Abstract
Background/objective
Disinfection of a 3D-printed surgical guide is of utmost importance as it comes into contact with hard and soft tissue during implant placement so it poses a potential risk of pathogenic transmission. Methods used for disinfection in the surgical field should be reliable, practical, and safe for the instruments and the patients. The objectives of this study were to compare the antimicrobial potential of 100% Virgin Coconut Oil, 2% Glutaraldehyde, and 70% Ethyl Alcohol used to decontaminate 3D-printed surgical guides.
Materials and methods
Thirty identical surgical guides were printed and cut into two halves (N = 60). Both halves were then contaminated with a defined amount of human saliva samples (2 ml). The first half (n = 30) was sub-grouped into three study groups which were immersed in one of the three disinfectants for 20 min as follows; group VCO was immersed in 100% Virgin Coconut Oil, group GA was immersed in 2% Glutaraldehyde, and group EA was immersed in 70% Ethyl Alcohol. The second half (n* = 30) was sub-grouped into three control groups which were immersed in sterile distilled water as follows group VCO*, group GA*, and group EA*. The microbial count was expressed as colony-forming units per plate and the comparison of the antimicrobial potential of the three tested disinfectants between the three study and three control groups was done using the One-Way ANOVA test.
Results
The culture results of three study groups revealed no bacterial growth with the highest % of reduction in the mean microbial count of the oral microorganisms (about100%) and an uncountable bacterial growth was shown between the three control groups (more than 100 CFU/plate) representing the baseline of the oral microorganisms. Therefore; statistically significant differences were found between the three control and three study groups (P < .001).
Conclusion
The antimicrobial potential of Virgin Coconut Oil was comparable and equivalent to Glutaraldehyde and Ethyl Alcohol with a significant inhibitory action against oral pathogens.
Similar content being viewed by others
Introduction
Prosthodontics has been cited as one of the dental specialties that most neglect infection control guidelines where the dental office-prosthesis laboratory connection represents a tremendous potential for infection pathway if effective disinfection procedures are not performed properly [1]. Cross-infection can be carried directly by blood or saliva and indirectly by contaminated equipment, surfaces, and airway which could transmit infectious diseases such as Human Immunodeficiency Virus (HIV), Hepatitis-B virus, Pneumonia, and Tuberculosis, thus contamination of the dental environment creates a great risk to patients and healthcare workers [2]. Moreover, the global outbreak of coronavirus disease (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) made infection control an essential practice for dental clinics and a crucial protocol for preserving the practice’s integrity [3, 4]. Currently, one of the growing technologies that lead to the birth of computer-guided surgery which is changing the manufacturing industry is additive manufacturing, rapid prototyping, or three-dimensional (3D) printing surgical guide, this medical device that aids in selecting the precise site for implant placement and minimizes the operators’ risk of error [5]. Surgical guide is not only known to be porous and has irregularities and crevices which may have a marked effect on microbial adherence and biofilm formation, but also, it comes in contact with the blood, bone, and oral fluids of the patient during surgical procedures and, causes serious infections if it is contaminated by any extraoral pathogens [6].
Accordingly, the United States Food and Drug Administration (FDA), Centers for Disease Control and Prevention (CDC), and the American Dental Association (ADA) categorized the surgical guide as a critical item that penetrates soft tissues or bones and has to be sterilized by steam heat. However, it was claimed that owing to the thermosensitivity of the surgical guide, it should not be sterilized but immersed in effective disinfecting solutions [7]. The existing disinfecting solutions, such as 2% Glutaraldehyde (GA) and 70% Ethyl Alcohol (EA), are potentially hazardous [8]. GA vapor caused serious adverse health effects such as asthma, rhinitis, and, bronchial hyperresponsiveness [9]. Although EA had substantial antimicrobial properties, it evaporated rapidly making extended exposure time difficult to achieve unless the items were immersed [10]. Additionally, certain pathogen populations were becoming more tolerant to EA exposure thus, the use of different antibacterial products in the clinical setting was highly suggested [11].
Subsequently, the emergence of antimicrobial resistance had become a frequent issue for practitioners therefore, researchers had significantly attracted attention to exploring novel antimicrobial compounds utilizing natural products of plant origin [12]. Nowadays, many herbs derived ingredients are being used as antiseptics, such as Cocos nucifera or “Virgin Coconut Oil’’ (VCO) which can be exceptionally advantageous, as it possessed antibacterial, antiviral, antioxidant, antifungal, and antiprotozoal characteristics towards a broad range of microorganisms [13, 14]. VCO is different from most other dietary oils because the predominant composition is a medium-chain fatty acid (MCFA), whereas, in the majority of other oils, the basic building blocks are almost entirely long-chain fatty acids [15]. In this regard, bactericidal activity could be decreased by increasing the chain length of the fatty acids and influencing their physical and chemical properties [16]. Notably, VCO is produced.from fresh coconut without the application of heat, which does not lead to alterationof the nature of the oil and its important content can be preserved [17]. The Medium Chain Triglyceride (MCT) content in VCO is lauric acid followed by myristic acid, palmitic acid, capric acid, caprylic acid, oleic acid, stearic acid, linoleic acid, and caproic acid, as shown in Table 1 [12, 18]. Consequently, VCO contains 92% saturated fatty acids, approximately 50% of which is lauric acids that can prevent infection and excessive cell damage due to activation of TGF-β cytokines which stimulate fibronectin in fibrin clots formation, then become the framework for re-epithelialization and accelerate the healing process [19]. Nevertheless, lauric acid is being formed into monolaurin in the human body, which is demonstrated to be an active antimicrobial monoglyceride [20]. It produces highly ordered membranes, which is thought to disrupt membrane function by affecting signal transduction due to blockage of promoters, uncoupling of energy systems, altered respiration state, and amino acid uptake Furthermore, it could solubilize lipids and phospholipids from bacterial cell membranes causing disintegration, inhibiting pathogen maturation, and preventing its binding to host cells [21]. This leads to the destruction of the cell membrane of DNA and RNA viruses such as HIV, Herpes, Cytomegalovirus, Influenza, various pathogenic bacteria such as Listeria monocytogene, and protozoa such as Giardia lamblia [22, 23]. Moreover, the alkalis in the saliva react with VCO resulting in saponification and the formation of a soap-like substance which reduces the adhesion of plaque. Hence, lauric acid reacts with salivary sodium hydroxide forming sodium laureate, the main constituent of soap which might be responsible for the cleansing action and decreased plaque accumulation [24]. Additionally, the acidic pH nature of VCO between 2.52 and 4.38 was considered to be an important attribute of its microbial inhibitory action against many gram-positive and gram-negative organisms such as Escherichia vulneris, Enterococcer species, Helicobacter pylori, Staphylococcus aureus, and Streptococcus mutans [25,26,27,28]. Remarkably, its glucolipid component, sucrose monolaurate has an anti-cariogenic property due to reduced glycolysis and sucrose oxidation and its viscous nature promotes lubrication, thereby inhibiting adhesion of bacteria or its by-products on the mucosal tissues [29]. It is worth noting that VCO has antipyretic and analgesic effects by stimulating the new blood vessels formation and by lowering the surface area of the wound., thus it has a miraculous healing power that acts as a natural antibiotic and also helps modulate immunity [30]. Few published research are reported about the effect of VCO as a disinfectant used for decontamination of 3D printed surgical guides. Thus, this study aimed to compare the antimicrobial potential of 100% VCO, 2%GA, and 70% EA used to decontaminate 3D-printed surgical guides. The null hypothesis was that no significant differences would be found in the antimicrobial potential of 100% of VCO, compared to 2% GA and 70% EA.
Materials and methods
Study setting
This in vitro comparative study was held at the Department of Prosthodontics, Faculty of Dentistry, and the Department of Microbiology, Medical Research Institute, Alexandria University. Prior to commencement, all the methods were approved by the Institutional Review Board (IRB) of Research Ethics Committee at the Faculty of Dentistry, Alexandria University, Egypt (IRB 00010556– IORG 0008839) after ensuring that all methods are in accordance with the Helsinki declaration.
Sample size calculation
The minimal sample size was calculated based on a previous study aimed to assess the antimicrobial effectiveness of 100% VCO, 2% GA, and 70% EA disinfectants used to decontaminate 3D-printed surgical guides. Khalil et al., [8] concluded that antimicrobial effectiveness was the same between the three tested disinfectants without showing any microbial growth. Based on their results, adopting a power of 80% to detect a standardized effect size in the dimensional accuracy (d = 0.9102) (large-sized standardized effect size), and level of significance 95% (alpha = 0.05), the minimum required sample size was found to be 60 surgical guides (30 surgical guides cut into two halves, number of groups per each half = 3, and number of surgical guides per group = 10 [31]. Any error in the procedure that may lead to the loss of any sample (guide) was compensated by replacing the lost guide with a new one to maintain the required minimum sample size of 60 and to control for attrition (withdrawal) bias [32].
Study design
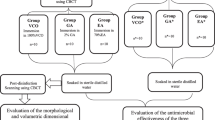
Thirty identical surgical guides were printed and cut into two halves (N = 60) then contaminated with a defined amount of human saliva samples (2 ml), collected from healthy partially edentulous participants attending the Department of Prosthodontics, Faculty of Dentistry, Alexandria University. The first half (n = 30) was sub-grouped into three study groups as follows: group VCO (n = 10), group GA (n = 10), and group EA (n = 10) which was immersed for 20 min in one of three disinfectants which were, 100%VCO, 2% GA, and 70% EA, then soaked in sterile distilled water. The second half (n* = 30) was sub-grouped into three control groups as follows: group VCO*(n* = 10), group GA*(n* = 10), and group EA*(n* = 10) which was soaked in sterile distilled water for 20 min for the assessment of the antimicrobial potential of the three tested disinfectants (Fig. 1).
Surgical guides production
A surgical guide was fabricated by making an impression of a mandibular dental arch with bilateral missing second premolars and first molars using polyether impression material and poured with dental stone to obtain a study cast. The study cast was scanned using CBCT (Acteon, X-mind Trium, Italy). The DICOM (Digital Imaging and Communications in Medicine) data were exported as a Standard Tessellation Language (STL) file to create a 3D model where the surgical guide was virtually designed using an open platform software (Blue Sky). Ten identical surgical guides were printed for each group using a clear photoreactive resin material (Resin cartridge form2; Formlabs Inc) using a desktop stereolithography 3D printer (formlabs 2, Formlabs Inc, Somerville, MA, USA) with the following parameters; layer thickness = 0.1 mm, layers number = 672, layer volume = 103.74 ml, offset (block out undercuts offset) = 0.15 and printing time = 6 h 45 min [33]. The 3D-printing works by adding layers of curable liquid photopolymer onto a build tray where fine layers accumulated to create 3D-surgical guides which were rinsed in a bath of 90% Isopropyl Alcohol for 10 min and then inserted in a bath of clean, unused, 90% Isopropyl Alcohol. The printed guides were left to dry for an additional 10 min and then exposed to 72 watts of Blue Ultraviolet light oven (315- 400 nm) for 10 min at 60 °C according to the manufacturer’s instruction, to achieve optimal mechanical properties. The support material was removed after curing using the flush cutter included in the Formlabs Standard Finish Kit. The surgical guides were then cut into two halves using a sterile cutting disk at low speed [34, 35] (Figs. 2 and 3a, b).
Collection of saliva samples and contamination protocol
Prior to the start of the microbiological trial, samples of saliva were obtained from healthy partially edentulous participants. Additionally, all test subjects were clear of any type of drugs/medicines in their systems and, attending the Department of Prosthodontics, Faculty of Dentistry, Alexandria University. The saliva samples were collected directly from the human mouths by instructing the participants to spit into sterile containers in the morning before breakfast and the surgical guides were then placed inside the containers. 2 ml of fresh saliva samples were applied on the surfaces of each half using a micropipette, and the containers were then manually shaken vigorously to facilitate the dispersion of the microorganisms [36].
Microbiological trial
After saliva contamination, the first half of the three study groups were immersed into one of the three disinfectants for 20 min then left to dry for an additional 10 min then soaked in sterile glass containers containing 100 ml. of sterile distilled water for 10 min. An extra step was done, in which a 50 µl (microliter) sample was taken directly from the surface of the surgical guides where the saliva was applied and disinfected with 100% VCO before its immersion in the sterile distilled water [37,38,39]. The second half of the three control groups were immersed in sterile glass containers containing 100 ml. of sterile distilled water for 20 min. From each half, a 15 ml falcon tube was filled and placed on the Scilogex Vortex mixer for 10 s (Fig. 4). Three samples were pipetted and cultured on three microbiological media; Blood, MacConkey, and Sabouraud dextrose agar plates then the microbial count was expressed as colony-forming units per plate (CFU/plate). The percentage (%) of reduction was calculated by the following equation: [40].
Statistical analysis
The comparison of the antimicrobial potential of the three tested disinfectants between the three study and three control groups was analyzed using the One-Way ANOVA (Analysis of variance) test that was used to verify the normality for all variables using descriptive statistics, plots, and normality tests. All variables showed normal distribution, so the mean, standard deviation (SD), and CFU/plate were calculated. The level of statistical significance was set at p < 0.05. Data were analyzed with IBM SPSS statistical software V 23.0, (SPSS Inc).
Results
The microbial count showed statistically significant differences between the three control and three study groups (P < 0.001) with the highest % of reduction in the mean number of oral microorganisms (about 100%) between the three study groups. The culture results showed an uncountable bacterial growth on the control plates (more than 100 CFU/ plate) representing the baseline of the oral microorganisms and the study plates showed no bacterial growth after the use of the three disinfectants (Tables 2, 3, 4 and Figs. 5, 6, 7 and 8). Regarding the direct sample that was collected from the surface of the surgical guides disinfected with 100% VCO, no bacterial growth was revealed (Table 5, Figs. 9, 10 and 11).
Discussion
The antimicrobial potential of the three disinfectants used to decontaminate the tested surgical guides was estimated and the null hypothesis was accepted as no significant differences were found in the antimicrobial potential of 100% VCO, compared to 2% GA and 70% EA.
The microbiological results showed that the investigated disinfectants had potentially considerable inhibitory capabilities against the oral pathogens detected in the saliva samples. The use of saliva biofilm extracted from partly edentulous subjects was required to identify the oral environment and to imitate different types of bacteria commonly prevalent in the oral cavity. Consequently, this investigation might provide the foundation for future in vivo studies evaluating the antibacterial properties of VCO as a mouthwash under preoperative surgery settings [40].
Indeed, the used concentration of VCO used in the present study was 100% because researchers were having difficulty dissolving or diluting the VCO during the microbiological trial. Organic solvents commonly used to dilute VCO include methanol, ethanol, and dimethyl sulfoxide. Nonetheless, because these solvents contain antibacterial capabilities, the test results are erroneous. Until recently, the majority of antimicrobial tests were conducted directly between the tested bacteria and the extract [12]. Consequently, these findings were supported by Gayatri et al. who revealed that the concentration of the antibacterial compounds in killing bacteria is linked with the effect caused. This correlation might be described using the hyperbolic curve; thus, increasing the concentration would raise the maximal effect of the agent [41].
The immersion technique was used as a disinfection method rather than spraying as spraying reduced disinfection effectiveness because the disinfectant could not reach the entire surface, especially given the porous nature of the surgical guide surface, where microorganisms could penetrate and survive [42]. On the contrary, immersion is regarded as the most reliable and safest procedure since disinfectants were applied to all surfaces of the surgical guide [43].
It is important to highlight that the contact time for the tested disinfectants was set to be 20 min as recommended by the fabricant/ manufacturer for an ideal disinfection outcome. As a result, the CDC considers 20 min at room temperature to be the minimal exposure period required to reliably kill bacteria with 2% GA and similarly, to disinfect contaminated surfaces with 70% EA. Moreover, VCO was found to decrease the total oral microbial count, after about 20 min of contact time [44].
The use of bench-top laboratory vortex mixers during the microbiological trial was proposed since it is a method often utilized in biofilm investigations and is more commonly employed for bacterial aggregation dispersion. Because the centrifugal rotator force was the main working mechanism of a vortex mixer, it minimized the time required for mixing and the results were instant. As a result, vortexing for 10 s or more reduced bacterial viability because it acted through typical tourbillon and swirling movements and by the effect of the water flow motion, removing or reducing the adherence of microorganisms on a surface, allowing proper shaking and mixing as well as a homogeneous distribution of the microorganisms within the solution [45, 46].
The extra step that was done where a direct sample was collected from the surfaces of the first half of the surgical guides that were disinfected with 100% VCO, was extremely essential to assess the local antimicrobial effect of VCO on the oral pathogens that would be present in the oral cavity (in vivo) and to evaluate its inhibitory action on various microorganisms found in the saliva samples. Moreover, the beneficial topical effect of VCO showed promising results by eradicating 100% of the microbes found on the surgical guides. These findings were coincided with the results of other investigations that indicated the local oral effect of VCO when used during the oil-pulling therapy – an alternate, substantial, cleansing method consisting of rinsing the oral cavity by ingesting a tablespoon of VCO for 20 min of contact time – to reduce the oral bacteria and improve oral health [37,38,39].
Comparatively, Menaka et al. [47] confirmed the findings of this study by illustrating that VCO oil pulling was as beneficial as Chlorhexidine mouthwash in decreasing plaque. It was assumed that because of agitation, VCO oil pulling generated mechanical shear load, resulting in oil emulsification and greater surface area, which inhibited plaque adherence and microbial coaggregation. Despite being commonly regarded as the "gold standard" among other orally administered treatments, chlorhexidine-containing mouthwash had a disagreeable flavor and unfavorable adverse effects such as tooth discoloration as well as mucosal irritation [48]. A randomized clinical trial conducted by Patel et al. [49] stated that oil pulling with VCO could be used as a preventive and therapeutic agent in gingivitis due to the significant reduction in values of plaque and gingival scores in patients with mild to moderate gingivitis. Besides that, a meta-analysis suggested by Peng et al. [24] indicated that oil pulling significantly reduced oral bacterial colony count compared to water or Chlorhexidine. Dewi et al. [50] recommended that the use of VCO could show a reduction in the number of Porphyromonas gingivalis and Treponema denticolaon at the margin of the porcelain-fused-metal crown.
The results were also in agreement with Ogboulu et al. [51] who reported VCO's antifungal activity was compared to fluconazole, a first-line treatment for drug-resistant Candida albicans. Also, VCO and its most active fatty acids were tested in vitro for their antibacterial properties against Clostridium difficile [52]. Subsequently, Abbas et al. [53] investigated the antimicrobial activity of VCO and affirmed that the powerful lauric acid was highly effective against Staphylococcus aureus, Streptococci, and Lactobacilli. Similarly, the current study was comparable to Widianingrum et al. [54] who concluded that the VCO has the potential to suppress the growth of Staphylococcus aureus and boost the ability of phagocytic immune cells to fight it, making it a viable alternative to antibiotics and a modulator of the cellular immune system. Interestingly, Horas et al. [55] showed that applying VCO to the palatal surgical wound during the palatoplasty hastened wound healing, raised the number/amount of fibroblast cells that developed in the wound, and reduced pain sensations. Research conducted by Silalahi et al. [56] proved that applying VCO topically to wounds obtained faster healing due to the decreased epithelialization time, increased fibroblast proliferation, and higher collagen turnover. Remarkably, Khalil et al. [8] documented that when 3D-printed surgical guides were examined at various time intervals from the manufacturing stage, the antibacterial efficiency of VCO was similar to 2% GA and 70% EA without displaying any microbial growth on the tested surgical guides. Another study was performed by Kamalaldin et al. [57] on a rabbit model of allergic asthma, where the effect of VCO inhalation on airway remodeling was studied. The percentage of inflammatory cells infiltrated, the thickness of the epithelium and mucosa regions, and the quantity of goblet and proliferative cells were all reduced, indicating that VCO inhalation was successful at alleviating airway inflammation and relieving asthma-related symptoms. Along with that, Luiz Henrique C. Vasconcelos et al. [58] demonstrated for the first time that VCO supplementation had a potential role in the adjuvant treatment for guinea pigs with persistent allergic lung inflammation, due to its impact on the inflammatory and oxidative processes of the airways. Moreover, Dayrit et al. [59] provided a scientific motivation for the adoption of VCO as prospective prophylactic therapy for COVID-19 patients and a general preventive medication against numerous microbial illnesses. However, it is crucial to note that the implementation of VCO as a disinfectant was not commonly discussed in dentistry, this is why its microbiological efficiency was compared with 2% GA and 70% EA, which are the most widely used disinfectants in the dental profession [42]. Although the current study lauded the disinfectants' antimicrobial efficiency in removing 100% of the organisms detected on the contaminated surgical guides, there were concerns regarding the occupational and environmental risks generated by GA and EA [8]. Shi et al. [60] identified latent GA to be a possible mutagen, inducing significant carcinogenic effects in mice lymphocytes. Furthermore, after 15 s of contact, SARS-CoV-2 could not be fully inactivated by EA in vitro research [61]. According to research, when administered to the skin, EA had no discernible long-term residual activity; nevertheless, the regeneration of bacteria happened slowly due to the devastating impact that EA might have on the persistent microorganisms [62]. In light of the aforementioned, the present work showed that VCO had completely eradicated many oral microorganisms and could function as an effective antimicrobial agent without showing adverse effects therefore, its risk-free application together with no documented or observed reports of harmful or toxic effects had expanded the possibilities of its use against different types of infections. Hence, further studies are required to prove that VCO is highly recommended and strongly desirable not only for the disinfection of 3D-printed objects but also as a surgical site disinfectant for providing a proper sterile operative area and thus, preventing infections during the surgical procedure [40]. However, the current research has some limitations. This study focused on the microbial count together with the presence/absence of the microbial species and did not focus on the identification of particular structural properties of the bacterial characteristics present on the surface of the surgical guides prior to and following the use of the antimicrobial agents. Thus, it could be interesting to discuss an atomic force microscopy study for evaluating guides surface before and after disinfection.
Conclusion
Based on the findings of this in vitro study, the antimicrobial potential of 100% VCO was comparable and equivalent to 2% GA and 70% EA with a significant inhibitory action against oral pathogens.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- HIV:
-
Human Immunodeficiency Virus
- SARS-COV-2:
-
Severe Acute Respiratory Syndrome Coronavirus
- 3D:
-
Three-dimensional
- FDA:
-
Food and Drug Administration
- CDC:
-
Center for Disease Control and Prevention
- ADA:
-
American Dental Association
- GA:
-
Glutaraldehyde
- EA:
-
Ethyl Alcohol
- MCT:
-
Medium Chain Triglyceride
- VCO:
-
Virgin Coconut Oil
- µl:
-
Microliter
- ANOVA:
-
Analysis of variance
- CFU:
-
Colony-forming units
- SD:
-
Standard deviation
References
Halawani R, Aboalshamat K, Alwsaidi R, Sharqawi S, Alhazmi R, Abualsaud Z, Alattallah A, Alamri M. Awareness and Practices of Dental Students and Dentists Regarding Infection Control in Prosthodontic Clinics. The Open Dent J 2020;16:184-90.
Abinaya K, Muthu Kumar B, Ahila SC. Evaluation of Surface Quality of Silicone Impression Materials after Disinfection with Ozone Water: An In vitro Study. Contemp Clin Dent. 2018;9:60–4.
Islam MS, Rahman KM, Sun Y, et al. Current knowledge of COVID-19 and infection prevention and control strategies in healthcare settings: A global analysis. Infect Control Hosp Epidemiol. 2020;41(10):1196–206. https://doi.org/10.1017/ice.2020.237.
K.S, Sumanth & Poovani, Shwetha & Shetty, Gautam & Sonnahalli, Nithin & Kumararama, Sindhu. Infection Control Protocol in Prosthodontics - A Review. Inter J Sci Res. 2019;8:1–3.
Ashtiani RE, Ghasemi Z, Nami M, Mighani F, Namdari M. Accuracy of static digital surgical guides for dental implants based on the guide system: A systematic review. J Stomatol Oral Maxillofac. 2021;122:600–7.
Remiszewski D. Effects of Various Decontamination Protocols on the Surface Microbial Load of Conventional and 3D Printed Surgical Guides for Dental Implants. 2020. Master's Theses. 1528. https://opencommons.uconn.edu/gs_theses/1528.
Marei HF, Alshaia A, Alarifi S, Almasoud N, Abdelhady A. Effect of Steam Heat Sterilization on the Accuracy of 3D Printed Surgical Guides. Implant Dent. 2019;28:372–7.
Khalil RT, Alshimy A, Elsherbini E, et al. The microbiological effect of virgin coconut oil on the morphological and volumetric dimensional changes of 3D printed surgical guides (in vitro study). BMC Oral Health. 2022;22:636. https://doi.org/10.1186/s12903-022-02671-8.
Chidambaranathan AS, Balasubramanium M. Comprehensive Review and Comparison of the Disinfection Techniques Currently Available in the Literature. J Prosthodont. 2019;28:e849–56.
Rutala WA, Weber DJ. Disinfection, sterilization, and antisepsis: An overview. Am J Infect Control. 2019; 47S:A3-A9. https://doi.org/10.1016/j.ajic.2019.01.018.
Bondurant SW, Duley CM, Harbell JW. Demonstrating the persistent antibacterial efficacy of a hand sanitizer containing benzalkonium chloride on human skin at 1, 2, and 4 hours after application. Am J Infect Control. 2019.S0196655319300082. https://doi.org/10.1016/j.ajic.2019.01.004.
Nasir NAMM, Abllah Z, Jalaludin AA, Shahdan IA, Manan WNHWA. Virgin Coconut Oil and Its Antimicrobial Properties against Pathogenic Microorganisms: A Review. International Dental Conference of Sumatera Utara 2017 (IDCSU 2017) 2018;8:192–9.
Dayrit FM. The properties of lauric acid and their significance in coconut oil. J Am Oil Chem Soc. 2015;92:1–5.
Umate N, Kuchewar V, Parwe S. A narrative review on the use of virgin coconut oil in dermatology. J Indian SysMedicine. 2022;10:86–9.
Wiyani L, Aladin A, Juniar ME. Antioxidant activity of Virgin Coconut Oil and Virgin Coconut Oil Emulsion. Syst Rev Pharm. 2020;11:973–6.
Nguyen V, Diep T. Efficacy of Enriched Medium-chain Fatty Acids in Virgin Coconut Oil in Their Antibacterial Activity against Food Pathogens. Philipp J Sci 2022;151:813–21. https://doi.org/10.56899/151.03.02.
Wiyani L, Aladin A, Putra B. Antidiabetic effect of the virgin coconut oil and the virgin coconut oil emulsions. Syst Rev Pharm. 2020;11:243–6.
Gurupadayya, B.M. & Spandana, T.Spandana.. Analytical Method Validation of Lauric Acid Present in Pure and Commercial Preparations of Coconut Oil using GC-FID Method. Int Res J Eng Technol. 2019;8:108–112. https://doi.org/10.17577/IJERTV8IS120081.
Thahir H, Irawaty Djais A, Nasir M, Rahayu Feblina A, Annisa A, Etriyani N, Achmad H. Virgin coconut oil as a new concept for periodontal tissue regeneration via expressions of TNF-α and TGF-β1. Int J Biomater. 2022;8:7562608.
Narayanankutty A, Illam SP, Raghavamenon AC. Health impacts of different edible oils prepared from coconut (Cocos nucifera): A comprehensive review. Trends Food Sci Technol. 2018;80:1–7.
do Couto MV, da Costa Sousa N, Abe HA, Dias JA, Cordeiro CA, Paixão PE, Santos TB, dos Santos Cunha F, Meneses JO, Bomfim CN, Honorato CA. Benefits of Virgin Coconut Oil in Diet to Colossoma macropomum (Cuvier, 1818). Aquacult Nutr. 2022;19;2022. https://doi.org/10.1155/2022/4387692.
Udensi, Justina & Umeh, Sarah & Mgbemena, Ifeyinwa & Emeka-Nwabunnia, Ijeoma & Ebe, Tochukwu & Aroh, Kenechukwu & O, Anyanwu. Antifungal Activities of Virgin Coconut Oil on Candida albicans, Aspergillus niger and Mould Species. Afr J Health Sci. 2021;7:889–93.
Siripaiboonpong N, Matangkasombut O, Pengcharoen H, Boonchaiyapluk B, Rujiraprasert P, Srithanyarat SS. Microbiological Effects of Virgin Coconut Oil Pulling in Comparison with Palm Oil Pulling as an Adjunctive Oral Hygiene Care for Patients with Gingival Inflammation: A Randomized Controlled Clinical Trial. J Indian Soc Periodontol. 2022;26:58.
Peng, T.-R.; Cheng, H.-Y.; Wu, T.-W.; Ng, B.-K. Effectiveness of Oil Pulling for Improving Oral Health: A Meta-Analysis. Healthcare. 2022;10:1991. https://doi.org/10.3390/healthcare1010199.
Kaliamoorthy S, Pazhani A, Nagarajan M, Meyyappan A, Rayar S, Mathivanan S. Comparing the effect of coconut oil pulling practice with oil pulling using sesame oil in plaque-induced gingivitis: A prospective comparative interventional study. J Nat Sc Biol Med. 2018;9:165–8.
Khor Y, Koh S, Long K, Long S, Ahmad S, Tan C. A comparative study of the physicochemical properties of a virgin coconut oil emulsion and commercial food supplement emulsions. Molecules. 2014;19:9187–202.
Parfene G, Horincar V, Tyagi AK, Malik A, Bahrim G. Production of medium chain saturated fatty acids with enhanced antimicrobial activity from crude coconut fat by solid state cultivation of Yarrowia lipolytica. Food Chem. 2013;136:1345–9.
Shino B, Peedikayil FC, Jaiprakash SR, Ahmed Bijapur G, Kottayi S, Jose D. Comparison of antimicrobial activity of chlorhexidine, coconut oil, probiotics, and ketoconazole on Candida albicans isolated in children with early childhood caries: An in vitro study. Scientifica (Cairo). 2016;2016:7061587.
Reddy U, Khijmatgar S, Hegde MN, Fabbro MD. Effects of coconut oil on oral health status of patients with poor oral hygiene: Systematic review and meta-analysis. J Int Oral Health. 2021;13:519–32.
Dafriani P, Nur SA, Morika HD, Marlinda R. Virgin coconut oil (VCO) accelerated wound healing process in diabetes mellitus (DM) patients with diabetic ulcer in dr. Rasidin Hospital, Padang, Indonesia. Jurnal Aisyah: Jurnal Ilmu Kesehatan. 2020;5:221–4.
Charan, J. and Biswas. T. How to Calculate Sample Size for Different Study Designs in Medical Research? Indian J Psychol Med Indian. 2013;35:121–6. https://doi.org/10.4103/0253-7176.116232.
Pannucci CJ, Wilkins EG. Identifying and avoiding bias in research. Plast Reconstr Surg. 2010;126:619–25. https://doi.org/10.1097/PRS.0b013e3181de24bc.
Ahmed MF, AbdelHamid AM, AlAbbasy FH. Accuracy of Implant Placement using two different types of CAD/CAM surgical guides. Alex Dent J. 2019;44:28–33. https://doi.org/10.21608/adjalexu.2019.63552.
Hada T, Kanazawa M, Iwaki M, Arakida T, Soeda Y, Katheng A, et al. Effect of Printing Direction on the Accuracy of 3D-Printed Dentures Using Stereolithography Technology. Material. 2020;13:3405. https://doi.org/10.3390/ma13153405.
Chepelev LL, Rybicki FJ. Sterilization of 3D Printed Parts Used as Medical Devices in the COVID-19 Pandemic. In 3D Printing in Medicine and Its Role in the COVID-19 Pandemic. Springer, Cham. 2021;12:107–13. https://doi.org/10.1007/978-3-030-61993-0_12.
Galo R, Maluta I, Contente MM, Torres CP, Borsatto MC. Effect of Saliva/Blood Contamination on Enamel Bond Strength. J Health Sci. 2021;23:277–81.
Seher F, Hosein M, Ahmed J. Role of coconut oil pulling on oral health–an overview. J Pakistan Dental Asso. 2018;27:94–9.
Zope SA. Effect of coconut oil pulling on plaque-induced gingivitis: A prospective clinical study. Int J Green Pharm. 2018;11:S750–5.
Shanbhag VK. Oil pulling for maintaining oral hygiene-A review. J Tradit Complement Med. 2017;7:106.
El-Sayed M, El-Dokky N, Eissa S. Evaluation of the antimicrobial effect of Coconut and Nigella Sativa oils on Streptococcous mutans, Lactobacilli, and Candida albicans an invitro-study. Egypt Dent J. 2017;63:2969–78. https://doi.org/10.21608/edj.2017.76077.
Gayatri A, Fauziah EV, Suharsini M. Antibacterial effect of virgin coconut oil on the viability of chromogenic bacteria that causes dental black stain in children. Int J App Pharm. 2018;90:83–6. https://doi.org/10.22159/ijap.2017.v9s2.20.
Török G, Gombocz P, Bognár E, Nagy P, Dinya E, Kispélyi B, Hermann P. Effects of disinfection and sterilization on the dimensional changes and mechanical properties of 3D printed surgical guides for implant therapy a pilot study. BMC Oral Health. 2020;20:102.
Altaf J, Malik MHA, Mir HA, Mushtaq MA, Munir MU, Shah AA. The effect of sodium hypochlorite disinfectant on the linear dimensional stability of alginate impression material. Professional Med J. 2022;29:1310–4. https://doi.org/10.29309/TPMJ/2022.29.09.6200.
Saher F, Hosein M, Ahmed J. Role of coconut oil pulling on oral health - an overview. J Pak Dent Assoc. 2018;27(3):94–9.
B, Webber, R. Canova & L.M. Esper, et al. The Use of Vortex and Ultrasound Techniques for the in vitro Removal of Salmonella spp. Biofilms Acta Scientiae Veterinariae. 2015;43:1332.
Totten AH, Xiao L, Crabb DM, Ratliff AE, Dybvig K, Waites KB, Atkinson TP. Shaken or stirred?: Comparison of methods for dispersion of Mycoplasma pneumoniae aggregates for persistence in vivo. J Microbiol Methods. 2017;132:56–62. https://doi.org/10.1016/j.mimet.2016.11.011.
Menaka V, Kavya G, Bhuvaneshwari R, Azali AS, Aparna S, Kumar PD. Effectiveness of coconut oil pulling as an adjuvant to oral hygiene procedure on plaque-induced gingivitis among middle-aged adults – An interventional study. J Global Oral Health. 2019;2(2):102–7.
James P, Worthington HV, Parnell C, Harding M, Lamont T, Cheung A, Whelton H, Riley P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst Rev. 2017;3:1–194. https://doi.org/10.1002/14651858.CD008676.pub2.
Patel P, Deshpande N. Comparative clinical evaluation of olive oil and coconut oil rinse on plaque levels in gingivitis patients. A Randomized clinical trial. J Orofac Res. 2020;7:63–7.
Dewi RS, Gita F, Bahtiar B, Kasim HB. Effect Of 12.5% Virgin Coconut Oil on Porphyromonas Gingivalis and Treponema Denticola Bacterial Colonization. Int J Appl Pharm. 2017;9:32–5.
Ogbolu DO, Oni AA, Daini OA, Oloko AP. In vitro antimicrobial properties of coconut oil on Candida species in Ibadan. Nigeria J Med Food. 2007;10:384.
Shilling M, Matt L. Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile. J Med Food. 2013;16:1079–85.
Abbas AA, Assikong EB, Akeh M, Upla P, Tuluma T. Antimicrobial activity of coconut oil and its derivative (lauric acid) on some selected clinical isolates. Int J Med Sci Clin Invent. 2017;4:3173–7.
Widianingrum DC, Noviandi CT, Salasia SI. Antibacterial and immunomodulator activities of Virgin Coconut Oil against Staphylococcus aureus. Heliyon. 2019;5: e02612. https://doi.org/10.1016/j.heliyon.2019.e02612.
Horas Rajagukguka, Sumaryati Syukurb, Sanusi Ibrahimc, Syafrizayantid. Beneficial Effect of Application of Virgin Coconut Oil (VCO) Product from Padang West Sumatra, Indonesia on Palatoplasty Wound Healing. Am Sci Res J Eng Technol Sci. 2017;34(1):231–6.
Silalahi J, Yuandani Y, Meliala DIPB, Margata L, Satria D. The Activity of Hydrolyzed Virgin Coconut Oil to Increase Proliferation and Cyclooxygenase-2 Expression towards on NIH 3T3 Cell Line in Wound Healing Process. Open Access Maced J Med Sci. 2019;7(19):3164–3168. Published 2019 Oct 14
Kamalaldin et al., Does Inhalation of Virgin Coconut Oil Accelerate Reversal of Airway Remodelling in an Allergic Model of Asthma? Int J Inflammation. 2017;Article ID 8741851, 11.
Luiz Henrique C. Vasconcelos, Maria da Conceição C. Silva, Alana C. Costa, Giuliana A. de Oliveira, Iara Leão Luna de Souza, Renato F. Righetti, Fernando R. Queiroga, Glêbia A. Cardoso, Alexandre S. Silva, Patrícia M. da Silva, Giciane C. Vieira, Iolanda de F. L. C. Tibério, Marta S. Madruga, Fabiana de A. Cavalcante, Bagnólia A. da Silva. Virgin Coconut Oil Supplementation Prevents Airway Hyperreactivity of Guinea Pigs with Chronic Allergic Lung Inflammation by Antioxidant Mechanism. Oxidative Med Cell Longevity. 2020; 2020Article ID 5148503:16. https://doi.org/10.1155/2020/5148503.
Dayrit FM, Angeles-Agdeppa I, Nacis JS, Capanzana MV, Tanda KV. Virgin coconut oil is effective in lowering C-reactive protein levels among suspect and probable cases of COVID-19. J Funct Foods. 2021;24: 104557.
Shi J, Lian H, Huang Y, et al. In vitro genotoxicity evaluation and metabolic study of residual glutaraldehyde in animal-derived biomaterials. Regen Biomater. 2020;7:619–25. https://doi.org/10.1093/rb/rbaa04.
Bidra AS, Pelletier JS, Westover JB, Frank S, Brown SM, Tessema B. Comparison of In Vitro Inactivation of SARS CoV-2 with Hydrogen Peroxide and Povidone-Iodine Oral Antiseptic Rinses. J Prosthodont. 2020;29:599–603. https://doi.org/10.1111/jopr.1322.
Gold NA, Mirza TM, Avva U. Alcohol Sanitizer. In: StatPearls. Treasure Island: StatPearls Publishing; 2022.
Acknowledgements
The authors would like to thank Dr. Akram Fathy Neena, Lecturer of Prosthodontics, Faculty of Dentistry, Alexandria University for his great assistance with the 3D-printing and designing process of the surgical guides, and Dr. Maged Ahmed Gadallah, Assistant Lecturer of Prosthodontics, Faculty of Dentistry, Alexandria University for his precious efforts during the study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors have no funding sources to declare.
Author information
Authors and Affiliations
Contributions
RTK, AA, EE and MEA contributed to conceptualization, methodology. RTK contributed to data collection, curation, material preparation and analysis of the laboratory data. EE supervised processing of microbiological samples, analysis and acquisition of microbiological data. MEA contributed to interpretation of data. RTK contributed to writing-original draft preparation, writing- review and editing final manuscript. All authors contributed to critical revision and approval of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Prior to the commencement of the study, ethical approval was obtained from the Institutional Review Board (IRB) of the Research Ethics Committee at the Faculty of Dentistry, Alexandria University, Egypt (IRB 00010556–IORG 0008839) after ensuring that all methods are in accordance with the Helsinki declaration. Owing to the fact that there is minimal risk involved in the study of using the saliva samples, informed verbal consent in front of a witness was obtained at the time of collection of the saliva samples declaring that these samples will be used for research purposes. The informed verbal consent was performed in compliance with the educational institution’s regulations and approved by the Institutional Review Board (IRB) of the Research Ethics Committee at the Faculty of Dentistry, Alexandria University, Egypt (IRB 00010556–IORG 0008839).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Khalil, R.T., Alshimy, A., Elsherbini, E. et al. Disinfection of 3D-printed surgical guides using virgin coconut oil (in vitro study). BMC Oral Health 23, 379 (2023). https://doi.org/10.1186/s12903-023-03092-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-023-03092-x