly compare ASC, adenocarcinoma, and squamous cell carcinoma. Second, patients who received prophylactic para-aortic irradiation were not randomized, and most received surgeon’s recommendations extended-field irradiation based on their intraoperative findings. Third, the proportion of patients enrolled in this study before 2010 was low, and the role of CCRT in cervical cancer treatment has yet to be confirmed. Multicenter, randomized, large-sample prospective studies should be conducted to assess the efficacy of concurrent para-aortic irradiation for ASC.
Conclusion
In summary, the incidence of ASC was extremely low and postoperative adjuvant therapy and lymph node metastasis were independent prognostic factors in patients with early-stage ASC who underwent surgery. Furthermore, patient prognosis was improved by postoperative CCRT, which is likely to become a standard therapy. Prophylactic irradiation of the para-aortic lymphatic drainage area can reduce relapse in patients with positive pelvic lymph nodes. The data presented herein could potentially provide new perspectives on clinical treatment.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Abbreviations
- HPV:
-
Human papillomavirus
- ASC:
-
Adenosquamous carcinoma
- CCRT:
-
Concurrent chemoradiotherapy
- CT:
-
Chemotherapy
- AUC:
-
Area under the plasma concentration time curve
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
References
Small W Jr, Bacon MA, Bajaj A, Chuang LT, Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR, Viswanathan AN, Gaffney DK. Cervical cancer: a global health crisis. Cancer. 2017;123(13):2404–12.
Yasuda S, Kojima A, Maeno Y, Oki N, Miyahara Y, Sudo T, Takekida S, Yamaguchi S, Nishimura R. Poor prognosis of patients with stage Ib1 adenosquamous cell carcinoma of the uterine cervix with pelvic lymphnode metastasis. Kobe J Med Sci. 2006;52(1–2):9–15.
Takac I, Ursic-Vrscaj M, Repse-Fokter A, Kodric T, Rakar S, Mozina A, Smrkolj S, Primic-Zakelj M, Strzinar V, Vakselj A, Arko D. Clinicopathological characteristics of cervical cancer between 2003 and 2005, after the introduction of a national cancer screening program in Slovenia. Eur J Obstet Gynecol Reprod Biol. 2008;140(1):82–9.
Cherry CP, Glucksmann A. Incidence, histology, and response to radiation of mixed carcinomas (adenoacanthomas) of the uterine cervix. Cancer. 1956;9(5):971–9.
Le J. Obstetrics and gynecology. 7th ed. Beijing: People’s Medical Publishing House; 2008.
Mathew A, George PS. Trends in incidence and mortality rates of squamous cell carcinoma and adenocarcinoma of cervix–worldwide. Asian Pac J Cancer Prev. 2009;10(4):645–50.
Nakanishi T, Ishikawa H, Suzuki Y, Inoue T, Nakamura S, Kuzuya K. A comparison of prognoses of pathologic stage Ib adenocarcinoma and squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 2000;79(2):289–93.
Shingleton HM, Bell MC, Fremgen A, Chmiel JS, Russell AH, Jones WB, Winchester DP, Clive RE. Is there really a difference in survival of women with squamous cell carcinoma, adenocarcinoma, and adenosquamous cell carcinoma of the cervix? Cancer. 1995;76(10 Suppl):1948–55.
Meng YH, Li S, Hu T, Ma D, Lu YP, Wang H. Clinical analysis of 132 cases of cervical adenosquamous carcinoma and cervical adenocarcinoma. Chin J Cancer. 2010;29(1):15–9.
Rudtanasudjatum K, Charoenkwan K, Khunamornpong S, Siriaunkgul S. Impact of histology on prognosis of patients with early-stage cervical cancer treated with radical surgery. Int J Gynaecol Obstet. 2011;115(2):183–7.
Mabuchi S, Okazawa M, Kinose Y, Matsuo K, Fujiwara M, Suzuki O, Morii E, Kamiura S, Ogawa K, Kimura T. Comparison of the prognoses of FIGO stage I to stage II adenosquamous carcinoma and adenocarcinoma of the uterine cervix treated with radical hysterectomy. Int J Gynecol Cancer. 2012;22(8):1389–97.
Lea JS, Coleman RL, Garner EO, Duska LR, Miller DS, Schorge JO. Adenosquamous histology predicts poor outcome in low-risk stage IB1 cervical adenocarcinoma. Gynecol Oncol. 2003;91(3):558–62.
Farley JH, Hickey KW, Carlson JW, Rose GS, Kost ER, Harrison TA. Adenosquamous histology predicts a poor outcome for patients with advanced-stage, but not early-stage, cervical carcinoma. Cancer. 2003;97(9):2196–202.
Lee JY, Lee C, Hahn S, Kim MA, Kim HS, Chung HH, Kim JW, Park NH, Song YS. Prognosis of adenosquamous carcinoma compared with adenocarcinoma in uterine cervical cancer: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. 2014;24(2):289–94.
Gouy S, Morice P, Narducci F, Uzan C, Gilmore J, Kolesnikov-Gauthier H, Querleu D, Haie-Meder C, Leblanc E. Nodal-staging surgery for locally advanced cervical cancer in the era of PET. Lancet Oncol. 2012;13(5):e212–20.
Lei J, Andrae B, Ploner A, Lagheden C, Eklund C, Nordqvist Kleppe S, Wang J, Fang F, Dillner J, Elfstrom KM, Sparen P. Cervical screening and risk of adenosquamous and rare histological types of invasive cervical carcinoma: population based nested case-control study. BMJ. 2019;365:l1207.
Valenti G, Vitale SG, Tropea A, Biondi A, Lagana AS. Tumor markers of uterine cervical cancer: a new scenario to guide surgical practice? Updates Surg. 2017;69(4):441–9.
Rossetti D, Vitale SG, Tropea A, Biondi A, Lagana AS. New procedures for the identification of sentinel lymph node: shaping the horizon of future management in early stage uterine cervical cancer. Updates Surg. 2017;69(3):383–8.
Barquet-Munoz SA, Cruz-Rodriguez E, Cantu De Leon DF, Isla-Ortiz D, Montalvo-Esquivel G, Herrera-Montalvo LA, Perez-Plasencia C, Perez-Montiel D, Herrera-Gomez A. Histology as prognostic factor in early-stage cervical carcinoma, experience in a third-level institution. Rev Investig Clin. 2017;69(5):286–92.
Baek MH, Park JY, Kim D, Suh DS, Kim JH, Kim YM, Kim YT, Nam JH. Comparison of adenocarcinoma and adenosquamous carcinoma in patients with early-stage cervical cancer after radical surgery. Gynecol Oncol. 2014;135(3):462–7.
Twu NF, Ou YC, Liao CI, Chang WY, Yang LY, Tang YH, Chen TC, Chen CH, Chen TH, Yeh LS, Hsu ST, Chen YC, Chang CC, Cheng YM, Huang CY, Liu FS, Lin YS, Hsiao SM, Kan YY, Lai CH. Prognostic factors and adjuvant therapy on survival in early-stage cervical adenocarcinoma/adenosquamous carcinoma after primary radical surgery: a Taiwanese Gynecologic Oncology Group (TGOG) study. Surg Oncol. 2016;25(3):229–35.
Corrado G, Vizza E, Legge F, Pedone Anchora L, Sperduti I, Fagotti A, Mancini E, Gallotta V, Zampa A, Chiofalo B, Scambia G. Comparison of different surgical approaches for stage IB1 cervical cancer patients: a multi-institution study and a review of the literature. Int J Gynecol Cancer. 2018;28(5):1020–8.
Costales A, Michener C, Escobar-Rodriguez PF. Radical trachelectomy for early stage cervical cancer. Curr Treat Options Oncol. 2018;19(12):75.
Zhou J, Wu SG, Sun JY, Li FY, Lin HX, Chen QH, He ZY. Comparison of clinical outcomes of squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma of the uterine cervix after definitive radiotherapy: a population-based analysis. J Cancer Res Clin Oncol. 2017;143(1):115–22.
Lee JY, Lee C, Hahn SK, Kim HS, Chung HH, Kim JW, Park NH, Song YS. A comparison of adenosquamous carcinoma and adenocarcinoma of the cervix after radical hysterectomy. Gynecol Obstet Investig. 2015;80(1):15–20.
Meng Q, Wang W, Liu X, Hou X, Lian X, Sun S, Yan J, Liu Z, Miao Z, Hu K, Zhang F. Escalated radiation and prophylactic extended field nodal irradiation are beneficial for FIGO IIIB cervical cancer patients’ prognosis. Radiat Oncol. 2018;13(1):223.
Lee J, Lin JB, Chang CL, Jan YT, Sun FJ, Wu MH, Chen YJ. Prophylactic lower para-aortic irradiation using intensity-modulated radiotherapy mitigates the risk of para-aortic recurrence in locally advanced cervical cancer: A 10-year institutional experience. Gynecol Oncol. 2017;146(1):20–6.
Wakatsuki M, Kato S, Ohno T, Banu PA, Hoang NC, Yadamsuren E, Supriana N, Cao J, Devi CRB, Calaguas MJ, Chansilpa Y, Cho CK, Adylkhanov T, Okonogi N, Nakano T, Tsujii H. Multi-institutional observational study of prophylactic extended-field concurrent chemoradiation therapy using weekly cisplatin for patients with pelvic node-positive cervical cancer in east and Southeast Asia. Int J Radiat Oncol Biol Phys. 2019;105(1):183–9.
Yoshida K, Kajiyama H, Yoshihara M, Ikeda Y, Yoshikawa N, Nishino K, Utsumi F, Niimi K, Suzuki S, Kikkawa F. Does postoperative prophylactic irradiation of para-aortic lymph nodes reduce the risk of recurrence in uterine cervical cancer with positive pelvic lymph nodes? Int J Clin Oncol. 2019;24(5):567–74.
Oh J, Seol KH, Lee HJ, Choi YS, Park JY, Bae JY. Prophylactic extended-field irradiation with concurrent chemotherapy for pelvic lymph node-positive cervical cancer. Radiat Oncol J. 2017;35(4):349–58.
Park SG, Kim JH, Oh YK, Byun SJ, Kim MY, Kwon SH, Kim OB. Is prophylactic irradiation to para-aortic lymph nodes in locally advanced cervical cancer necessary? Cancer Res Treat. 2014;46(4):374–82.
Twu N-F, Ou Y-C, Liao C-I, Chang W-Y, Yang L-Y, Tang Y-H, Chen T-C, Chen C-H, Chen T-H, Yeh L-S. Prognostic factors and adjuvant therapy on survival in early-stage cervical adenocarcinoma/adenosquamous carcinoma after primary radical surgery: a Taiwanese Gynecologic Oncology Group (TGOG) study. Surg Oncol. 2016;25(3):229–35.
Kasamatsu T, Onda T, Sawada M, Kato T, Ikeda S, Sasajima Y, Tsuda H. Radical hysterectomy for FIGO stage I-IIB adenocarcinoma of the uterine cervix. Br J Cancer. 2009;100(9):1400–5.
Look KY, Brunetto VL, Clarke-Pearson DL, Averette HE, Major FJ, Alvarez RD, Homesley HD, Zaino RJ. An analysis of cell type in patients with surgically staged stage IB carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1996;63(3):304–11.
Acknowledgements
We would like to thank the patients and their families and all the investigators, including the physicians, nurses and workers in the study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LL designed the study, YWL collected the data and wrote the manuscript, LLZ and HYT finished the follow-up, MLZ and YNW performed the analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol and content of this research were approved by the Ethical board of the Institute of the Jiangxi Maternal and Child Health Hospital. Written informed consent to participate in the study was obtained from all the patients.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Y., Tu, H., Zhang, L. et al. The best postoperative adjuvant therapy for patients with early stage cervical adenosquamous carcinoma. BMC Women's Health 22, 112 (2022). https://doi.org/10.1186/s12905-021-01588-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-021-01588-8