Abstract
Background
SGLT2 inhibitors and DPP4 inhibitors have been suggested to affect lipid metabolism. However, there are few randomized controlled trials comparing the effects on the lipid metabolism between the two types of antidiabetic drugs. The SUCRE study (UMIN ID: 000018084) was designed to compare the effects of ipragliflozin and sitagliptin on serum lipid and apolipoprotein profiles and other clinical parameters.
Methods
This is a multicenter, open-label, randomized, controlled trial. Patients with type 2 diabetes (20–74 years old) with HbA1c levels of 7.0-10.5% and serum triglyceride levels of 120–399 mg/dL (1.35–4.50 mmol/L) on diet and/or oral hypoglycemic agents were enrolled. Subjects were randomized to treatment with ipragliflozin (50 mg/day, n = 77) or sitagliptin (50 mg/day, n = 83). Laboratory measurements were performed at 0, 1, 3, and 6 months of treatment.
Results
Ipragliflozin and sitagliptin reduced fasting plasma glucose, glycoalbumin, and HbA1c almost equally. Ipragliflozin increased HDL-C and decreased apo E. Sitagliptin decreased TG, apo B48, CII, and CIII, but increased LDL-C. The between-treatment differences were significant for HDL-C (P = 0.02) and apo B48 (P = 0.006), and nearly significant for apo A1 (P = 0.06). In addition, ipragliflozin reduced body weight, blood pressure, serum liver enzymes, uric acid, and leptin, and increased serum ketones compared with sitagliptin.
Conclusions
While ipragliflozin and sitagliptin showed similar effects on glycemic parameters, the effects on serum lipid and apolipoprotein profiles were different. Ipragliflozin may have an anti-atherogenic effect through modulation of HDL-C and apo E compared to sitagliptin through TG and apo B48, CII, and CIII in patients with type 2 diabetes.
Similar content being viewed by others
Background
Diabetes is a major risk factor for the development of atherosclerosis and is associated with a 2- to 4-fold increased risk of cardiovascular disease [1]. Hyperglycemia, especially postprandial hyperglycemia, has been shown to cause atherosclerosis [2]. Adequate glycemic control is therefore important for the prevention of cardiovascular disease. Both sodium-glucose cotransporter 2 (SGLT2) and dipeptidyl peptidase-4 (DPP4) inhibitors are promising drugs for this purpose [3]. SGLT2 inhibitors promote urinary glucose excretion by inhibiting renal tubular SGLT2 and improve hyperglycemia in an insulin-independent manner [4]. On the other hand, DPP4 inhibitors maintain blood incretin levels by inhibiting the incretin-degrading activity of DPP4, promote insulin secretory secretion in response to blood glucose levels, and improve glucose metabolism [5]. SGLT2 inhibitors are expected to improve hyperglycemia even in the presence of insulin deficiency, while DPP4 inhibitors are effective in those with some preservation of insulin secretion [3].
Both SGLT2 and DPP4 inhibitors exhibit diverse advantages beyond their glucose-lowering properties. DPP4 inhibitors have a neutral influence on body weight, minimal risk of hypoglycemia [6], and safety in terms of major cardiovascular outcomes [7], excluding heart failure with saxagliptin [8] and possibly alogliptin [9]. Conversely, SGLT2 inhibitors provide significant benefits, including body weight reduction [10], decreased risk of atherosclerotic cardiovascular disease and heart failure [11, 12], beneficial effects on kidney function [13], and a reduction in all-cause mortality [14]. Furthermore, both agents have been shown to affect lipid metabolism. For example, SGLT2 inhibitors have been reported to increase low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) [15, 16] and DPP4 inhibitors to decrease triglycerides (TG) and remnants [17, 18]. Since dysregulation of lipid metabolism plays a key role in the development of atherosclerosis associated with diabetes [19], it is of great importance to elucidate the pleiotropic effects of these drugs on lipid and apolipoprotein profiles. This understanding is crucial in formulating an optimal treatment strategy for diabetes, particularly in conjunction with atherosclerosis. However, there are few randomized controlled trials comparing the effects of SGLT2 inhibitors and DPP4 inhibitors on lipid and apolipoprotein profiles. Here, we conducted a multicenter, randomized trial to compare the effects of a SGLT2 inhibitor (ipragliflozin) and a DPP4 inhibitor (sitagliptin) in patients with type 2 diabetes. The SUCRE study was designed to analyze the effects of ipragliflozin and sitagliptin primarily on lipid metabolism, and secondarily on glucose metabolism and other clinical parameters related to atherosclerosis, to clarify the anti-atherogenic benefits of the two drugs. The primary outcome measures were serum lipids and apolipoproteins. Secondary outcome measures included glucose metabolism parameters, body weight, systolic and diastolic blood pressure, and renal function parameters.
Methods
Study design
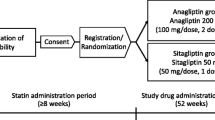
The present study was designed as a multicenter, open-label, randomized controlled trial. This study was registered in the University Hospital Medical Information Network (UMIN) as the Sodium-GlUcose Co-transporteR 2 inhibitor, Ipragliflozin, Suglat®, Effect on Lipid and Glucose Metabolism (SUCRE) Study (UMIN ID: 000018084). Recruitment and follow-up of participants took place from June 2015 to September 2019 at 14 hospitals and clinics across Japan. The study adhered to the principles of the Declaration of Helsinki and was approved by the ethics committees at Fukujuji Hospital, Japan Anti-Tuberculosis Association and, where appropriate, at each of the participating hospitals and clinics. Written informed consent was obtained from all patients.
Eligible patients were outpatients with type 2 diabetes aged 20–74 years with inadequate glycemic control (HbA1c of 7.0-10.5%) and serum TG of 120–399 mg/dL (1.35–4.50 mmol/L). Patients treated with lifestyle modification and/or antidiabetic agents, excluding insulins, GLP-1 receptor agonists, SGLT2 inhibitors, and DPP4 inhibitors, were included in the study. Glycemic control requires HbA1c variability to be stable within 1% in the absolute value over the past 2 months. Patients were ineligible if they received continuous treatment with lipid-lowering agents (statins, ezetimibe, fibrates, nicotinic acid, probucol, anion exchange resins, or eicosatetraenoic acid); if they had type 1 diabetes, proliferative retinopathy, dysuria, urinary and genital infections, chronic renal impairment (serum creatinine (Cr) > 1.5 mg/dL), severe liver dysfunction (Child-Pugh class C), severe infection, trauma, or possible pregnancy; if they have a medical history of ketosis, diabetic coma, cardiovascular disease (cerebral infarction, myocardial infarction, unstable angina, angioplasty, or heart disease, NYHA class III-IV) in the past 6 months; treated with diuretics, steroids or immunosuppressive therapy for the past 6 months; or if they were allergic to SGLT2 or DPP4 inhibitors. Patients were also ineligible if the study physicians deemed it inappropriate for them to participate.
Eligible patients were enrolled and randomized (1:1) to the ipragliflozin group or the sitagliptin group. Randomization was performed using a computer-generated randomization list generated by an independent statistician using the permuted block method with a block size of 10 and equal allocation to the two treatments. Both patients and study physicians were informed of their treatment assignment. Patients received 50 mg of ipragliflozin or 50 mg of sitagliptin once daily for at least 6 months in addition to their previous treatment. The doses were determined according to the usual doses of both drugs in Japan. The glycemic target was HbA1c < 7.0%. Study physicians were asked not to change the antidiabetic regimen during the first 3 months. Thereafter, dose escalation of sitagliptin or ipragliflozin up to 100 mg/day was allowed to achieve the glycemic target. The addition, dose titration, or discontinuation of oral antidiabetic medications other than SGLT2 inhibitors, DPP4 inhibitors, and GLP-1 receptor agonists was also allowed as needed. No medications for dyslipidemia were prescribed during the study period.
Measurements
Venous blood and urine samples were collected under fasting conditions at 0, 1, 3, and 6 months of treatment, and the samples were transported to the central laboratory (SRL Inc., Tokyo, Japan), where venous blood was centrifuged to separate serum or plasma for biochemical measurements. Blood pressure and body weight were measured at the same time of blood sampling. Peripheral blood cell counts were measured at 0, 1, 3, and 6 months.
The primary outcome measures were serum lipids and apolipoproteins. Lipid-related parameters included serum total cholesterol (TC), HDL-C, LDL-C, and TG and apolipoprotein (apo) AI, AII, B, B48, CII, CIII, and E. Secondary outcome measures included glucose metabolism parameters [FPG, insulin, HbA1c, and glycoalbumin (GA)], body weight, systolic and diastolic blood pressure, renal function parameters such as estimated glomerular filtration rate (eGFR) and urine albumin-to-Cr ratio (UACR). Serum Cr and cystatin C (CysC) concentrations were used to calculate Cr-based eGFR and cystatin CysC-based eGFR, respectively [20, 21]. Other laboratory measurements included blood cell counts, serum liver enzymes, uric acid, electrolytes, compliments, leptin, non-cholesterol sterols, selected fatty acids, and ketones.
All the laboratory measurements were performed at a central laboratory (SRL, Tokyo, Japan) using the standard methods. Details of the methods and resources are provided in Table S1. Briefly, plasma glucose was measured by the hexokinase UV method; serum insulin concentration and HbA1c by chemiluminescence enzyme immunoassays for each; and GA by the visible absorption spectrometry enzymatic method. Serum TC and TG were measured by enzymatic methods for each; HDL-C by direct assay. LDL-C was calculated using the Friedewald formula. Apo AI, AII, B, CII, CIII, and E were measured by turbidimetric immunoassays by using corresponding individual kits. Apo B48 was measured by chemiluminescent enzyme-linked immunosorbent assay using apo B48 CLEA (Fujirebio Inc., Tokyo, Japan).
Statistical analysis
We did not statistically determine a required sample size because the present study was exploratory with respect to serum apolipoproteins. There was no information on potential differences in changes in apolipoproteins between ipragliflozin and sitagliptin treatment at the time of protocol development. The target number of patients to be enrolled was set at 200 (100 for each group) based on a previous study that reported statistically significant changes in some of the serum apolipoproteins with sitagliptin treatment [22].
The full analysis set for the assessment of outcome variables included randomized patients who had a baseline measurement and at least one follow-up measurement and who adhere to the study protocol. We did not use measurements of FPG, insulin, and TG if the blood sample was reported to have been collected in postprandial condition or if plasma insulin was ≥ 25 µU/mL (175 pmol/L). LDL-C was not calculated if TG was ≥ 400 mg/dL. Therefore, the number of patients varied at different time points during the follow-up varied (Table S2).
The distribution of each continuous variable was tested for normality using the skewness-kurtosis test. Baseline characteristics of patients were presented as mean with standard deviation (SD) for normally distributed variables, median with interquartile range (IQR, 25th and 75th percentiles) for continuous variables with a non-normal distribution, and proportions for categorical variables. Comparisons between groups at baseline were made using the unpaired t-test for variables with a normal distribution, the Wilcoxon rank-sum test for continuous variables with a non-normal distribution, and Fisher’s exact test for categorical variables. A mixed-model repeated-measures analysis was used to assess the between-treatment difference in the change during treatment from baseline in an outcome parameter (dependent variable). Adjustment was made for sex, age, and baseline measurement of a parameter of interest. Fixed effects were treatment, month of visit, variables used for adjustment, and treatment by month interaction, and the random effect was patient. Month of visit was treated as a categorical variable, and an unstructured variance-covariance matrix was used. The robust method was used to estimate the standard errors to allow for potential departure from the model assumption. Changes from baseline were used as such in the mixed model analysis, except for the urine albumin-to-creatinine ratio, which had an extremely skewed distribution and was transformed to natural logarithm. Marginal means and 95% confidence intervals were estimated. The analysis of adverse events was performed on all the patients who started treatment, and the comparison was based on the actual treatment received. The occurrence of adverse events was compared using Fisher’s test. P values < 0.05 (two-tailed) were considered statistically significant. Statistical analysis was performed using Stata version 13 software (StataCorp LLC, College Station, TX, USA).
Results
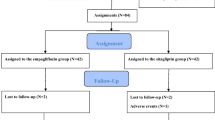
A total of 175 patients were enrolled and randomized to ipragliflozin or sitagliptin. Of the participants, a total of 15 patients were excluded due to protocol violations (n = 8), no baseline measurements (n = 2), withdrawal of informed consent (n = 3), and no follow-up measurements (n = 2). Finally, 160 patients (n = 77 and n = 83 in the ipragliflozin and sitagliptin groups, respectively) were remained in the analysis (Fig. 1).
Flowchart of subject enrollment. A total of 175 patients were enrolled and randomized to ipragliflozin or sitagliptin. Of the 175 participants, 15 were excluded from the efficacy analysis due to protocol violations, no baseline measurements, withdrawal of informed consent, and no follow-up measurements. I.C., informed consent
Baseline characteristics and measurements
There were 44 (57.1%) and 44 (53.0%) men in the ipragliflozin and sitagliptin groups, respectively. There were no significant differences in the frequency of alcohol consumption, smoking, comorbidities other than hypertension, and medical history (Table 1). Antidiabetic medications were used by 52 (67.5%) and 56 (67.5%) patients in the ipragliflozin and sitagliptin groups, respectively (P = 1.00). The number and class of the medications did not differ between the two groups. For example, the number of participants using pioglitazone was 4 (5.2%) and 3 (3.6%) cases in the ipragliflozin and sitagliptin groups, respectively (P = 0.71). Except for systolic blood pressure, there were no significant differences in the clinical and laboratory measurements among the participants (Table 2). Among the other laboratory measurements, serum complement C3 were higher in the ipragliflozin group (Table S3).
Glucose metabolism parameters
Changes in glucose metabolism parameters from baseline are shown in Fig. 2; Table 3. FPG, HbA1c, and GA were significantly decreased by the treatments to almost the same extent in both groups (Fig. 2A and C, and 2D). Serum insulin decreased at 1 and 3 months in the ipragliflozin group (Fig. 2B).
Treatment effects of ipragliflozin and sitagliptin on glucose metabolism parameters. Changes from baseline in fasting plasma glucose (FPG, mmol/L) (A), serum insulin (pmol/L) (B), HbA1c (%) (C), and glycoalbumin (GA, %) (D) in the ipragliflozin and sitagliptin groups are shown as closed circles with blue lines and closed squares with red lines, respectively. Data are expressed as mean ± 95% confidence interval of the mean. *, P < 0.05; ***, P < 0.001 vs. baseline in each group in the mixed-effects model analysis. §, P < 0.05 for interaction during the overall study period, vs. the sitagliptin group in the mixed-effects model analysis
Serum lipids and apolipoproteins
Changes in serum lipids and apolipoproteins from baseline are shown in Figs. 3 and 4; Table 3. Although TC did not change in either group (Fig. 3A), LDL-C increased consistently in the sitagliptin group; TG decreased transiently in both groups (Fig. 3C and D); and HDL-C increased fairly consistently in the ipragliflozin group (Fig. 3B). A significant between-treatment difference was observed only for HDL-C (Table 3).
Treatment effects of ipragliflozin and sitagliptin on serum lipids. Changes from baseline in serum total cholesterol (TC, mg/dL) (A), high-density lipoprotein cholesterol (HDL-C, mg/dL) (B), low-density lipoprotein cholesterol (LDL-C, mg/dL) (C), and triglycerides (TG, mg/dL) (D) in the ipragliflozin and sitagliptin groups are shown as closed circles with blue lines and closed squares with red lines, respectively. Data are expressed as mean ± 95% confidence interval of the mean. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. baseline in each group; †, P < 0.05 for difference at the follow up visit; ‡, P < 0.05 for difference during the overall study period, vs. the sitagliptin group in the mixed-effects model analysis
Treatment effects of ipragliflozin and sitagliptin on serum apolipoproteins. Changes from baseline in serum apolipoprotein (Apo) AI (mg/dL) (A), AII (mg/dL) (B), B (mg/dL) (C), B48 (µg/dL) (D), CII (mg/dL) (E), CIII (mg/dL) (F), and E (mg/dL) (G) in the ipragliflozin and sitagliptin groups are shown as closed circles with blue lines and closed squares with red lines, respectively. Data are expressed as mean ± 95% confidence interval of the mean. *, P < 0.05; **, P < 0.01, ***, P < 0.001 vs. baseline in each group; ††, P < 0.01 for difference at the follow up visit; ‡‡, P < 0.01 for difference during the overall study period, vs. the sitagliptin group in the mixed-effects model analysis
Apo AI increased gradually and apo AII decreased transiently in the ipragliflozin group (Fig. 4A and B). Although apo B did not change in either group, apo B48 decreased consistently in the sitagliptin group (Fig. 4C and D). Apo CII decreased consistently and apo CIII decreased almost consistently in the sitagliptin group (Fig. 4E and F). Apo E decreased transiently in the ipragliflozin group (Fig. 4G). Consequently, overall reductions in apo E were observed in the ipragliflozin group, and in apo B48, CII, and CIII in the sitagliptin group (Table 3). A significant between-treatment difference was observed for apo B48 and a near-significant difference for apo A1 (Fig. 4D; Table 3).
Body weight, blood pressure, and renal function
Changes from baseline in the secondary outcome measures other than glucose metabolism parameters are shown in Table 4. Body weight and blood pressure decreased significantly in the ipragliflozin group but not in the sitagliptin group, resulting in overall significant between-treatment differences. In the ipragliflozin group, the reduction in body weight was greater in the later months of follow-up (P < 10− 3 for interaction). Regarding renal function parameters, eGFR decreased consistently in both groups during the treatment period, although the decrease in Cr-based eGFR was less pronounced in the ipragliflozin group. The overall reduction in UACR was significant in the ipragliflozin group, but there was no between-treatment difference.
Other laboratory measurements
Overall changes in other laboratory measurements during the treatment period are summarized in Table S4. Between-treatment differences were observed in blood cell counts except for white blood cells and platelets, serum liver function parameters (AST, ALT, GGT, and LDH), uric acid, leptin, and ketones. Most of the differences were induced by ipragliflozin treatment, such as increases in blood cell counts and ketones, and decreases in liver function parameters, uric acid, and leptin.
Adverse events
The analysis of adverse events included 171 patients who received actual treatment with each drug; 80 and 91 patients with ipragliflozin and sitagliptin, respectively. Reported adverse events are summarized in Table S5. The number of patients reporting at least one adverse event was 20 (25.0%) in the ipragliflozin group and 7 (7.7%) in the sitagliptin group. Skin lesions and elevated blood ketones were reported only in the ipragliflozin group. Two patients in the ipragliflozin group discontinued treatment due to the occurrence of rash or cholelithiasis/cholecystitis.
Discussion
In this multicenter, open-label, randomized, controlled trial, we found that although both ipragliflozin and sitagliptin reduced glucose metabolism parameters to a similar extent, the effects on serum lipid and apolipoprotein profiles were completely different. Ipragliflozin increased HDL-C and decreased apo E, whereas sitagliptin decreased TG, apo B48, CII, and CIII but increased LDL-C. Ipragliflozin was more likely to improve cardiovascular disease risk factors, i.e., HDL-C, body weight, blood pressure, and uric acid, compared with sitagliptin.
To date, three randomized controlled trials have compared ipragliflozin and sitagliptin head-to-head in patients with type 2 diabetes [23,24,25]. Contrary to the present finding, these studies reported greater reductions in FPG with ipragliflozin than with sitagliptin at 12 [23], 24 [24], and 52 weeks [25] of treatment, whereas reductions in HbA1c did not show a measurable difference between the two treatments [23,24,25]. These findings may indicate that sitagliptin primarily act on the improvement of postprandial blood glucose levels compared to ipragliflozin. However, under the conditions of the present study, both ipragliflozin and sitagliptin reduced FPG and HbA1c to a similar extent. Furthermore, GA, which reflects postprandial blood glucose levels more accurately than HbA1c, was similarly decreased by both drugs. We do not have a direct explanation for the present findings that differ from previous observations, but the discrepancy in FPG may be due to differences in study design regarding the use of other antidiabetic drugs. The present study allowed adjustment of existing antidiabetic drugs after the first 3 months of treatment, whereas the previous studies did not allow such adjustment. However, the reductions in FPG did not differ between the two treatments even at 1 month and 3 months when adjustment for antidiabetic drugs was not allowed. The previous studies [23, 24] and the present study showed that ipragliflozin reduced body weight more, accompanied by increased levels of ketone bodies, as compared with sitagliptin. This could be due to increased fat oxidation as a result of accelerated urinary glucose excretion caused by the SGLT2 inhibitory effect of ipragliflozin. In addition, it has been suggested that body weight loss with ipragliflozin can be partly explained by a reduction in extracellular water [26], as SGLT2 inhibitors have an osmotic diuretic effect with urinary excretion of sodium accompanied by glucose [27]. This consideration may be supported by the reductions in blood pressure and serum uric acid, and increases in red blood cell count and hematocrit in the ipragliflozin group in the present study.
The present study showed that ipragliflozin and sitagliptin had different effects on lipid and apolipoprotein profiles despite similar hypoglycemic effects. Ipragliflozin increased HDL-C without affecting TC and LDL-C, whereas sitagliptin decreased TG but increased LDL-C without affecting TC and HDL-C. In a meta-analysis by Li, et al. [16]. , SGLT2 inhibitors increased TC, LDL-C, and HDL-C and decreased TG. However, the effects of SGLT2 inhibitors on TC and TG were not consistent across studies. On the other hand, an increase in HDL-C with SGLT2 inhibitors was consistently found in meta-analyses [28, 29]. Several possible mechanisms have been proposed for the HDL-C increase with SGLT2 inhibitors; (1) increased lipolysis of triglycerides, (2) decreased CETP activity, and (3) de novo production of nascent HDL [30, 31]. According to the finding that apo A1 was gradually increased by ipragliflozin in the present study, the HDL increase by SGLT2 inhibitors is more likely explained by de novo synthesis of apo AI and subsequent production of nascent HDL. Notably, although there was no difference between treatments, apo E was significantly reduced in the ipragliflozin group. The liver is a major source of apo E [32], and elevated plasma apo E levels have been reported in patients with non-alcoholic fatty liver disease (NAFLD) regardless of apo E genotype [33]. Since ipragliflozin has been shown to improve hepatic steatosis [34], and serum liver enzymes were reduced in the present study, circulating apo E may have been reduced as hepatic steatosis was improved. On the other hand, sitagliptin reduced TG and apo B48 without affecting HDL-C and apo AI. These results suggest that the effect of sitagliptin on triglyceride metabolism can be explained by an inhibition of lipoprotein production from the small intestine, as manifested by decreases in apo B48 and CII, and not by a lipolytic activity associated with an increase in HDL-C. These findings are consistent with previous reports that sitagliptin suppressed postprandial triglyceride elevation [17] and reduced fasting apo B48 in addition to CII [22]. In addition, a single-arm, observational study of sitagliptin treatment in drug naïve patients with type 2 diabetes suggested that sitagliptin might improve atherogenic lipids in a glycemic efficacy dependent manner [35]. However, the present study would not be able to address such a hypothesis based on patients with a baseline HbA1c of 10.2%, which was much higher than the baseline HbA1c of 7.4% in the sitagliptin group.
SGLT2 inhibitors slow the decline in eGFR in patients with type 2 diabetes, even with a transient reduction in eGFR early in treatment [36]. DDP-4 inhibitors have been reported to be associated with a continuous decline in eGFR [37]. The present study showed that both ipragliflozin and sitagliptin decreased eGFR, particularly CysC-based eGFR, to a similar extent up to 6 months of treatment. In contrast to eGFR reduction, both SGLT2 and DDP-4 inhibitors are protective against worsening of albuminuria and may even reduce UACR [36,37,38]. In the present study, we could show a statistically significant UACR reduction with ipragliflozin treatment, but not with sitagliptin treatment, although the between-treatment difference was not appreciable. The findings are compatible with the recent observation in the network meta-analysis on SGLT2 inhibitors and DDP-4 inhibitors [39].
The present study has several limitations. First, it was an open-label study. Second, the number of cases was insufficient to detect between-treatment differences in changes in apolipoproteins when within-treatment differences were detected. Because more adverse events were observed in the ipragliflozin group, the smaller number of cases in this group may have influenced the study results. Third, dietary control was not strictly prescribed during the follow-up period. Fourth, the study protocol allowed for the addition, dose titration, or discontinuation of oral antidiabetic drugs other than SGLT2 inhibitors, DPP4 inhibitors, and GLP-1 receptor agonists after 3 months of treatment. In fact, changes in drug regimens were only made within the insulin sensitizer category. For example, metformin was started in four cases in the ipragliflozin group and pioglitazone in one case in the sitagliptin group. In addition, metformin and/or pioglitazone were discontinued in two cases in the sitagliptin group. However, this may have allowed us to evaluate the effects of ipragliflozin and sitagliptin on lipid and apolipoprotein profiles independent of glycemic levels. Fifth, we did not have detailed information on antihypertensive drugs while the prevalence of hypertension was higher in the ipragliflozin group. In fact, the participants using antihypertensive drugs were more frequent in the ipragliflozin group (n = 54, 70.1%) than in the sitagliptin group (n = 43, 51.8%) (P = 0.02). Some antihypertensive drugs have a neutral or beneficial effect on the lipid profile, while others have an adverse effect. Different use of antihypertensive drugs may influence the lipid profile. Finally, although the intent-to-treat (ITT) analysis is a principle in the randomized trial, the present study excluded patients with no follow-up measurements and those who did not follow to the protocol, necessarily causing the so-called attrition bias. The present study was exploratory, and the observed findings should be validated in the ITT analysis in larger randomized trials in the future. Further investigations, including animal or ex vivo studies, are also needed to fully understand the pharmacological effects of these drugs on lipid metabolism.
Conclusions
In summary, although ipragliflozin and sitagliptin showed similar effects on glycemic parameters in the present study, the effects of these drugs on lipid and apolipoprotein profiles were different. Both drugs have favorable effects on the profiles, i.e., increased HDL-C and decreased apo E with ipragliflozin and decreased TG, apo B48, CII, and CIII with sitagliptin. Ipragliflozin is more likely than sitagliptin to contribute to plaque reduction by improving cardiovascular disease risk factors, including HDL-C, body weight, blood pressure, and uric acid. These findings in the present study suggest a strong need for further large clinical trials to validate the protective effects of ipragliflozin on cardiovascular outcomes.
Data availability
Some or all datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- SGLT2:
-
Sodium-glucose cotransporter 2
- DPP4:
-
Dipeptidyl peptidase-4
- SUCRE:
-
Sodium-GlUose Co-transporteR 2 inhibitor, Ipragliflozin, Suglat®, Effect on Lipid and Glucose Metabolism
- UMIN:
-
University hospital Medical Information Network
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholestero
- TG:
-
Triglycerides
- HbA1c:
-
Glycohemoglobin
- Cr:
-
Creatinine
- NYHA:
-
New York Heart Association
- GLP-1:
-
Glucagon-like peptide-1
- Apo:
-
Apolipoprotein
- FPG:
-
Fasting plasma glucose
- GA:
-
Glycoalbumin
- eGFR:
-
Estimated glomerular filtration rate
- UACR:
-
Urine albumin-to-Cr ratio
- CysC:
-
Cystatin C
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- LDH:
-
Lactate dehydrogenase
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- GGT:
-
Gamma-glutamyl transferase
- NAFLD:
-
Non-alcoholic fatty liver disease
- ITT:
-
Intent-to-treat
References
Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241(19):2035–8.
Selvin E, Lazo M, Chen Y, Shen L, Rubin J, McEvoy JW, Hoogeveen RC, Sharrett AR, Ballantyne CM, Coresh J. Diabetes mellitus, prediabetes, and incidence of subclinical myocardial damage. Circulation. 2014;130(16):1374–82.
Lamos EM, Hedrington M, Davis SN. An update on the safety and efficacy of oral antidiabetic drugs: DPP-4 inhibitors and SGLT-2 inhibitors. Expert Opin Drug Saf. 2019;18(8):691–701.
Hedrington MS, Davis SN. Ipragliflozin, a sodium-glucose cotransporter 2 inhibitor, in the treatment of type 2 diabetes. Expert Opin Drug Metab Toxicol. 2015;11(4):613–23.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705.
Salvo F, Moore N, Arnaud M, Robinson P, Raschi E, De Ponti F, Begaud B, Pariente A. Addition of dipeptidyl peptidase-4 inhibitors to sulphonylureas and risk of hypoglycaemia: systematic review and meta-analysis. BMJ. 2016;353:i2231.
Liu D, Jin B, Chen W, Yun P. Dipeptidyl peptidase 4 (DPP-4) inhibitors and cardiovascular outcomes in patients with type 2 diabetes mellitus (T2DM): a systematic review and meta-analysis. BMC Pharmacol Toxicol. 2019;20(1):15.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–26.
Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385(9982):2067–76.
Liu XY, Zhang N, Chen R, Zhao JG, Yu P. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2years. J Diabetes Complications. 2015;29(8):1295–303.
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Comparison of the effects of Glucagon-Like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for Prevention of Major adverse Cardiovascular and renal outcomes in type 2 diabetes Mellitus. Circulation. 2019;139(17):2022–31.
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9.
Nuffield Department of Population Health Renal Studies, Consortium SiM-AC-RT G. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788–801.
Marilly E, Cottin J, Cabrera N, Cornu C, Boussageon R, Moulin P, Lega JC, Gueyffier F, Cucherat M, Grenet G. SGLT2 inhibitors in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials balancing their risks and benefits. Diabetologia. 2022;65(12):2000–10.
Basu D, Huggins LA, Scerbo D, Obunike J, Mullick AE, Rothenberg PL, Di Prospero NA, Eckel RH, Goldberg IJ. Mechanism of increased LDL (low-Density lipoprotein) and decreased triglycerides with SGLT2 (sodium-Glucose cotransporter 2) inhibition. Arterioscler Thromb Vasc Biol. 2018;38(9):2207–16.
Li D, Wu T, Wang T, Wei H, Wang A, Tang H, Song Y. Effects of sodium glucose cotransporter 2 inhibitors on risk of dyslipidemia among patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Pharmacoepidemiol Drug Saf. 2020;29(5):582–90.
Tremblay AJ, Lamarche B, Deacon CF, Weisnagel SJ, Couture P. Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13(4):366–73.
Tremblay AJ, Lamarche B, Kelly I, Charest A, Lepine MC, Droit A, Couture P. Effect of sitagliptin therapy on triglyceride-rich lipoprotein kinetics in patients with type 2 diabetes. Diabetes Obes Metab. 2014;16(12):1223–9.
Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes Mellitus-Atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 2020;21(5):1835.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–92.
Kakuta Y, Okumi M, Ichimaru N, Abe T, Nonomura N, Okuyama A, Kojima Y, Isaka Y, Takahara S, Imai E, et al. Utility of the Japanese GFR estimation equation for evaluating potential donor kidney function. Clin Exp Nephrol. 2010;14(1):63–7.
Tanimura-Inagaki K, Nagao M, Harada T, Sugihara H, Moritani S, Sasaki J, Kono S, Oikawa S. Slim study investigators: Sitagliptin improves plasma apolipoprotein profile in type 2 diabetes: a randomized clinical trial of sitagliptin effect on lipid and glucose metabolism (SLIM) study. Diabetes Res Clin Pract. 2020;162:108119.
Tsurutani Y, Nakai K, Inoue K, Azuma K, Mukai S, Maruyama S, Iizuka T, Matsuzawa Y, Saito J, Omura M, et al. Comparative study of the effects of ipragliflozin and sitagliptin on multiple metabolic variables in Japanese patients with type 2 diabetes: a multicentre, randomized, prospective, open-label, active-controlled study. Diabetes Obes Metab. 2018;20(11):2675–9.
Kang SM, Yun HM, Sohn M, Lim S. Vascular and metabolic effects of ipragliflozin versus sitagliptin (IVS) in type 2 diabetes treated with sulphonylurea and metformin: IVS study. Diabetes Obes Metab. 2023;25(7):1922–31.
Kitazawa M, Katagiri T, Suzuki H, Matsunaga S, M HY, Ikarashi T, Yamamoto M, Furukawa K, Iwanaga M, Hatta M, et al. A 52-week randomized controlled trial of ipragliflozin or sitagliptin in type 2 diabetes combined with metformin: the N-ISM study. Diabetes Obes Metab. 2021;23(3):811–21.
Kawata T, Iizuka T, Iemitsu K, Takihata M, Takai M, Nakajima S, Minami N, Umezawa S, Kanamori A, Takeda H, et al. Ipragliflozin improves Glycemic Control and decreases body Fat in patients with type 2 diabetes Mellitus. J Clin Med Res. 2017;9(7):586–95.
Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8(4):262–275e269.
Mazidi M, Rezaie P, Gao HK, Kengne AP. Effect of sodium-glucose Cotransport-2 inhibitors on blood pressure in people with type 2 diabetes Mellitus: a systematic review and Meta-analysis of 43 randomized control trials with 22 528 patients. J Am Heart Assoc. 2017;6(6):e004007.
Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab. 2016;18(8):783–94.
Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Boren J, Catapano AL, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32(11):1345–61.
Krauss RM, Wojnooski K, Orr J, Geaney JC, Pinto CA, Liu Y, Wagner JA, Luk JM, Johnson-Levonas AO, Anderson MS, et al. Changes in lipoprotein subfraction concentration and composition in healthy individuals treated with the CETP inhibitor anacetrapib. J Lipid Res. 2012;53(3):540–7.
Davignon J, Cohn JS, Mabile L, Bernier L. Apolipoprotein E and atherosclerosis: insight from animal and human studies. Clin Chim Acta. 1999;286(1–2):115–43.
van den Berg EH, Corsetti JP, Bakker SJL, Dullaart RPF. Plasma ApoE elevations are associated with NAFLD: the PREVEND Study. PLoS ONE. 2019;14(8):e0220659.
Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Li Q, Tomiyama H, et al. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715(1–3):246–55.
Kutoh E, Kuto AN, Wada A, Hayashi J, Kurihara R. Sitagliptin as an initial therapy and Differential regulations of metabolic parameters depending on its glycemic response in subjects with type 2 diabetes. Drug Res (Stuttg). 2021;71(3):157–65.
Seidu S, Kunutsor SK, Cos X, Gillani S, Khunti K, For, on behalf of Primary Care Diabetes E. SGLT2 inhibitors and renal outcomes in type 2 diabetes with or without renal impairment: a systematic review and meta-analysis. Prim Care Diabetes. 2018;12(3):265–83.
Bae JH, Kim S, Park EG, Kim SG, Hahn S, Kim NH. Effects of Dipeptidyl Peptidase-4 inhibitors on renal outcomes in patients with type 2 diabetes: a systematic review and Meta-analysis. Endocrinol Metab (Seoul). 2019;34(1):80–92.
Khoo CM, Deerochanawong C, Chan SP, Matawaran B, Sheu WH, Chan J, Mithal A, Luk A, Suastika K, Yoon KH, et al. Use of sodium-glucose co-transporter-2 inhibitors in Asian patients with type 2 diabetes and kidney disease: an Asian perspective and expert recommendations. Diabetes Obes Metab. 2021;23(2):299–317.
Bae JH, Park EG, Kim S, Kim SG, Hahn S, Kim NH. Comparative renal effects of Dipeptidyl Peptidase-4 inhibitors and sodium-glucose cotransporter 2 inhibitors on individual outcomes in patients with type 2 diabetes: a systematic review and network Meta-analysis. Endocrinol Metab (Seoul). 2021;36(2):388–400.
Acknowledgements
The authors thank Dot World Co., Ltd. (Tokyo) for data management and Dr. Suminori Kono (MedStat Corporation, Fukuoka) for assistance with statistical analysis and preparation of the manuscript. We thank all of the members of SUCRE study group; Dr. Sano R (Sano Clinic, Yamagata), Osonoi T (Naka Kinen Clinic, Ibaraki), Dr. Takahashi S (Ageo Central General Hospital, Saitama), Dr. Shuto H (Minamikoshigaya-Kennshinkai Clinic, Saitama), Dr. Moritani H (Moritani Clinic, Tokyo), Dr. Nakajima Y (Nakajima Clinic, Tokyo), Dr. Kimura K (Hachijyo Hospital, Tokyo), Dr. Suzuki J (University of Fukui Faculty of Medical Science, Fukui), Dr. Yamashita S (Rinku General Medical Center, Osaka), Dr. Ikeda Y (Obase Hospital, Fukuoka), Dr. Otonari T (Otonari Clinic, Fukuoka), Dr. Sawayama Y (Fukuoka Red Cross Hospital, Fukuoka), Dr. Hashiguchi Y (Tenpozan Naika Clinic, Kagoshima), Dr. Biro S (Tsukasa Health Care Hospital, Kagoshima), Dr. Yamakawa K (Chubu Tokushukai Hospital, Okinawa), Dr. Higa M (Tomishiro Central Hospital, Okinawa), and Dr. Yamada M (Yamada Clini, Okinawa). We also thank Dr. Tada N (Jikei University School of Medicine, Tokyo) and Dr. Kawakami M (Nerimahikarigaoka Hospital, Tokyo) for monitoring the safety of the participants during the study.
Funding
This trial was funded by Astellas Pharma Inc. The company had no role in the study design, selection of patients, data collation and analysis, interpretation of the results, or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
MN: data analysis, interpretation, and drafting and editing of the manuscript; JS: creation of the study design; KTI: data analysis and interpretation; SI: Patient recruitment and follow-up; HS: data interpretation; SO: study design, data analysis and interpretation, and critical revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study adhered to the principles of the Declaration of Helsinki and was approved by the ethics committees at Fukujuji Hospital, Japan Anti-Tuberculosis Association and, where appropriate, at each of the participating hospitals and clinics. Written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
^Deceased 1 March 2019.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nagao, M., Sasaki, J., Tanimura-Inagaki, K. et al. Ipragliflozin and sitagliptin differentially affect lipid and apolipoprotein profiles in type 2 diabetes: the SUCRE study. Cardiovasc Diabetol 23, 56 (2024). https://doi.org/10.1186/s12933-024-02149-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02149-7