Abstract
Objective
The gold standard for a soft tissue augmentation around implants is a subepithelial connective tissue graft (CTG), but the xenogeneic collagen matrices (XCM) started to be used as an alternative. This systematic review aimed to assess the effectiveness XCM in comparison to CTG for the increasing the thickness of the soft tissue around implants.
Data
All studies included at least two parallel groups comparing the use of CTG and XCM with a minimum follow-up of 3 months. As the primary outcome, the amount of soft tissue thickness gain after soft tissue augmentation with XCM or CTG was assessed. Secondary outcomes were clinical and patient-related outcomes; evaluation of aesthetic outcomes, patient-reported outcomes measures (PROMs) and complications. Eligible studies were selected based on the inclusion criteria. Meta-analysis was applied whenever possible. The quality of the evidence of studies including in meta-analysis was assessed using the GRADE approach.
Source
A systematic literature search up to January 2022 was conducted using the following electronic databases: PubMed (MEDLINE), Scopus, Cochrane Library, LILACS, eLIBRARY.RU. Unpublished researches, the gray literature, nonprofit reports, government studies and other materials were reviewed electronically using an EASY search. An additional manual search was carried out in November 2022.
Study selection
Of the 1376 articles from the initial search, 8 randomized controlled trials (RCTs) (306 patients and 325 implants) were included in this systematic review, and 7 studies were part of the meta-analysis. Meta-analysis revealed that XCM is less effective than the CTG in increasing soft tissue thickness around dental implants. However, XCM also provides soft tissue thickness gain and can be recommended for use in various clinical situations.
Clinical significance
Previous systematic reviews and meta-analyses have shown that autologous grafts are more effective than collagen matrices in increasing soft tissue thickness, however, the latter can be used as an alternative. Studies included in previous systematic reviews varied in design, which could lead to limitations. The present systematic review and meta-analysis includes for the first time only randomized controlled clinical trials with collagen matrix of xenogeneic origin in the test group. Tight eligibility criteria were established, and the main parameter studied was soft tissue thickness. It was found that xenogeneic collagen matrix is effective for increasing soft tissue thickness around dental implants, however, the results obtained using an autogenous connective tissue graft are superior.
Similar content being viewed by others
Introduction
Nowadays, the use of dental implants can be regarded as widespread and predictable procedure for the replacement of missing teeth [1]. The presence of sufficient amount of bone and soft tissues surrounding dental implants allows attaining long-term outcomes of implant treatment [2, 3]. An adequate keratinized mucosa width (KMW) of ≥ 2 mm around dental implants refers to crucial factor preventing inflammatory complications and unsatisfactory esthetic result [4, 5].
It has been reported that soft tissue thickness is also regarded as decisive volumetric parameter affecting peri-implant marginal bone level. In 1996, Berglundh and Lindhe revealed that a minimum of 2 mm gingival thickness is required for establishment of biological width around implants, and if the thickness is insufficient, bone loss will occur [6]. These findings were further confirmed by Linkevicius et al. [7, 8]. It should also be noted that the thin mucosal tissue in the area of the implants might have a grayish color due to the visibility of the abutment [9].
In recent times the topic of peri-implant soft tissue management gains increasingly more interest in the scientific literature. Currently, autogenous connective tissue graft (CTG) taken from the palate or maxillary tuberosity is represented as the gold standard for soft tissue thickening [10]. However, such disadvantages of harvesting procedure as complexity and duration of manipulation, limitations in graft size, as well as the possibility of prolonged bleeding, infection risk and postoperative discomfort at the donor site have been reported [11, 12].
In order to simplify the clinical procedure, the acellular dermal matrix, amniotic membrane of human origin, as well as a range of synthetic materials can be alternatively applied [13, 14]. The efficacy of xenogeneic collagen matrices (XCM) has been also described, demonstrating volume stability over a period of time, good tissue integration, promotion of new blood vessels formation and cellular ingrowth [15, 16].
The main representatives of XCMs are bilayered collagen matrix (CM), volume-stable collagen matrix (VCMX), acellular dermal matrix (ADM) and extracellular matrix. Each of them has certain advantages, for instance, the bilayered CM has an excellent color match with the surrounding tissue, while maintaining stability and elasticity distinguishes the VCMX from others. The application of ADM contributes to enhanced proliferation of fibroblasts, endothelial cells and tissue revascularization, while extracellular matrix is also capable to stimulate cell adhesion, differentiation, and vascular ingrowth, which aids a more predictable outcome [14].
Recent studies have been focused on comparative analysis between XCMs and CTGs in terms of their aid to the quality and quantity of soft tissues around dental implants after soft tissue augmentation [17,18,19]. Investigations were aimed to assess changes in soft tissue dimensions, periodontal status, and patient satisfaction after augmentation by XCM or CTG [20]. The leading volumetric parameter that was given attention in previous systematic reviews was KMW, while data on changes in soft tissue thickness were often of secondary importance [12, 21]. Whereas there have been a sufficient variety of studies to the soft tissue management, their results remain controversial with a low number of randomized clinical trials, resulting in a poor evidence to the present topic. Consequently, the present systematic review and meta-analysis aimed to evaluate the effectiveness of XCM in comparison to CTG for increasing the soft tissue thickness around dental implants.
Materials and methods
Study registration and protocol development
The protocol of the present review was registered and allocated the identification number CRD42022297500 in the International Prospective Register of Systematic Reviews (PROSPERO). This manuscript was prepared following the Cochrane Collaboration guidelines [22] and reported in accordance to the standards of the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) Statement [23].
Objectives
The goal of this review was to analyze and compare effectiveness of modern XCM and autogenous subepithelial CTG in increasing soft tissue thickness around dental implants.
PICOT Question and Focused Question
Population (P): patients who underwent soft tissue grafting (XCM, CTG) under the full or split-thickness flap to increase the thickness (volume) of soft tissues in the area of dental implants (during implant placement or during the healing abutment placement) for aesthetic, biological and functional reasons.
Intervention (I): surgical intervention that was performed by placing the graft (XCM) in the implant area under the full or split-thickness flap.
Comparison (C): in the control group the operation was performed with the use of CTG. The grafts from different donor areas are included in the analysis: CTG or subepithelial connective tissue graft (SCTG) harvested from the palate or maxillary tuberosity, de-epithelized free gingival graft (FGG).
Outcome (O): the main outcome was to evaluate increasing soft tissue thickness (volume) in the area of implants, additional outcomes included changes in clinical and radiographic peri-implant outcomes (width of keratinized mucosa, plaque index, bleeding indices, probing depth, marginal bone levels, surgery time), professional evaluation of aesthetic outcomes (pink and white esthetic scores), PROMs – pain syndrome, quality of life and complications.
Time (T): Minimum follow-up of 3 months after the surgical intervention.
The focused question is: Can modern XCMs provide results comparable to autogenous CTGs in increasing soft tissue thickness in the area of dental implants?
Eligibility criteria
Inclusion criteria
-
XCM or CTG augmentation at implant site
-
Prospective randomized controlled interventional human studies
-
Evaluation and reporting of clinical outcomes of increasing soft tissue thickness in the implant area using XCM and/or autogenous CTG over a minimum follow-up period of 3 months.
Exclusion criteria
-
Retrospective and cross-sectional clinical studies, reviews, case reports or animal studies
-
Increasing solely the KMW in the area of the implants
-
Application of FGG, allogenic and alloplastic (synthetic) materials for soft tissue augmentation
-
Increasing soft tissue volume or thickness around natural teeth
-
Soft tissue augmentation with immediate implant placement into the socket of the extracted tooth
-
Augmentation of soft tissues simultaneously with bone grafting
Information sources and search strategy
Selection of studies
A detailed systematic literature search was conducted by three researchers (IA, MZ and MM) using the following electronic databases: The National Library of Medicine (MEDLINE via PubMed); Scopus, the Cochrane Library, Latin American & Caribbean Health Sciences Literature (LILACS), eLIBRARY.ru. For examining unpublished trials, the grey literature, nonprofit reports, government research or other materials were also electronically explored through searching in EASY.
The search strategy was primarily designed for the PubMed (MEDLINE) database with advanced search and free text terms, and then adapted accordingly the other databases. There were no restrictions on publication date, language or journal in all databases, except for the LILACS database, where a language restriction was applied (only articles in English). The search was conducted on January 5, 2022. The PubMed (MEDLINE) search was rerun in July 2022. Also in November 2022, an additional manual search was carried out. Details regarding the search strategy are reported in Table 1. In addition, reference lists of relevant studies and full-texts of previous systematic reviews investigating soft tissue management around dental implants were also screened [10, 12, 17, 21, 24]. The search results were downloaded to a Rayyan QCRI (Qatar Computing Research Institute, Doha, Qatar) to facilitate duplicate removal and cross-reference checks.
After duplicates removal, two investigators (IA and ST) independently screened the titles and content of the abstracts (if available) to choose potentially suitable studies for set inclusion criteria. At the second stage, full-text versions of studies that met the inclusion criteria and for which the decision to be included in the review could not be made based on the title or abstract, were assessed in detail against the eligibility criteria. If some studies required more information or full-text versions were not available, the authors were contacted by the investigators. Disagreements were solved by discussion and involving a third reviewer, whose decision was determinative. All articles that did not meet the criteria were excluded with an indication of the reason.
Data extraction
Information was extracted from each included study on the following parameters:
-
Study characteristics and conclusions (author(s), year of publication, number of centers, study design, groups, time of augmentation, immediate healing abutment placement (yes/no) and its characteristics, treatment sites, number of participants and treated sites (baseline/follow-up), age of participants, smokers acceptance, follow-up period, outcomes and summary results) (Table 2).
-
Intervention characteristics (surgical technique, time of augmentation, recipient site formation, type of XCM, size/volume/thickness of XCM, donor site, harvesting technique, size/volume/thickness of CTG) (Table 3).
-
Soft tissue thickness outcome (time of augmentation, follow-up period, measurement technique, outcomes of soft tissue thickness/volume, changes in soft tissue thickness/volume) (Table 4).
-
Secondary outcomes (KMW, surgery time, PROMs, aesthetic outcomes, peri-implant tissue health, complications) (Tables 5 and 6).
Assessment of the risk of bias
Two reviewers (SB and MM) independently performed the assessment of risk of bias for included studies using the Cochrane Collaboration’s tool for assessing risk of bias in randomized controlled clinical studies [22]. Seven domains were analyzed, including sequence generation, allocation concealment, blinding of participants and personnel, blinding of the outcome assessor, incomplete outcome data, selective reporting and other bias. Risk of bias judgments were categorized as low, unclear and high risk of bias.
Quality of evidence assessment
The confidence in the evidence of effects found in the meta-analysis was assessed using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. Quality was assessed as high, moderate, low or very low according to GRADE. The summary table of results was constructed using GRADEpro GDT software.
Statistical analysis
The research results were pooled using the meta library v. 5.5–0 with R programming environment v. 4.1.2 and RStudio v. 2022.07.2 (PBC, Boston). A random effects models were used for meta-analysis. Data are presented as mean or mean differences (MD) and 95% confidence interval (CI). The I2 statistic was used to assess the heterogeneity of studies. Study bias was assessed using a funnel plot of MD of soft tissue thickness gain at 3 months between groups versus standard error. The significance was considered at p < 0.05 [32].
Results
Study selection
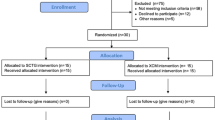
The study search process is presented in Fig. 1. This review identified 1376 records in total. Following removal of duplicates, titles and abstracts of 1115 records were screened. Full-text versions of 28 articles were assessed for eligibility. After exclusion of articles that did not meet eligibility criteria, 8 studies [15, 25,26,27,28,29,30,31] were included in qualitative synthesis, and 7 studies [15, 25,26,27,28, 30, 31] were included in meta-analysis. Reasons for exclusion are reported in Table 7.
Study characteristics
All the included studies [15, 25,26,27,28,29,30,31] had RCT design. Soft tissue augmentation procedures at implant sites were described in studies involving 306 patients and 325 implants in total. All studies [15, 25,26,27,28,29,30,31] included at least two parallel groups comparing the use of CTG with XCM. The follow-up time ranged from 1 to 12 months. More detailed characteristics of the included studies are summarized in Tables 2 and 3.
Qualitative analysis
The result of bias risk assessment for the included randomized clinical trials, using The Cochrane Risk of Bias Tool [22] is shown in Fig. 2. Five [15, 25, 27, 28, 30] of the included studies were considered a low risk of bias, one trial [26] was classified as a moderate risk of bias, and two studies had a high risk of bias [29, 31].
Statistical analyses
Meta-analysis of included studies [15, 25,26,27,28, 30, 31] revealed that in the CTG group, the pooled mean 3-month soft tissue thickness gain in the buccal subgroup was μ = 1.0704 mm [95% CI: 0.8325 – 1.3084, I2 = 53.1%]. Heterogeneity has been found in studies. Pooled mean of CTG group 3-month soft tissue thickness gain in the crestal subgroup was μ = 0.7428 mm [95% CI: 0.0280 – 1.4577, I2 = 41.8%]. No differences were observed between subgroups (Chi2 = 2.90, df = 1, p-value = 0.0888). Total pooled mean of CTG was μ = 0.9881 [95% CI: 0.7803 – 1.1959] with heterogeneity I2 = 54.8% [95% CI: 8.0% – 77.8%, Chi2 = 19.92, df = 9, p-value = 0.0184] (Fig. 3).
Meta-analysis revealed that the pooled mean 3-month soft tissue thickness gain in buccal subgroup of XCM group was μ = 0.7778 mm [95% CI: 0.5446 – 1.0109, I2 = 55.3%]. Heterogeneity has been found in studies. Pooled mean of CTG group 3-month soft tissue thickness gain in the crestal subgroup was μ = 0.5661 mm [95% CI: -0.676 – 1.8084, I2 = 75.2%]. No differences were observed between subgroups (Chi2 = 0.48, df = 1, p-value = 0.4864). Total pooled mean of CTG was μ = 0.6972 [95% CI: 0.4659 – 0.9284] with heterogeneity I2 = 76.2% [95% CI: 55.9% – 87.1%, Chi2 = 37.76, df = 9, p-value < 0.0001] (Fig. 4).
When comparing groups, it has been revealed that in the buccal subgroup mean difference (MD) between XCM (experimental) and CTG (control) 3-month soft tissue thickness tissue gain was -0.3187 mm [95% CI: -0.5080 – -0.1295, I2 = 0%]. In crestal subgroup MD was -0.3026 mm [95% CI:—0.6074 – 0.0021, I2 = 22.6%.]. No differences were observed between subgroups (Chi2 = 0.01, df = 1, p-value = 0.9299) (Fig. 5).
Total MD between CTG and XCM 3-month soft tissue thickness gain was -0.3199 mm [95% CI:—0.4746 – -0.1653, z = -4.06, p-value < 0.0001]. Heterogeneity has not been found in studies I2 = 0% [95% CI: 0.0 – 62.4%, Chi2 = 5.15, df = 9, p-value = 0.8212] (Fig. 5).
In order to evaluate the impact and heterogeneity of measurement methods (analog, digital) on soft tissue thickness gain in the studies, a meta-analysis was undertaken, incorporating research studies [15, 25,26,27,28, 30, 31]. A meta-analysis revealed that within CTG group, the pooled mean 3-month soft tissue thickness gain in the analog subgroup was μ = 0.9501 mm [95% CI: 0.8668–1.0334, I2 = 0.0%]. In the digital subgroup of the CTG group, the pooled mean 3-month in soft tissue thickness gain was μ = 0.9992 mm [95% CI: 0.6173 – 1.3811, I2 = 73.9%]. Heterogeneity was identified in the studies. No significant differences were observed between the subgroups (Chi2 = 0.11, df = 1, p-value = 0.7450) (Fig. 6).
Meta-analysis revealed that the pooled mean 3-month soft tissue thickness gain within the analog subgroup of the XCM group was μ = 0.7208 mm [95% CI: 0.1804 – 1.2611, I2 = 48.1%]. Heterogeneity has been found in studies. The pooled mean within the digital subgroup of the CTG group in 3-month soft tissue thickness gain was μ = 0.6771 mm [95% CI: 0.3321 – 1.0221, I2 = 84.1%]. No significant differences were observed between the subgroups (Chi2 = 0.04, df = 1, p-value = 0.8402) (Fig. 7).
Furthermore, when comparing the study groups, it was revealed that in the analog subgroup mean difference (MD) between XCM (experimental) and CTG (control) 3-month soft tissue thickness tissue gain was -0.3463 mm [95% CI: -0.6001; -0.0924– -0.1295, I2 = 0%]. In the digital subgroup MD was -0.3044 mm [95% CI: -0.4994 – -0.1095, I2 = 0.0%]. No heterogeneity was found in the studies, and no significant differences were observed between the subgroups (Chi2 = 0.07, df = 1, p-value = 0.7979) (Fig. 8).
Analysis of publication bias in studies reporting difference of 3-month soft tissue thickness gain in CTG versus XCM groups was performed by funnel plot. No significant asymmetrical distribution was found. Statistical evaluation of publication bias was not performed due to the limited number of subgroup studies (buccal n = 7, crestal n = 3) (Fig. 9).
Quality of evidence
The quality of the evidence was assessed using the GRADE approach. The following outcomes were considered important: soft tissue thickness gain at 3 months in total and in subgroups divided by position (buccal and crestal) and by methodology (analogue and digital). As recommended, the baseline level of evidence for outcomes is high, reasons for reducing the quality of evidence are summarised in ‘Summary of findings’ table. Two authors (IA and AG) worked independently to assess the quality of evidence, disagreements were resolved by consensus. Quality of two outcomes was graded as high and quality of three outcomes was assessed as moderate according to GRADE criteria.
Question: Can modern XCMs provide results comparable to autogenous CTGs in increasing soft tissue thickness in the area of dental implants?
Soft tissue thickness
Measurement methods of soft tissue thickness
Measurements of the mucosal thickness were performed by various methods. The analog measurement method was used by 4 authors, [15, 25, 29, 31] 3 authors used the digital method, [26, 28, 30] and in 1 study [27] ultrasound device was used.
Analog techniques for measuring soft tissue thickness differed from each other. Thus, Cairo et al. used an injection needle with a silicon stop and digital caliper with 0.01 mm of accuracy. The measurement was carried out at 1 point, which was located 1.0 mm coronal to the mucogingival junction (MGJ) [25]. Hélio et al. measured supracrestal soft tissue thickness by a puncture with a short carpule needle with an endodontic rubber cursor in the center of the future prosthetic crown [31]. Thoma et al. also measured by transmucosal probing with an endodontic instrument. There were 3 points of measurement: the occlusal, buccal and apical aspects, which were standardized by an individualized stent fabricated by CAD/CAM technology [15]. Baldi et al. conducted evaluation of facial soft tissue level (FST) measured as the distance in mm between the mid-facial soft tissue level and a reference line connecting the FST of the adjacent teeth [29].
Digital techniques for measuring soft tissue thickness were presented in three studies [26, 28, 30]. Zeltner et al. used digital-assisted technique with impressions [28]. After impressions were taken, dental stone casts were fabricated and optically scanned with a desktop 3D scanner. After importing digital models into a digital imaging software program, crestal and buccal regions of interest were identified [28]. The same method was used by Ashurko et al. who investigated buccal contour changes at 3 equidistant points (in 1-mm step) in the coronally-apical direction at the center of the alveolar ridge [30].
To analyze volumetric and profilometric changes Cosyn et al. took an optical scan by intra-oral scanner. Study-relevant areas were from 0.5 mm below the soft tissue margin to 4 mm more apical and from the mesial to the distal line angle of the implant crown [26].
Puzio et al. used an ultrasonic device (Pirop®, Echoson) [27]. The thickness of the mucosa was measured at two points: the first point was located on the line connecting the cemento-enamel junctions of adjacent teeth on the gingival margin. The second point was on the MGJ along the axis of the future implant. The volumetric changes measured in mm (in the software), which corresponded to the mean distance between the three surfaces representing the evaluated time-points [27].
Soft tissue thickness outcomes
The baseline was defined as the time of the first measurement before any augmentation took place, except for the study by Baldi et al., [29] in which baseline mucosal thickness was not determined. In 5 studies, [15, 25, 27, 30, 31] the initial soft tissue thickness in mm was determined, except for the studies by Zeltner et al. [28] and Cosyn et al., [26] where the researchers used mucosal volume parameter in mm3 in a specific area as an initial measurement (Tables 5 and 6).
In the study by Cairo et al., in the XCM and CTG groups, the initial gingival thickness was comparable and amounted to 2.1 ± 0.63 and 2.1 ± 0.59, respectively [25]. After three months the mean thickness for the XCM group increased to 2.8 ± 0.7, the CTG group showed 3.1 ± 0.5. The final thickness after six months in the XCM and CTG groups was 3.0 ± 0.7 and 3.4 ± 0.6, respectively. A significant gain in soft tissue thickness was observed in both groups compared to baseline (p < 0.0001). Soft tissue increase was 0.9 ± 0.2 in the XCM group and 1.2 ± 0.3 mm in the CTG group, and was lower in the test group (difference -0.3 mm; 95%CI 0.5 – 0.2; p = 0.0001). At the same time, 79% of XCM-treated sites and 93% of CTG-treated sites achieved final soft tissue thickness ≥ 2.5 mm [25].
In the study by Puzio et al., baseline soft tissue thickness before augmentation procedure was below 2 mm in all groups [27]. Mean values of gingival thickness at point 1 were between 1.15 ± 0.40 to 1.39 ± 0.65 and at point 2 were between 0.9 ± 0.77 to 1.10 ± 0.44. There was no significant difference between all groups in case of point 1 and 2. At the 3-months follow-up at the point 1 statistically significant mucosal thickness gain was observed only between groups with no graft area (I) and CTG group 3 months after implant placement (IIIb) (0.23 mm vs. 0.95 mm; p = 0.042). At point 2 soft tissue thickness increased significantly in the XCM group (IIIa) and CTG group 3 months after implant placement (IIIb) (0.48 mm vs. 1.01 mm; p = 0.042). But there were no significant differences between XCM group (IIa) and CTG group 3 months before implant placement (IIb) (p = 0.654). Significant differences in thickness gain between groups I–IIIb, IIa–IIIb and IIIa–IIIb were also observed. Ultrasonic assessment after 12 months showed the highest soft tissue gain at point 1 in CTG groups: 1.76 ± 0.7 (IIb) and 1.52 ± 1.0 (IIIb). The highest increase (1.76 ± 0.7) was in the group CTG before implantation (IIb), but the difference was not significant (p = 0.928). The smallest increase was recorded in the group where XCM was used 3 months after implant placement (0.89 ± 0.6). But there was no significant statistical difference at point 1 after 12 months between the groups with XCM and CTG before (p = 0.241) and after (p = 0.188) implant placement. At point 2 the highest soft tissue gain was also observed in CTG groups: 1.36 ± 0.6 (IIb) and 1.15 ± 0.5 (IIIb). The smallest increase was obtained in XCM groups: 1.0 ± 0.7 (IIa) and 0.57 ± 0.6 (IIIa). There were no significant differences in soft tissue thickness gain between the groups with XCM (IIa) and CTG (IIb) before implant placement (p = 0.654). But the difference between the groups with XCM (IIIa) and CTG (IIIb) 3 months after implant placement was significant (p = 0.042). Regardless of the time of augmentation, there was no statistically significant difference between groups using the same material [27].
In the study by Thoma et al., baseline soft tissue thickness was above 2 mm in all groups: 3.5 ± 1.0 (XCM) and 4.2 ± 1.9 (CTG) at the occlusal site (p = 0.442), 2.9 ± 1.5 (XCM) and 4.1 ± 2.0 (CTG) at the buccal site (p = 0.211), 2.6 ± 2.3 (XCM) and 3.4 ± 1.8 (CTG) at the apical site (p = 0.246) [15]. There were no statistically significant differences between XCM and CTG (p = 0.987; p = 0.953; p = 0.481) at 1 month after soft tissue augmentation. The increase in soft tissue thickness 3 months after augmentation procedure was observed mainly at the occlusal site: 1.4 ± 1.4 (XCM) and 0.8 ± 1.8 (CTG), but there were no statistically significant differences between two groups (p = 0.359). For all other sites, similar increases were observed: 1.1 ± 1.4 (XCM) and 0.8 ± 2.2 (CTG) at the buccal site (p = 0.281), 0.9 ± 1.9 (XCM) and 1.6 ± 2.6 (CTG) at the apical site (p = 1.000; p = 0.470) [15].
Zeltner et al. [28] demonstrated the results obtained using a numerical analysis of the same patient population which was reported in a previous publication by Thoma et al. [15] The median crestal regions of interest (ROI) was 24.8 mm2 and 23.7 mm2 for XCM and CTG, respectively. The corresponding values for the buccal ROI were 32.2 mm2 for XCM and 29.2 mm2 for CTG. The differences between groups were not statistically significant (crestal p = 0.278; buccal p = 0.113). The linear changes from baseline to 3 months in the crestal ROI amounted to 0.27 ± 0.26 for XCM and to 0.42 ± 0.74 for CTG. The change in the XCM group was significant (p = 0.002), whereas the change in the CTG group was not significant (p = 0.129). The differences between the two groups did not differ significantly (p = 0.287). The gain in soft tissue volume from baseline to 3 months in the buccal ROI was 0.77 ± 0.74 for XCM and 0.79 ± 0.45 for CTG. Changes for both XCM (p = 0.002) and CTG (p = 0.004) were significant. The differences between groups were not significant (p = 0.534) [28].
In the study by Baldi et al., baseline measurements of mucosal thickness were not provided [29]. The dynamics of changes in the level of the vestibular mucosa (FST) showed an increase in the first 1.5 months by 0.34 ± 0.13 (SE) in the XCM group (p = 0.0218) and by 0.50 ± 0.22 (SE) in the CTG group (p = 0.0756). The difference was statistically significant only in XCM group. After 6 months, the level of the vestibular mucosa in both groups decreased, and the difference from the initial level was 0.32 ± 0.21 (SE) and 0.35 ± 0.30 (SE) in the XCM and CTG groups, respectively. The mean differences in FST levels between group without any augmentation material and CTG group were significant for the 1.5 and 6 month follow-up, being 0.93 ± 0.76 (p = 0.01) and 1.32 ± 1.03 mm (p = 0.008). Other differences between groups were not statistically significant [29].
In the study by Cosyn et al., the initial soft tissue thickness was defined as a volume parameter of study-relevant area of interest (AOI) [26]. The mean AOI amounted to 28.63 and 28.07 mm3 in the control and test group, respectively. There was no significant difference between the groups (p = 0.553). Directly after the augmentation, a change in the vestibular contour of the soft tissues was observed. In addition, a significant time effect (within group difference) was observed in both groups. In the CTG group, the increase in buccal soft tissue profile was 1.43 mm (95% CI 1.15 – 1.70). After 3 months, the increase in buccal soft tissue profile was 1.15 mm (95% CI 0.88 – 1.43), compared to the initial level. In the XCM group, the increase in buccal soft tissue profile immediately post-surgery was 1.90 mm (95% CI 1.63 – 2.18). After 3 months, the increase in buccal soft tissue profile from baseline was 0.85 mm (95% CI 0.58 – 1.13). Three months after surgery, no significant effect of the treatment was observed, although there was a trend towards an additional increase in the buccal soft tissue profile by 0.30 mm (95% CI 0.01 to 0.61; p = 0.054) in favor of the control group. A significant shrinkage of CTG and XCM was defined to be 0.27 mm (95% CI 0.01 – 0.53; p = 0.039) and 1.05 mm (95% CI 0.79 – 1.31; p < 0.001). Sites treated with XCM demonstrated 0.78 mm (95% CI 0.41 – 1.14; p < 0.001) more shrinkage between than sites treated with CTG [26]. A 1-year follow-up study was recently published. The difference between groups in increase was 0.41 mm (98.3% CI: 0.12 – 0.69) and was significant in favor of CTG [50].
In the study by Ashurko et al., in the XCM and CTG groups, the initial mucosal thickness was comparable and amounted to 1.61 ± 0.07 and 1.63 ± 0.07, respectively [30]. After three months the mean thickness for the XCM group increased to 2.81 ± 0.11, the CTG group showed 3.16 ± 0.11. A significant gain in soft tissue thickness was observed in both groups compared to baseline. Soft tissue increasing was 1.18 ± 0.11 in the XCM group and 1.55 ± 0.11 mm in the CTG group. The difference between groups was -0.366 (− 0.66 to − 0.07; p = 0.016) in favor to CTG. A soft tissue thickness at least 2 mm was achieved in 93.33% (70.18 to 99.69%) of CTG group patients and 60% (35.75 to 80.18%) of XCM group (p = 0.08). A soft tissue thickness at least 3 mm was achieved in 46.67% (24.81 to 69.89%) of SCTG group patients and 33.33% (15.18 to 58.29%) of XCM group (p = 0.71) [30].
In a study by Hélio et al., the initial soft tissue thickness was 2.12 ± 0.33 for XCM group and 2.05 ± 0.33 for CTG group [31]. After three months the mean thickness for the XCM group increased to 2.61 ± 0.43, the CTG group showed 2.98 ± 0.5. A significant gain in soft tissue thickness was observed in both groups compared to baseline (for XCM p = 0.013; for CTG p < 0.001). There was no statistically significant difference between the two groups (p = 0.065) [31].
Patient-Reported Outcome Measures (PROMs)
Only five [15, 25, 26, 29, 30] of the eight included studies examined patient-reported outcome measures (PROMs) (Tab 7). The intensity of post-operative pain, general discomfort and patient satisfaction with the result were recorded using a visual analogue scale (VAS). Two studies [15, 30] used the Oral Health Impact Profile Questionnaire (OHIP-G14) to assess quality of life.
In a study by Baldi et al., the patients’ mean aesthetic satisfaction was high in all three groups, with no statistically significant differences between groups found after 6 months [29]. Cairo et al. [25] found that patients tolerated surgical procedure using a collagen matrix more easily. However, there was no statistically significant difference in perceived pain between two groups. Seven days after the intervention, patients of the test group using VAS noted: lower intensity of post-operative pain, the least number of days with discomfort and less intake of anti-inflammatory drugs. After 2 weeks, the CTG group had more sites with edema. There were no statistically significant differences in other indicators in the post-operative period and after 6 months; all patients reported their high satisfaction with the result [25].
Post-operative bleeding was assessed dichotomously (yes/no) in the study by Cosyn et. al. [26] VAS was used to record the severity of post-operative pain, edema and hematoma, the number of analgesics taken and aesthetic satisfaction. In both study groups, after 7 days, no statistically significant difference was observed between the groups in terms of post-operative bleeding, pain, edema and in the use of analgesics. However, the mean score for post-operative hematoma was lower in the test group (XCM) than in the control group (CTG). Three months after the operation, there was no significant difference between the groups in the patients’ aesthetic satisfaction with the condition of the peri-implant soft tissues [26].
Thoma et al. calculated VAS scores 4 h after surgery and then daily until suture removal, as well as on days 30 and 90 [15]. When sutures were removed, the mean total scores on the OHIP-G14 questionnaire for CTG were higher than for XCM. The difference in both criteria was not statistically significant. But the results obtained for these indicators correlated with the high use of analgesics in the CTG group from the day of surgery to the day of suture removal. The median physical pain was 100% higher in the CTG group compared to the XCM group. The largest differences between groups were found for physical pain and social disability [15].
Ashurko et al. demonstrated a slightly higher VAS score for CTG between day 1 and day 7 post-surgery without being statistically significantly different at any time point (p > 0.05) [30]. By the 7th day, patients with XCM showed a more pronounced decrease in the quality of life (2.22 ± 0.77) compared with patients with CTG (1.87 ± 0.74); however, the difference between the groups was not statistically significant (p > 0.05). By the 90th day, the difference between the groups changed and amounted to 0.67 ± 0.62 and 0.73 ± 0.46, respectively (p > 0.05). No other significant difference was detected in the postoperative period [30].
Peri-implant tissue health
The condition of peri-implant tissues was assessed in four [15, 25, 26, 29] of eight studies included in this systematic review. The condition of peri-implant was analyzed according to the following indicators: bone level, probing depth, bleeding on probing, plaque, clinical attachment level.
In the study by Cairo et al., during 6 months after intervention, there was no statistically significant difference in mean bone level between groups (0.1 mm difference; 95% Cl – 0.1 to 0.3; p = 0.022) [25]. There was also no significant difference in other parameters between the groups [25].
In Cosyn et al. study, the probing depth and bleeding on probing were assessed after 3 months after implantation [26]. Plaque and bleeding on probing were between 20 and 30% after 3 months and did not vary between groups, however the difference in probing depth between groups was 0.30 mm (95% CI 0.06 – 0.54; p = 0.017) and was statistically significant in favor of control group (CTG). Mean marginal bone loss was 0.34 mm in control group (CTG) and 0.72 in test group (XCM) after 3 months. The difference was 0.38 mm (95% CI 0.15—0.60; p = 0.001) and was statistically significant in favor of control group [26]. In the study by Thoma et al., clinical and periodontal measurements were assessed before surgery, 30 and 90 days after the intervention. As a result, there was no significant difference between groups considering all measurements [15]. Baldi et al. assessed only bone level around implant after 6 weeks after soft tissue augmentation and at the time of permanent crowns installation. There was no statistically significant difference between groups [29].
Width of keratinized Mucosa
The width of keratinized mucosa (KMW) was evaluated in five [15, 25, 29,30,31] of the eight included studies. In a study by Cairo et al. at the 6-month follow-up visit surgery resulted in a significant increase in KMW (1.1 and 0.9 mm for XCM and CTG respectively). The difference between groups was not statistically significant [25]. In study by Baldi et al. there was not statistically difference between XCM and CTG, and both treatments demonstrated a significant increase (1.05 and 0.80 mm respectively) from baseline to six-month follow-up [29].
In study by Thoma et al. there was no statistically significant difference in KMW between groups at the mesial neighboring tooth (p = 0.264) and implantation site (p = 0.624). At the distal neighboring tooth the difference in KMW between groups was statistically significant (1.1 mm; Q1: 2.0; Q3: 0.1) (p = 0.029) [15].
In study by Ashurko et al. the amount of KMW was not changed in both procedures 3 months after intervention and the similar final amount of KMW was observed with no significant difference [30].
Hélio et al. showed increasing of KMW from 3.00 ± 0.65 to 3.67 ± 0.69 (p = 0.003) for XCM and from 3.21 ± 0.71 to 4.41 ± 0.55 (p < 0.001) for CTG [31]. When comparing the 2 types of grafts in study, it was observed that the final KMW was higher in CTG group, with statistically significant difference between the two types of grafts (p < 0.014) [31].
Esthetic Outcomes
Three [25, 26, 29] of eight included studies evaluated aesthetic outcomes. The Cosyn et al. assessed mid-facial recession (MFR), pink aesthetic score (PES) and mucosal scarring index (MSI) [26]. Mid-facial recession was calculated 3 months after soft tissue augmentation by subtracting the level of mid-facial soft tissues (the distance from the incisal edge of the crown to the buccal edge of the mucosa in the center of the implant) from the postoperative level of mid-facial soft tissues. Positive values indicated the onset of a recession, while negative values indicated a vertical regrowth. PES and MSI were assessed at 3 months from occlusal and anterior photographs by scores: pink aesthetic score from 0 to 14 (worst to perfect aesthetic result), and mucosal scarring index from 0 to 10 (from no scar to worst). Mid-facial recession was significantly higher in XCM group than in CTG group. The authors attributed this result to the large thickness of XCM, which could provoke a more coronal location of the flap in the test group. In addition, it was concluded that there was no clinical significance in more pronounced mid-facial recession in the control group, as there was no significant difference between PES in the control and test groups. The MSI was low in both groups, as evidenced by the sufficient peri-implant esthetic outcome in both groups [26].
In a study by Baldi et al., aesthetics was evaluated both by the operating physician and by the patient at the stage of permanent crown installation [29]. The surgeon assessed aesthetics using a pink-aesthetic score, which included evaluation of each variable: medial and distal papilla, soft tissue level, alveolar ridge deficiency, and soft tissue color and texture. Patients also expressed their degree of satisfaction with implant treatment in general, answering questions from the questionnaire using a visual analogue scale. As a result, there were no statistically significant differences between the three study groups in PES, but there was a significant difference in interdental papilla index between CTG group and no graft group. Mean esthetic patient satisfaction was high in all groups, and no statistical differences were found between them [29].
Cairo et al. collected outcomes from patients regarding aesthetics (soft tissue and crown appearance) and results of VAS [25]. It was found that after 6 months (at the last appointment), patients were highly satisfied with the aesthetic results with no significant difference between the groups [25].
Histological findings
In this systematic review, among the included studies only three [15, 30, 31] reported histological findings.
Thoma et al. evaluated obtained biopsies 90 days after grafting XCM (volume stable collagen matrix) and CTG histologically to study the amounts of remaining XCM and new connective tissue formations [15]. In both groups’ vascularization was observed throughout the specimens with limited amount of inflammatory cells. In the CTG group biopsies there was a relatively loose network of collagen fibers with and no differentiation between grafted and newly formed connective tissue. A dense collagen fiber network as well as identification of remaining XMC with the remodeling processes was observed in the XCM group. The histomorphometric assessment showed 32.1% (± 18.5%) of a remaining matrix body, and 30.1% (± 11.8%) mean amount of connective tissue in XCM group, while in the group with CTG the mean amount of newly formed and grafted connective tissue reached 77.6% (± 11.6%) [15].
In the study by Ashurko et al., it was revealed that in both groups, after 3 months the newly formed mucous membrane of biopsy specimens was lined with stratified squamous epithelium of various thicknesses, which was delimited from the papillary layer by a basement membrane [30]. The indicators of the average and maximum thickness of the epithelial layer were less than in CTG group than in XCM group. There were no differences in the true average thickness (relative area) of the layers in the two groups. The CTG group had significantly longer rete ridges. At the same time, this index did not significantly affect the length of the basal membrane, although its relative length was slightly longer than in the XCM group. The indicators of the proliferative capacity of the epithelium, represented by the relative cellularity of the basal layer and the proportion of mitoses in the basal layer in the XCM group were also significantly lower than in the CTG group [30].
Hélio et al. found that 3 months after soft tissue augmentation XCM and CTG did not present a statistically significant difference in the number of fibroblasts per area close to the epithelium (with no grafted material present) and an area close to the periosteum (within the graft area) with no clinical and histological signs of inflammatory process [31].
Complications
All included studies were analyzed for reported complications. Cosyn et al. reported complications in the CTG group: intolerable pain and edema, removal of the implant due to mobility after 1 week, wound dehiscence after 1 week, and in the XCM group: severe post-operative bleeding and wound dehiscence after 1 week [26]. Cairo et al. described 1 mm soft tissue recession 6 months post-op [25]. Ashurko et al. described complicated healing in 2 patients of the XCM group [30]. At the time of suture removal (day 14), the discrepancy of the wound edges with exposure of the granulating surface of the collagen matrix was determined. In both cases, the patients did not notice any discomfort during healing, no exposure of the implant cap screws was detected, and the wound healed by secondary intention [30].
Discussion
Summary of main results
The present systematic review and meta-analysis addresses the question: “Can modern XCM provide results comparable to autogenous CTG in increasing soft tissue thickness around dental implants?” Furthermore, such parameters as KMW, PROMs, aesthetic outcomes, peri-implant health, and histological findings have been analyzed.
Based on our systematic review and meta-analysis, it can be argued that the use of XCM, as well as CTG, leads to an increase in the thickness of soft tissues in the implant area. Measurement of soft tissue changes after augmentation procedures is associated with certain difficulties, primarily because the changes are shallow and sometimes hard to detect. The standard or analog measurement method has been used in most studies (periodontal probe, endodontic probe, etc.), [18, 31, 34, 36] although recently, more modern methods using digital technologies, [26, 28, 30, 35] and ultrasonic devices [27] find their application. Still, in the most of the studies analog techniques were utilized, which have their drawbacks compared to more modern digital methods.
Baldi et al. used an analog method to measure changes in mucosal thickness along a reference line, which characterizes changes in soft tissue contour in height [29]. The article does not describe the measurement methodology itself in sufficient detail: in which part of the ridge the measurement was made, how the reproducibility of the measurement at the same point was controlled, with what accuracy the measurement was made and what instrument was used. There might have been some bias in the study outcomes. For the same reason, the author has no data on the initial thickness of the soft tissues since the used technique was non-invasive.
A more classic technique with an injection needle was used in a study by Cairo et al. [25]. The main disadvantage of this method is that the data obtained refer to only one specific point of the dental implant site. In addition, it is difficult to determine the same point at different periods of time, which may produce bias. The same problems should be noted in the study by Hélio et al. [31].
Fabrication of a customized stent with openings on the occlusal and buccal sides ensures reproducibility of gathered measurements, as demonstrated in the study by of Thoma et al. [15]. Furthermore, such approach makes it possible to receive data from several points providing a better overview of tissue reaction.
The utilization of digital technologies allows performing a volumetric assessment based on the comparison of digital models received in different time periods. This method allows obtaining data regarding the volume changes of the entire augmentation area [35]. A similar approach is described in the studies by Zeltner et al. and Ashurko et. al. [28, 30]. Despite the fact that Zeltner et al. [28] conducted measurements on a group of Thoma et al. [15], we decided to include it in this review, since the author used a more modern, digital version of the measurement of volumetric changes after augmentation and obtained completely new results. This once again tells us about the difficulty of comparing.
Digital method is non-invasive and does not cause radiation exposure. A disputable issue in the study by Zeltner et al. and Ashurko et al. is that taking analog impressions may produce some inaccuracies of the entire analysis. This is especially true for areas with mucosal reflections [28, 30]. The utilization of an intraoral scanner may overcome this problem, as demonstrated by Cosyn et al. [26]. As the author of the publication noted, the main drawback of this technique is that the area of interest in all patients was different and varied depending on individual differences in anatomical structures [26]. This problem was solved in a special way in the study by Célien Eeckhout et al., which was not included in this review. In order to make a direct comparison between patients with areas of interest differed in size, the researchers converted the mean volume change per area into a mean linear change in buccal soft tissues profile in mm [35]. Another problem in the study by Cosyn et al. is that restorations were installed at the same time with soft tissue grafting [26]. It is related to the limitation of this method, since the technique of comparing 3D models makes it possible to adequately compare only equivalent areas, without any superstructures. After placing the crown, the soft tissues are usually displaced, which can create the illusion of an increase in volume of the tissues. No randomized trials have been found to confirm or reject this assumption and this is a subject for further investigation.
The use of an ultrasonic device to measure soft tissue thickness is also of interest. This technique was used in a study by Puzio et al., but there is a problem with reproducibility of the reference points in the study due to the availability of only conditional landmarks [27].
In the present study, an attempt has been made to analyse the data on mucosal thickness gain in the area of dental implants depending on the thickness assessment method used: digital or analogue. However, since there were few studies that fits the inclusion and exclusion criteria, we had to summarise the data of buccal and occlusal measurements for the analysis. Based on the meta-analysis we can cautiously conclude that, there is no statistically significant difference between the methods used. However, more studies with subgrouping are needed for a definitive understanding.
According to the meta-analysis, soft tissue growth occurred more on the vestibular surface than on the occlusal one. Zeltner et al. assumes that this may be due to an increased pressure on the transplants in the crestal region caused by the primary wound closure and sutures position. XCM has high elasticity, and it is not as resistant to mechanical load as CTG. Possibly for this reason, there was a more pronounced difference in volume between the buccal and crestal regions of interest in the XCM group [28]. In addition, deficiency in the occlusal site may be due to more frequent soft tissue dehiscence defects after suture removal (30% (VCMX) and 10% (SCTG)) and healing by secondary intention [15].
In most of the reviewed studies, the long-term follow-up was 3 months [15, 19, 26, 28, 30, 31]. It is known, that volume decrease may be associated with remodeling during the initial phase of wound healing, which is most pronounced in the first three months after soft tissue augmentation [34, 51]. However, only minimal changes are observed between 3 and 6 months [16, 52]. Nevertheless, according to some researchers, 6 months is the optimal time to assess the effectiveness of soft tissue augmentation. Because the collagen matrix is completely degraded at this time, that allows a more objective assessment of the result [36, 44, 53].
In high number of RCT, which were included in this systematic review, standard periodontal tests were assessed: bone level, probing depth, bleeding on probing, plaque, clinical attachment level. In should be noted that the majority of clinicians agree on the lack of statistically significant difference in these tests between groups [15, 18, 25, 29, 48].
Most researchers also agree that sufficient width of the keratinized attached mucosa reduces the risk of plaque accumulation, the occurrence of mucosal recession and peri-implantitis, and plays an important role in maintaining peri-implant health [54, 55]. Therefore, in studies, in addition to the actual increase in the thickness of the peri-implant soft tissues, it is also necessary to pay attention to the increase in the width of the efficiency factor. Analyzing the results of the included studies, it can be noted that most clinicians reveal a significant increase in KMW using collagen matrix and autogenous graft, but do not indicate statistically significant differences in this parameter between groups [15, 25, 29]. In recent years, several recent systematic reviews and meta-analyses also did not reveal a statistically significant difference in KMW gain between the use of XCM and CTG [17, 21].
The studies of most authors report a more comfortable postoperative period with the use of soft tissue substitutes, which is accompanied by less overall pain and less consumption of painkillers compared with the use of CTGs [15, 18, 25, 46, 56]. However, it should be noted that the severity of postoperative discomfort and pain depends on the localization of the donor area and the method of harvesting CTG. According to some studies, the absence of morbidity in the donor area is due to more comfortable healing after CTG harvesting from the region of the maxillary tuberosity, compared to the area of the hard palate [57]. Despite the difference in the methods of assessing aesthetic results, in most studies, patients were highly satisfied with the results of treatment, which is confirmed by the questionnaire data given above.
An adequate soft tissue volume around dental implants is an important component in achieving not only the protection from mechanical damage and the occurrence of inflammatory complications, but also to obtain a higher aesthetic result of implant treatment [42, 58]. In this regard, aesthetics assessment is included in the secondary outcomes of some studies focusing on peri-implant soft tissue augmentation. Despite the difference in methods for assessing aesthetic outcomes, in most studies, [25, 26, 29] patients who underwent soft tissue augmentation were highly satisfied with the results of the treatment. Objective methods for assessing aesthetic outcomes (PES, MSI, MFR) also showed no statistically significant differences between CTG and XCM groups [25, 26, 29].
It should be mentioned, that it is important to assess the outcomes in the long term, as was done by Thoma et al., who evaluated results 3 years after installation of permanent restorations and found minor differences and stable results in terms of buccal contour, marginal bone level and aesthetics [47].
Agreements and disagreements with other reviews
A systematic review by Gargallo-Albiol et al. published in 2019 found no significant difference between the use of XCM and CTG to increase soft tissue thickness [17]. In contrast, the present study showed a higher effectiveness of CTG compared to XCM. It can be assumed, that this discrepancy may be due to several reasons. First, Gargallo-Albiol al. reviewed included RCTs with a different surgical approaches (bilaminar technique and apically positioned flap), which could contribute to some heterogeneity. Second, 4 RCTs [26, 29,30,31] included in this systematic review were published after the Gargallo-Albiol et al. study.
Despite this, the results of this review correspond to the results of most of the previous studies [12, 21, 59, 60]. It is worth noting that the authors of most reviews included not only RCT, but also CCT, which may have influenced the results of the study. In addition, in some studies, the authors compared the soft tissue thickness gain after various surgical techniques (bilaminar technique, apically positioned flap, using of a free gingival graft, etc.), which can be compared with each other only by using a network meta-analysis [12]. A recently published systematic review by Valles et al. found a statistically significant difference in the increase of soft tissue thickness in the group using CTG compared to soft tissue substitutes. It should be noted that the authors included not only collagen matrix, but also ADM, PRF, T-PRF in the group of soft tissue substitutes, which diverges from the objectives of our review. It should also be mentioned that during the period indicated by the authors, there were several RCTs [28, 29, 31] matching the inclusion criteria that were not added to the systematic review [59]. In order to expand the available evidence, we have added more recent studies, allowing us to conduct a review and meta-analysis of the maximum number of RCTs to date.
Quality of evidence
The certainty of evidence from the most important outcomes of this study was also analyzed. The GRADE assessment is an essential point in determining the methodological quality of the articles included in the systematic review. The GRADE approach includes five domains for rating down certainty (risk of bias, inconsistency, indirectness, imprecision and publication bias) [61]. The results obtained from the GRADE criteria-based assessment of the studied outcomes showed that the articles included in this systematic review and meta-analysis had a moderate or high certainty level. The decrease in the confidence level for three of the five outcomes from high to moderate was influenced by a high risk of bias in one of the included studies [31].
Limitations
This systematic review presents some limitations that should be considered.
First, during the study we conducted literature search in the following databases: PubMed (MEDLINE), Scopus, Cochrane Library, LILACS, eLIBRARY.RU. Although other databases are known to exist (e.g. Embase and Web of Science). Thus it can be assumed that there are more scientific publications that were not included in this study, although this was compensated for by the manual search that was conducted. Unpublished studies, the gray literature, nonprofit reports, government studies and other materials were also reviewed electronically using EASY search.
Second, few studies with different design that involved recording changes in soft tissue thickness by different methods and at different control points were included.
Third, the analysis cannot be considered completely objective due to the fact that in some studies soft tissue augmentation was accompanied by full wound closure, and in another part of the studies healing abutment was immediately installed, which could potentially affect the reliability of the results.
The small number of included studies does not allow to provide an objective assessment of the amount of soft tissue gain from the occlusal surface, because this parameter was analysed only in 3 studies. Moreover, the same patient population was analysed using different methods in the Thoma and Zeltner studies [15, 28]. Furthermore, when performing subgroup meta-analyses and attempting to compare digital and analogue measurement methods, we encountered an even greater reduction in the power of subgroup comparison. Therefore, we decided to combine the results of measurements and subsequent analyses of the vestibular and occlusal subgroups, which also represents a limitation of this paper.
In our study we conclude that XCM is less effective than CTG in increasing soft tissue thickness around dental implants. However, our statement must be interpreted with caution given the limited number of articles included in the meta-analysis and high variability in the outcome measures in the studies. Consequently, there is a requirement for further long-term researches.
Conclusion
Within the limitations associated with the insufficient number of studies analysed in this study, the present systematic review and meta-analysis suggest that XCM is less effective than CTG in increasing soft tissue thickness around dental implants. However, XCM also provides soft tissue thickness gain and can be recommended for use in various clinical situations.
Availability of data and materials
The data is available on reasonable request.
References
Moraschini V, Poubel LA, Ferreira VF, et al. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int J Oral Maxillofac Surg. 2015;44:377–88. https://doi.org/10.1016/j.ijom.2014.10.023.
Thoma DS, Naenni N, Figuero E, et al. Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin Oral Implants Res. 2018;29:32–49. https://doi.org/10.1111/clr.13114.
Steigenga JT, Al-Shammari KF, Nociti FH, et al. Dental implant design and its relationship to long-term implant success. Implant Dent. 2003;12:306–17. https://doi.org/10.1097/01.ID.0000091140.76130.A1.
Moraschini V, Luz D, Velloso G, et al. Quality assessment of systematic reviews of the significance of keratinized mucosa on implant health. Int J Oral Maxillofac Surg. 2017;46:774–81. https://doi.org/10.1016/j.ijom.2017.02.1274.
Tarasenko S, Ashurko I, Taschieri S, et al. Comparative analysis of methods to increase the amount of keratinized mucosa before stage-two surgery: a randomized controlled study. Quintessence Int. 2020;51:374–87. https://doi.org/10.3290/j.qi.a44216.
Berglundh T, Lindhe J. Dimension of the periimplant mucosa Biological width revisited. J Clin Periodontol. 1996;23:971–3. https://doi.org/10.1111/j.1600-051X.1996.tb00520.x.
Linkevicius T, Apse P, Grybauskas S, et al. The influence of soft tissue thickness on crestal bone changes around implants: a 1-year prospective controlled clinical trial. Int J Oral Maxillofac Implant. 2009;24:712–9. https://doi.org/10.1111/cid.12222.
Linkevicius T, Puisys A, Steigmann M, et al. Influence of vertical soft tissue thickness on crestal bone changes around implants with platform switching: a comparative clinical study. Clin Implant Dent Relat Res. 2015;17:1228–36.
Jung RE, Sailer I, Hämmerle CHF, et al. In vitro color changes of soft tissues caused by restorative materials. Int J Periodontics Restorative Dent. 2007;27:251–7. https://doi.org/10.11607/prd.00.0745.
Lissek M, Boeker M, Happe A. How thick is the oral mucosa around implants after augmentation with different materials: A systematic review of the effectiveness of substitute matrices in comparison to connective tissue grafts. Int J Mol Sci. 2020;21:1–19. https://doi.org/10.3390/ijms21145043.
Griffin TJ, Cheung WS, Zavras AI, et al. Postoperative Complications Following Gingival Augmentation Procedures. J Periodontol. 2006;77:2070–9. https://doi.org/10.1902/jop.2006.050296.
Vallecillo C, Toledano-Osorio M, Vallecillo-Rivas M, et al. Collagen matrix vs. autogenous connective tissue graft for soft tissue augmentation: a systematic review and meta-analysis. Polymers (Basel). 2021;13:1810. https://doi.org/10.3390/polym13111810.
Fénelon M, Catros S, Fricain JC. What is the benefit of using amniotic membrane in oral surgery? A comprehensive review of clinical studies. Clin Oral Investig. 2018;22:1881–91. https://doi.org/10.1007/s00784-018-2457-3.
Tavelli L, McGuire MK, Zucchelli G, et al. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J Periodontol. 2020;91:17–25. https://doi.org/10.1002/JPER.19-0351.
Thoma DS, Zeltner M, Hilbe M, et al. Randomized controlled clinical study evaluating effectiveness and safety of a volume-stable collagen matrix compared to autogenous connective tissue grafts for soft tissue augmentation at implant sites. J Clin Periodontol. 2016;43:874–85. https://doi.org/10.1111/jcpe.12588.
Schmitt CM, Brückbauer P, Schlegel KA, et al. Volumetric soft tissue alterations in the early healing phase after peri- implant soft tissue contour augmentation with a porcine collagen matrix versus the autologous connective tissue graft: A controlled clinical trial. J Clin Periodontol. 2021;48:145–62. https://doi.org/10.1111/jcpe.13387.
Gargallo-Albiol J, Barootchi S, Tavelli L, et al. Efficacy of xenogeneic collagen matrix to augment peri-implant soft tissue thickness compared with autogenous connective tissue graft: A systematic review and meta-analysis. Int J Oral Maxillofac Implant. 2019;34:1059–69. https://doi.org/10.11607/jomi.7497.
De Angelis P, De Angelis S, Passarelli PC, et al. Clinical comparison of a xenogeneic collagen matrix versus subepithelial autogenous connective tissue graft for augmentation of soft tissue around implants. Int J Oral Maxillofac Surg. 2021;50:956–63. https://doi.org/10.1016/j.ijom.2020.11.014.
Ashurko I, Esayan A, Magdalyanova M, et al. Current concepts of surgical methods to increase mucosal thickness during dental implantation. J Adv Pharm Educ Res. 2021;11:37–41. https://doi.org/10.51847/bY9xiuZKJf.
Zuiderveld EG, Meijer HJA, Vissink A, et al. The influence of different soft-tissue grafting procedures at single implant placement on esthetics: A randomized controlled trial. J Periodontol. 2018;89:903–14. https://doi.org/10.1002/JPER.18-0061.
Moraschini V, Guimarães HB, Cavalcante IC, et al. Clinical efficacy of xenogeneic collagen matrix in augmenting keratinized mucosa round dental implants : a systematic review and meta-analysis. Clin Oral Investig. 2020;24:2163–74. https://doi.org/10.1007/s00784-020-03321-5.
Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343. https://doi.org/10.1136/bmj.d5928.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. https://doi.org/10.7326/0003-4819-151-4-200908180-00135.
Tavelli L, Barootchi S, Avila-Ortiz G, et al. Peri-implant soft tissue phenotype modification and its impact on peri-implant health: a systematic review and network meta-analysis. J Periodontol. 2021;92:21–44. https://doi.org/10.1002/JPER.19-0716.
Cairo R, Barbato L, Tonelli P, et al. Xenogeneic Collagen Matrix versus Connective Tissue Graft for buccal soft tissue augmentation at implant site. A randomized, controlled clinical trial. J Clin Periodontol. 2017;44:769–76. https://doi.org/10.1111/jcpe.12750.
Cosyn J, Eeckhout C, Christiaens V, et al. A multi-centre randomized controlled trial comparing connective tissue graft with collagen matrix to increase soft tissue thickness at the buccal aspect of single implants: 3-month results. J Clin Periodontol. 2021;48:1502–15. https://doi.org/10.1111/jcpe.13560.
Puzio M, Błaszczyszyn A, Hadzik J, et al. Ultrasound assessment of soft tissue augmentation around implants in the aesthetic zone using a connective tissue graft and xenogeneic collagen matrix – 1-year randomised follow-up. Ann Anat. 2018;217:129–41. https://doi.org/10.1016/j.aanat.2017.11.003.
Zeltner M, Jung RE, Hämmerle CHF, et al. Randomized controlled clinical study comparing a volume-stable collagen matrix to autogenous connective tissue grafts for soft tissue augmentation at implant sites: linear volumetric soft tissue changes up to 3 months. J Clin Periodontol. 2017;44:446–53. https://doi.org/10.1111/jcpe.12697.
Baldi N, Buti J, Mensi M, et al. Xenogeneic dermal matrix versus autologous connective tissue graft versus no graft at abutment connection for improving aesthetics: 6-month outcomes of a randomised controlled trial. Clin Trials Dent. 2020;2:49–62. https://doi.org/10.36130/CTD.03.2020.05.
Ashurko I, Tarasenko S, Esayan A, et al. Clinical Oral Investigations Connective tissue graft versus xenogeneic collagen matrix for soft tissue augmentation at implant sites : a randomized-controlled clinical trial. Clin Oral Investig. 2022;26:7191–208. https://doi.org/10.1007/s00784-022-04680-x.
Hélio M, Daiane P, Elizabeth M, et al. Peri-implant soft tissue augmentation with palate subepitelial connective tissue graft compared to porcine collagen matrix: A randomized controlled clinical study and histomorphometric analysis. Int J Appl Dent Sci. 2019;5:319–25.
Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Wiley. 2019.
Zafiropoulos G-G, John G. Use of collagen matrix for augmentation of the peri-implant soft tissue at the time of immediate implant placement. J Contemp Dent Pract. 2017;18:386–91. https://doi.org/10.5005/jp-journals-10024-2052.
Schallhorn RA, McClain PK, Charles A, et al. Evaluation of a porcine collagen matrix used to augment keratinized tissue and increase soft tissue thickness around existing dental implants. Int J Periodontics Restorative Dent. 2015;35:99–103. https://doi.org/10.11607/prd.1888.
Eeckhout C, Bouckaert E, Verleyen D, et al. A 3-year prospective study on a porcine-derived acellular collagen matrix to re-establish convexity at the buccal aspect of single implants in the molar area: a volumetric analysis. J Clin Med. 2020;9:1568. https://doi.org/10.3390/jcm9051568.
Verardi S, Orsini M, Lombardi T, et al. Comparison between two different techniques for peri-implant soft tissue augmentation: Porcine dermal matrix graft versus tenting screw. J Periodontol. 2020;91:1011–7. https://doi.org/10.1002/JPER.19-0447.
Liu X, Xu YY, Lai HC, et al. Comparison of acellular dermal matrix graft and palatal connective tissue graft in the augmentation of labial tissue around implants. Shanghai kou qiang yi xue [Shanghai J Stomatol]. 2007;16:484–8.
Amin M. Comparison between acellular dermal matrix and connective tissue graft on vertical soft tissue thickness during submerged implant insertion, https://en.irct.ir/trial/59383 (2021).
Lai HC, Xu YY, Zhang ZY, et al. Buccal soft tissue augmentation using acellular dermal matrix in implant therapy. Zhonghua Kou Qiang Yi Xue Za Zhi. 2006;41:395–6.
Tavelli L, Barootchi S, Vera Rodriguez M, et al. Early soft tissue changes following implant placement with or without soft tissue augmentation using a xenogeneic cross-link collagen scaffold: A volumetric comparative study. J Esthet Restor Dent. 2022;34:181–7. https://doi.org/10.1111/jerd.12856.
Froum SJ, Khouly I, Tarnow DP, et al. The use of a xenogeneic collagen matrix at the time of implant placement to increase the volume of buccal soft tissue. Int J Periodontics Restor Dent. 2015;35:179–89. https://doi.org/10.11607/prd.2226.
Maiorana C, Beretta M, Pivetti L, et al. Use of a collagen matrix as a substitute for free mucosal grafts in pre-prosthetic surgery: 1 year results from a clinical prospective study on 15 patients. Open Dent J. 2016;10:395–410. https://doi.org/10.2174/1874210601610010395.
Artzi Z, Renert U, Netanely E, et al. Histologic composition of marginal mucosal tissue augmented by a resorbable volume-stable collagen matrix in soft tissue thickening procedures in humans: a morphometric observational study. Clin Oral Investig. 2022;26:427–35. https://doi.org/10.1007/s00784-021-04016-1.
Zafiropoulos G-G, Deli G, Hoffmann O, et al. Changes of the peri-implant soft tissue thickness after grafting with a collagen matrix. J Indian Soc Periodontol. 2016;20:441–5. https://doi.org/10.4103/0972-124X.181245.
Santagata M, Tartaro G, D’Amato S. Clinical and histologic comparative study of subepithelial connective tissue graft and extracellular matrix membrane. A preliminary split-mouth study in humans. Int J Periodontics Restor Dent. 2015;35:85–91. https://doi.org/10.11607/prd.1849.
Sanz M, Lorenzo R, Aranda JJ, et al. Clinical evaluation of a new collagen matrix (Mucograft® prototype) to enhance the width of keratinized tissue in patients with fixed prosthetic restorations: A randomized prospective clinical trial. J Clin Periodontol. 2009;36:868–76. https://doi.org/10.1111/j.1600-051X.2009.01460.x.
Thoma DS, Gasser TJW, Jung RE, et al. Randomized controlled clinical trial comparing implant sites augmented with a volume-stable collagen matrix or an autogenous connective tissue graft: 3-year data after insertion of reconstructions. J Clin Periodontol. 2020;47:630–9. https://doi.org/10.1111/jcpe.13271.
Huber S, Zeltner M, Hämmerle CHF, et al. Non-interventional 1-year follow-up study of peri-implant soft tissues following previous soft tissue augmentation and crown insertion in single tooth gaps. J Clin Periodontol. 2018;45:504–12. https://doi.org/10.1111/ijlh.12426.
Puzio M, Hadzik J, Błaszczyszyn A, et al. Soft tissue augmentation around dental implants with connective tissue graft (CTG) and xenogenic collagen matrix (XCM) 1-year randomized control trail. Ann Anat. 2020;230:151484. https://doi.org/10.1016/j.aanat.2020.151484.
Cosyn J, Eeckhout C, De Bruyckere T, et al. A multi-centre randomized controlled trial comparing connective tissue graft with collagen matrix to increase soft tissue thickness at the buccal aspect of single implants: 1-year results. J Clin Periodontol. 2022;49:911–21. https://doi.org/10.1111/jcpe.13691.
Studer SP, Lehner C, Bucher A, et al. Soft tissue correction of a single-tooth pontic space: A comparative quantitative volume assessment. J Prosthet Dent. 2000;83:402–11. https://doi.org/10.1016/S0022-3913(00)70034-5.
Thoma DS, Naenni N, Benic GI, et al. Soft tissue volume augmentation at dental implant sites using a volume stable three-dimensional collagen matrix – histological outcomes of a preclinical study. J Clin Periodontol. 2017;44:185–94. https://doi.org/10.1111/jcpe.12635.
Fickl S, Nannmark U, Schlagenhauf U, et al. Porcine dermal matrix in the treatment of dehiscence-type defects – an experimental split-mouth animal trial. Clin Oral Implants Res. 2015;26:799–805. https://doi.org/10.1111/clr.12355.
Chackartchi T, Romanos GE, Sculean A. Soft tissue-related complications and management around dental implants. Periodontol. 2019;2000(81):124–38. https://doi.org/10.1111/prd.12287.
Roccuzzo M, Grasso G, Dalmasso P. Keratinized mucosa around implants in partially edentulous posterior mandible: 10-year results of a prospective comparative study. Clin Oral Implants Res. 2016;27:491–6. https://doi.org/10.1111/clr.12563.
Lorenzo R, García V, Orsini M, et al. Clinical efficacy of a xenogeneic collagen matrix in augmenting keratinized mucosa around implants: A randomized controlled prospective clinical trial. Clin Oral Implants Res. 2012;23:316–24. https://doi.org/10.1111/j.1600-0501.2011.02260.x.
Sanz-Martín I, Rojo E, Maldonado E, et al. Structural and histological differences between connective tissue grafts harvested from the lateral palatal mucosa or from the tuberosity area. Clin Oral Investig. 2019;23:957–64. https://doi.org/10.1007/s00784-018-2516-9.
Bassetti RG, Stähli A, Bassetti MA, et al. Soft tissue augmentation procedures at second-stage surgery: a systematic review. Clin Oral Investig. 2016;20:1369–87. https://doi.org/10.1007/s00784-016-1815-2.
Valles C, Vilarrasa J, Barallat L, et al. Efficacy of soft tissue augmentation procedures on tissue thickening around dental implants: A systematic review and meta-analysis. Clin Oral Implants Res. 2022;33:72–99. https://doi.org/10.1111/clr.13920.
Cairo F, Barbato L, Selvaggi F, et al. Surgical procedures for soft tissue augmentation at implant sites. A systematic review and meta-analysis of randomized controlled trials. Clin Implant Dent Relat Res. 2019;21:1262–70. https://doi.org/10.1111/cid.12861.
Zeng L, Brignardello-Petersen R, Hultcrantz M, et al. GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. J Clin Epidemiol. 2021;137:163–75. https://doi.org/10.1016/j.jclinepi.2021.03.026.
Acknowledgements
None.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization, I.A. and S.T.; Methodology, I.A., M.M. and S.T.; Software, M.B., I.A., A.G. and M.M.; Validation, I. A and S.T.; Formal Analysis, M.B and I.A.; Investigation, I.A., S.B., M.K., M.Z., A.G. and M.M.; Data curation, I.A and S.T.; Writing—Original Draft, I.A., S.B., M.M., M.K., A.G., M.B. and M.Z.; Writing—Review & Editing, I.A., S.T., M.M. and A.G.; Visualization, I.A., M.B., A.U. and S.T.; Supervision, I.A. and M.M.; Project administration, I.A and A.U.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors gave their written consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ashurko, I., Tarasenko, S., Magdalyanova, M. et al. Comparative analysis of xenogeneic collagen matrix and autogenous subepithelial connective tissue graft to increase soft tissue volume around dental implants: a systematic review and meta-analysis. BMC Oral Health 23, 741 (2023). https://doi.org/10.1186/s12903-023-03475-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-023-03475-0