Abstract
Background
Polycystic ovary syndrome (PCOS) is the most common cause of anovulatory infertility in women of childbearing age. Randomized controlled trials (RCTs) have reported that exenatide and metformin are effective in the treatment of PCOS. In this meta-analysis, we aimed to compare the effectiveness and safety of exenatide alone or in combination with metformin versus metformin in patients suffering from PCOS.
Methods
RCTs of exenatide therapy were identified through a search of electronic databases in November 2022 and updated in October 2023. Eligible studies were identified independently by the reviewers. Outcomes were analysed with Revman 5.4.
Results
Nine RCTs among 214 studies on 1059 women with PCOS were included in the analysis, and among the nine RCTs, eight studies compared exenatide with metformin. Our meta-analysis demonstrated that exenatide was more effective than metformin in terms of pregnancy rate (RR 1.85 [95% CI 1.19,2.86] P = 0.006), sex hormone-binding globulin (SHBG) (MD 5 [95% CI 3.82,6.18] P < 0.001), and follicle-stimulating hormone (FSH) (MD 0.82 [95% 0.41,1.24] P < 0.001). The reductions in total testosterone (TT) (SMD -0.43 [95% CI -0.84, -0.03] P = 0.04) was more significant after treatment with exenatide than after treatment with metformin. In terms of safety, exenatide had a lower diarrhea rate (RR 0.11 [95% CI 0.01, 0.84]) than metformin. In the other three studies, exenatide plus metformin was compared with metformin. Exenatide combined with metformin was more effective in improving SHBG (MD 10.38[95%CI 6.7,14.06] P < 0.001), Matsuda index (MD 0.21[95%CI 0.05,0.37]) and reducing free androgen index (FAI) (MD -3.34 [-4.84, -1.83] P < 0.001), Weight (MD -2.32 [95%CI -3.89, -0.66]) and WC (MD-5.61[95%CI -8.4, -2.82] P < 0.001). The incidence of side effects between exenatide plus metformin and metformin was not statistically significant.
Conclusions
Exenatide alone or in combination with metformin is more effective than metformin for women with PCOS. Considering the evidence on effectiveness and safety, exenatide alone or in combination with metformin may be a better treatment approach than metformin for women with PCOS.
Trial registration
INPLASY https://inplasy.com/inplasy-protocols/ ID: 10.37766/inplasy2022.11.0055.
Similar content being viewed by others
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder among women of childbearing age and is associated with high androgen levels, oligomenorrhea or amenorrhea, anovulation, and polycystic morphology of the ovaries, according to the Rotterdam criteria [1]. Although insulin resistance and obesity are not necessary criteria for diagnosing PCOS, they are important pathophysiological alterations in PCOS patients [2, 3]. Insulin resistance and obesity alone or in combination with sex hormone disorder [4, 5] may gradually give rise to reproductive and metabolic abnormalities [6, 7], cardiovascular disease [8], diabetes mellitus [9], and non-alcoholic fatty liver [10].
Clinical studies have demonstrated that metformin improves anovulation, menstrual disturbances, and hyperandrogenism in patients with PCOS [11, 12]. Metformin has also been prescribed for obese women with PCOS who suffer from metabolic irregularity on basis of the guidelines for the assessment and management of PCOS [13]. However, metformin has a slow onset of action, and it is difficult to achieve satisfactory weight control with metformin in patients with PCOS.
Exenatide, which belongs to the glucagon-like peptide-1 (GLP-1) receptor agonist family, has been used to treat diabetes and obesity chiefly owing to its prominent effects on insulin resistance, weight loss, and metabolic disorders [14, 15]. Some clinical randomized controlled trials (RCTs) have demonstrated that exenatide also has beneficial effects on pregnancy rate [16, 17], the menstrual frequency ratio (MFR) [18], insulin resistance (IR) [19], and weight reduction in women who are suffering from PCOS [16, 20]. Whether exenatide is the preferred treatment option for patients with PCOS still needs to be confirmed by large-scale clinical studies and adequate evidence-based data. When choosing a drug regimen, it is necessary to consider efficacy and safety. The most common adverse reactions associated with exenatide treatment are gastrointestinal reactions [21]. Other side effects include sclerosis at the injection site, headache, and allergic reactions [22]. Therefore, in this meta-analysis, we aimed to compare effectiveness and safety of exenatide or exenatide plus metformin versus metformin for women suffering from PCOS.
Methods
Search strategy
Original studies published in English for this meta-analysis were sought from electronic databases (PubMed, Embase, Web of Science, Cochrane library, the Web of Science, Scopus, and Google Scholar). We searched for RCTs on humans in databases from their establishment date to October 2023 by utilizing a strategy combining MeSH words with free words. The utilized terms involved "Polycystic Ovary Syndrome" [MeSH], Ovary Syndrome, Polycystic [Title/Abstract], Syndrome, Polycystic Ovary [Title/Abstract], "Exenatide"[MeSH], Exendin-4 [Title/Abstract], Peptide, Ex4 [Title/Abstract], randomized controlled trial [Publication Type], and randomized [Title/Abstract]. The search history on PubMed is presented in Table S1.
Inclusion and exclusion criteria
All searched articles were independently screened by 2 authors according to the inclusion criteria, which were as follows: 1) participants: females diagnosed with PCOS on the basis of the Rotterdam criteria; 2) intervention(s): exenatide alone or plus metformin in the treatment of PCOS; 3) comparison(s): exenatide versus metformin; 4) outcomes: effectiveness estimated by pregnancy rate, sex hormone levels, change in body weight, and metabolic disorders and safety assessed by the incidence of side effects; 5) study type: human-based RCTs. The exclusion criteria were as follows: one-arm study with no control group, placebo-controlled trials, and studies with no results.
Data extraction
Two authors (XS and SH) selected articles independently based on the inclusion and exclusion criteria and then reviewed the full texts to extract data from the eligible articles. In the case of disputes, a discussion meeting was organized by a third author (YM). Necessary information for outcomes included pregnancy rate, MFR, total testosterone (TT), follicle-stimulating hormone (FSH), luteinizing hormone (LH), dehydroepiandrosterone sulfate (DHEAS), sex hormone-binding globulin (SHBG), the free androgen index (FAI), homeostasis model assessment-insulin resistance (HOMA-IR), Matsuda index, fasting insulin (FINS), fasting blood glucose (FBG), weight, body mass index (BMI), waist circumference (WC), the waist-hip ratio (WHR), and side effects (diarrhea, constipation, nausea, vomiting, stomach pain, headache, and fatigue). Information on the trials included basic information (authors, publication year, title, criteria for PCOS, number of attendees, mean age, and total sample sizes), interventions (drugs, dose, and duration), and study design. The MFR was calculated using the ratio of expected menses to observation weeks. Matsuda index is an indicator of insulin sensitivity calculated from the glucose tolerance test.
Risk of bias assessment and quality assessment
The risk of bias for each RCT was evaluated by using the Cochrane Collaboration’s risk of bias assessment, which includes the aspects of sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other bias. Studies were characterized as “low risk”, “unclear risk”, and “high risk”. Quality assessment of the RCTs was performed with the Grading of Recommendations and Evaluation (GRADE) system. Quality was defined as “high”, “moderate”, “low”, or “very low” according to the study design, risk of bias, indirectness, inconsistency, imprecision, and publication bias [23].
Statistical analysis
Meta-analysis plus forest plots were applied by utilizing Rev Man 5.4. Continuous variables were presented as the standardized mean difference (SMD) or mean difference (MD) with a 95% confidence interval (CI) on basis of the agreement of research units. For dichotomous data, the risk ratio (RR) with 95% CI for every original study was gathered for meta-analysis utilizing the Mantel/Haenszel model. Heterogeneity was appraised through the Chi-squared (X2) test and presented as I-squared (I2) values. I2 < 50% and P > 0.1 indicate the existence of little heterogeneity among original studies. Analyses were employed by utilizing fixed-effects models. I2 ≥ 50% indicates high heterogeneity, and subgroup analysis or sensitivity analysis was performed. If no reason for the heterogeneity could be determined and the heterogeneity was within the relevant limits, a random-effects model could be utilized. P values < 0.05 were recognized to indicate significant differences.
Results
Study identification
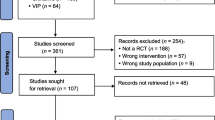
All 214 articles were obtained after initial examination of the electronic databases. Nine RCTs were ultimately included after removing duplicates, screening the titles and abstracts, and performing the whole study assessment [21, 24,25,26,27,28,29,30]. The procedures used to search for and identify RCTs are shown in Fig. 1. Details of excluded articles in the supplementary Excel sheet.
Detailed information on the selected trials
A total of 1149 women with PCOS were included in the 9 RCTs. One of the 9 trials had an intervention time of 24 weeks [31], while the remaining trials had an intervention time of 12 weeks. The total sample sizes ranged from 40 to 176. The dose of exenatide ranged from 10 µg/day (5 µg bid) to 20 µg/day (10 µg bid), while the dose of metformin ranged from 1000 mg/day (500 mg bid) to 3000 mg/day (1000 mg tid). Detailed information on the selected trials is reported in Table 1.
Quality assessment
The risk of bias estimation of the RCTs is displayed in Fig. 2, and the details can be found in Table S2. All 9 selected trials had a clear description of random sequence generation and allocation concealment. Four trials clearly stated that interventions were not blinded to participants and personnel [16, 18, 31, 32], one trial was blinded to participants and personnel [33], and the other four trials did not report details of the blinding method [10, 17, 20, 34]. One trial clearly stated that the blinding method was not applied to outcome assessors [33], while the remaining eight trials did not report whether blinding was applied to outcome assessors. The risk of incomplete outcome data in one study was uncertain [17], while in the other eight studies, it was low. The risk of reporting bias and other bias was low in all trials. The quality of the included studies was assessed by using GRADE and is presented in Table 2. The included studies were of moderate to low quality, and the main negative points were risk of bias and imprecision.
Comparison between exenatide and metformin
Comparison of the effectiveness between exenatide and metformin
Three trials reported the outcome of pregnancy rate which included 265 women with PCOS. There was no heterogeneity between trials (I2 = 0%, P = 0.52), and the fixed-effects model was used for examination. Exenatide had a higher pregnancy rate (RR 1.85 [95% CI 1.19,2.86] P = 0.006) than metformin (Fig. 3) (Table 3).
Four studies including 327 women reported TT, and the heterogeneity among studies was significant (I2 = 65%, P = 0.04). The random effects model of the meta-analysis indicated that exenatide is superior to metformin in TT (SMD -0.43 [95% CI -0.84, -0.03] P = 0.04) (Fig. 3) (Table 4). Sensitivity analysis was conducted due to the significant heterogeneity observed among the four included studies (I2 = 65%, P = 0.04). This analysis indicated that the study "Elkind-Hirsch K 2008" greatly contributed to the observed heterogeneity. After excluding this study, heterogeneity testing was insignificant (I2 = 0%, P = 0.51). The heterogeneity in the excluded study could possibly be attributed to its small sample size and the distinct population compared to the other three studies. The Meta-analysis results remained consistent after sensitivity analysis, suggesting the stability of the findings. (MD = -0.16 [95% CI (-0.26, -0.07)] P < 0.001) (Fig S3).
The pooled outcomes for SHBG in four studies and FSH in two studies were reported between the two groups. The heterogeneity between trials was low, and a fixed-effects model was used for analysis. Exenatide was more beneficial than metformin in terms of SHBG (MD 5 [95% CI 3.82,6.18] and FSH (MD 0.82 [95% 0.41,1.24] P < 0.001) (Fig. 3) (Table 4).
The exenatide and metformin groups exhibited similar effects on FAI (SMD -0.84 [95% CI -2.69,1.01] P = 0.38) and DHEAS (SMD 0.03 [95% CI -0.21,0.27] P = 0.82) (Fig. 3) (Table 4). Due to the significant heterogeneity among the four included studies for FAI (I2 = 65%, P = 0.03), a sensitivity analysis was performed. This analysis identified the study "Elkind-Hirsch K 2008" as a major contributor to the observed heterogeneity. Upon excluding this study, the heterogeneity testing was insignificant (I2 = 0%, P = 0.99). The observed heterogeneity may be due to its small sample size and unique population compared to the other three studies. The Meta-analysis results remained consistent after conducting the sensitivity analysis, indicating the robustness of the findings (MD = 0.14 [95% CI (-0.97, 1.26)] P = 0.8) (Fig S3).
The MFR was reported in two studies comparing exenatide and metformin in 256 patients with PCOS. Exenatide showed greater effectiveness than metformin in improving MFR, however, there was significant heterogeneity among the two studies included (I2 > 97%) (Table 4).
Meta-analysis demonstrated a greater reduction in HOMA-IR, FINS, weight, BMI, WC and WHR in the exenatide group than in the metformin group. The difference in FBG between the two groups was insignificant (Fig S1, Fig S2). Owing to the heterogeneity among the four studies included in the meta-analysis of FBG, we conducted subgroup analyses based on the dosages of exenatide and metformin (Fig S4). Similarly, the five studies included for BMI also exhibited heterogeneity, hence, they were subjected to subgroup analyses as well (Fig S5).
Comparison of the safety between exenatide and metformin
Three trials including 122 women with PCOS reported the outcome of diarrhea. There was no heterogeneity among trials (I2 = 0%, P = 0.64), and the fixed-effects model was used for analysis. Exenatide had a lower diarrhea rate than metformin (RR 0.11 [95% CI 0.01, 0.84] P = 0.03) (Fig. 4) (Table 5). The incidence of constipation, nausea, vomiting, stomach pain, and headache in the two groups was not significantly different (Fig. 4) (Table 5).
Comparison between exenatide plus metformin and metformin
Comparison of the effectiveness between exenatide plus metformin and metformin
Pooled outcomes of SHBG and FAI in three RCTs were reported in two groups. The heterogeneity among trials was low, fixed-effects model was utilized for analysis. Exenatide plus metformin was more effective in terms of SHBG (MD 10.38[95%CI 6.7,14.06] P < 0.001), and FAI (MD -3.34 [-4.84, -1.83] P < 0.001) (Fig. 5) (Table 4).
68 women with Matsuda index were reported in two studies, in which the heterogeneity was low. The fixed-effects model of meta-analysis revealed that exenatide plus metformin was superior to metformin in Matsuda index (MD 0.21[95%CI 0.05,0.37] P = 0.01) (Fig. 5) (Table 4).
Weight was reported in 3 RCTs involving 168 patients. Meta-analysis utilizing the fixed effects model demonstrated greater reduction in weight (MD -2.32 [95%CI -3.89, -0.66] P = 0.006) in the group of exenatide plus metformin (Fig. 5) (Table 4).
WC was reported in 2 studies in the comparison between exenatide plus metformin and metformin containing 68 patients with PCOS. The fixed-effects model was applied for meta-analysis. Results revealed that exenatide was more effective in reducing WC (MD-5.61[95%CI -8.4, -2.82] P < 0.001) (Fig. 5) (Table 4).
Comparison of the effectiveness between exenatide plus metformin and metformin
The incidence of diarrhea, nausea, vomiting, stomach pain, constipation, headache, and fatigue between two group was not statistically significant (Fig. 6) (Table 5).
Discussion
PCOS is commonly associated with infertility, menstrual cycle disorders and abnormal sex hormone levels [5]. In addition, obesity and insulin resistance are frequently seen in women with PCOS and aggravate the adverse features of the syndrome [2]. Exenatide, a GLP-1 receptor agonist, was originally used for glycaemic control in diabetes and was later used for the treatment of obesity [35]. Recently, clinical studies have proven that exenatide also has various beneficial effects on menstrual disorders, anovulation, and androgen excess in patients with PCOS [18, 31, 33]. RCTs reported that exenatide alone or combined with metformin was superior to metformin for weight loss in PCOS patients [16, 18]. However, there is a lack of evidence-based recommendations related to the effects of exenatide on PCOS. This meta-analysis showing that exenatide or exenatide plus metformin is more effective than metformin. Exenatide might affect the human reproductive system through different mechanisms (Fig S6).
The ability of exenatide to improve sex hormone disorders could be one of the reasons for this effect. It is well known that PCOS is the most common cause of anovulatory infertility [36]. Initial data indicate that sex hormone disorders and derangement of early follicle development may be partly responsible for anovulation and infertility in PCOS patients [37]. High levels of circulating androgens can prevent the development of dominant follicles [37]. In addition, adipose tissue aromatizes androgens into oestrogens and affects gonadotropin production by inhibiting the hypothalamic-pituitary-ovarian axis through negative feedback [38]. Reduced hepatic production of SHBG in PCOS patients further limits the bioavailability of peripheral androgens [32]. Therefore, the increase in FSH and SHBG along with the decrease in testosterone results in better regularity of menses and fertility potential. Exenatide was superior to metformin in reducing serum testosterone concentrations and improving FSH and SHBG levels in women with PCOS. Finally, the fertility utility of the exenatide group was better than that of the metformin group, and the pregnancy rate of the exenatide group was significantly higher than that of the metformin group.
The second mechanism for the improvement of reproductive function is based on the improvement of IR and hyperinsulinaemia. PCOS is associated with a high prevalence of IR, hyperinsulinaemia, and high androgen levels [39], particularly in those with abdominal obesity [3, 40, 41]. In addition, PCOS patients with IR are most at risk of infertility, type 2 diabetes (T2DM) and cardiovascular disease [28]. The continuous increase in insulin levels stimulates the ovaries to secrete excessive androgens [42] and decrease hepatic SHBG, ultimately aggravating abnormalities in the hypothalamus-pituitary-ovarian axis, ovulation disorders and infertility [43]. HOMA-IR calculated from FPG and FINS and the Matsuda index derived from fasting and postprandial insulin assays are both indicators of IR [44]. Both metformin and exenatide can reduce IR [21, 45]. Exenatide improves IR partly associated with weight loss and improved glucose metabolism in patients with PCOS [15]. At the same time, exenatide improves IR by inhibiting appetite and glucagon secretion, which delays gastric emptying [28]. The pooled outcomes for HOMA-IR in 5 RCTs were consistent with those of Han Y et al. [40], who reported that HOMA-IR showed better improvements with GLP-1 receptor agonists than with metformin in patients with polycystic ovary syndrome. Based on 4 studies, our meta-analysis showed a greater reduction in FINS in the exenatide group than in the metformin group.
The third mechanism for the improvement of reproductive function involves weight reduction [18], and this effect is mainly achieved through the reduction of the total fat rate, the improvement of IR, and the downregulation of inflammatory factors accompanied by weight loss [31]. The prevalence of obesity or overweight in patients with PCOS is nearly 50% [46]. IR, abdominal fat deposition, and increased androgen secretion in PCOS are exacerbated when obesity is present [47]. Studies have shown that obesity leads to lower implantation and clinical pregnancy rates and higher miscarriage rates [48, 49]. According to international guidelines, women with obesity are recommended to lose weight before spontaneous conception or conception through in vitro fertilization (IVF) [50]. As a short-acting GLP-1 agonist, exenatide has demonstrated positive effects on weight reduction, BMI, WC, and body fat content in overweight/obese PCOS patients [28, 32]. The mechanism by which exenatide mediates weight loss is primarily a reduction in food intake due to direct hypothalamic effects. Exenatide can also delay gastric emptying through central action mediated by the autonomic nervous system. The present meta-analysis confirmed that exenatide showed greater reductions in weight, BMI, WC, and the WHR than metformin.
Strengths and limitations
The present study has several strengths. Our study compared the efficacy and safety between exenatide alone or plus metformin and metformin in patients with PCOS from several perspectives. We not only focused on exenatide, but also summarized the effectiveness of exenatide plus metformin in PCOS patients, which previous meta-studies on GLP-1 receptor agonists have not done.
Our research also has some limitations. To date, the clinical randomized controlled trials of exenatide in the treatment of PCOS have been short-term trials of 12–24 weeks, and there are no studies of longer duration. In addition, the quality of the included studies was low to moderate. Therefore, the long-term effect needs to be established by longer, larger, multicentre, and higher-quality clinical studies.
Conclusion
This meta-analysis demonstrates that exenatide, either alone or in combination with metformin, exhibits superior performance over metformin in managing PCOS, while maintaining a comparable side effect profile. Exenatide, either alone or in combination with metformin, appears to be a safe and effective treatment choice for patients diagnosed with PCOS. Further studies with larger sample sizes and longer durations are warranted to investigate the combined treatment approach when obesity is linked to PCOS.
Availability of data and materials
The paper contains all the evidence that supports the results. The corresponding author will provide more in-depth information and raw data upon reasonable request.
Abbreviations
- PCOS:
-
Polycystic ovary syndrome
- GLP-1:
-
Glucagon-like peptide-1
- RCTs:
-
Randomized controlled trials
- MFR:
-
Menstrual frequency ratio
- IR:
-
Insulin resistance
- TT:
-
Total testosterone
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- DHEAS:
-
Dehydroepiandrosterone sulfate
- SHBG:
-
Sex hormone-binding globulin
- FAI:
-
Free androgen index
- HOMA-IR:
-
Homeostasis model assessment-insulin resistance
- FINS:
-
Fasting insulin
- FBG:
-
Fasting blood glucose
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- WHR:
-
Waist-hip ratio
- GRADE:
-
Grading of Recommendations and Evaluation
- SMD:
-
Standardized mean difference
- MD:
-
Mean difference
- CI:
-
Confidence interval
- RR:
-
Risk ratio
References
Rotterdam EA-SPcwg. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7. https://doi.org/10.1093/humrep/deh098.
Liu S, Hu W, He Y, Li L, Liu H, Gao L et al. 2020 Serum Fetuin-A levels are increased and associated with insulin resistance in women with polycystic ovary syndrome. BMC Endocrine Disorders.20(1). https://doi.org/10.1186/s12902-020-0538-1.
McBreairty LE, Chilibeck PD, Gordon JJ, Chizen DR, Zello GA. Polycystic ovary syndrome is a risk factor for sarcopenic obesity: a case control study. BMC Endocr Disord. 2019;19(1):70. https://doi.org/10.1186/s12902-019-0381-4.
Mansour A, Hashemi Taheri AP, Moradi B, Mohajeri-Tehrani MR, Qorbani M, Ghorbani Pashakolaee S, et al. Ovarian volume, not follicle count, is independently associated with androgens in patients with polycystic ovary syndrome. BMC Endocr Disord. 2022;22(1):298. https://doi.org/10.1186/s12902-022-01224-y.
El-Eshmawy MM, Ibrahim A, Bahriz R, Shams-Eldin N, Mahsoub N. Serum uric acid/creatinine ratio and free androgen index are synergistically associated with increased risk of polycystic ovary syndrome in obese women. BMC Endocr Disord. 2022;22(1):315. https://doi.org/10.1186/s12902-022-01240-y.
Jin C, Zou K, Xu Y, Yang H, Pan J. Elevated plasma pentraxin-3 in polycystic ovary syndrome is associated with hyperandrogenism: a case-control study. BMC Endocr Disord. 2021;21(1):240. https://doi.org/10.1186/s12902-021-00886-4.
Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–97. https://doi.org/10.1016/S0140-6736(07)61345-2.
Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metab. 2021;106(3):e1071–83. https://doi.org/10.1210/clinem/dgaa839.
Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–31. https://doi.org/10.1038/nrendo.2010.217.
Zheng S, Zhang Y, Long T, Lu J, Liu X, Yan J, et al. Short term monotherapy with exenatide is superior to metformin in weight loss, improving insulin resistance and inflammation in Chinese overweight/obese PCOS women. Obesity Medicine. 2017;7:15–20. https://doi.org/10.1016/j.obmed.2017.06.003.
Garzia E, Galiano V, Marfia G, Navone S, Grossi E, Marconi AM. Hyperandrogenism and menstrual imbalance are the best predictors of metformin response in PCOS patients. Reprod Biol Endocrinol. 2022;20(1):6. https://doi.org/10.1186/s12958-021-00876-0.
Bahadur A, Arora H, Ravi AK, Naithani M, Bahurupi Y, Chaturvedi J, et al. Comparison of Clinical, Metabolic and Hormonal Effects of Metformin Versus Combined Therapy of Metformin With Myoinositol Plus D-Chiro-Inositol in Women With Polycystic Ovary Syndrome (PCOS): A Randomized Controlled Trial. Cureus. 2021;13(6):e15510. https://doi.org/10.7759/cureus.15510.
Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110(3):364–79. https://doi.org/10.1016/j.fertnstert.2018.05.004.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705. https://doi.org/10.1016/S0140-6736(06)69705-5.
Dushay J, Gao C, Gopalakrishnan GS, Crawley M, Mitten EK, Wilker E, et al. Short-term exenatide treatment leads to significant weight loss in a subset of obese women without diabetes. Diabetes Care. 2012;35(1):4–11. https://doi.org/10.2337/dc11-0931.
Li R, Mai T, Zheng S, Zhang Y. Effect of metformin and exenatide on pregnancy rate and pregnancy outcomes in overweight or obese infertility PCOS women: long-term follow-up of an RCT. Arch Gynecol Obstet. 2022;306(5):1711–21. https://doi.org/10.1007/s00404-022-06700-3.
Li RY, Zheng S, Mai T, Xue J, Zhang Y. 2020 1341-P: Comparison of the Effects of Metformin and Exenatide on Pregnancy Rate and Outcomes in Overweight or Obese PCOS Women. Diabetes.69(Supplement_1). https://doi.org/10.2337/db20-1341-P.
Liu X, Zhang Y, Zheng SY, Lin R, Xie YJ, Chen H, et al. Efficacy of exenatide on weight loss, metabolic parameters and pregnancy in overweight/obese polycystic ovary syndrome. Clin Endocrinol (Oxf). 2017;87(6):767–74. https://doi.org/10.1111/cen.13454.
Wen Q, Fang S, Liang Y, Tian Y, Chen Y, Yuan J et al. 2023 Short-term effect of beinaglutide combined with metformin versus metformin alone on weight loss and metabolic profiles in obese patients with polycystic ovary syndrome: a pilot randomized trial. Front Endocrinol (Lausanne).14:1156521. https://doi.org/10.3389/fendo.2023.1156521.
Zheng S, Liu E, Zhang Y, Long T, Liu X, Gong Y, et al. Circulating zinc-alpha2-glycoprotein is reduced in women with polycystic ovary syndrome, but can be increased by exenatide or metformin treatment. Endocr J. 2019;66(6):555–62. https://doi.org/10.1507/endocrj.EJ18-0153.
Ma RL, Deng Y, Wang YF, Zhu SY, Ding XS, Sun AJ. 2021 Short-term combined treatment with exenatide and metformin for overweight/obese women with polycystic ovary syndrome. Chin Med J (Engl).134(23):2882–9. https://doi.org/10.1097/CM9.0000000000001712.
Di Dalmazi G, Coluzzi S, Baldassarre MPA, Sorbo SE, Dell'Aquila S, Febo F et al. 2020 Exenatide Once Weekly: Effectiveness, Tolerability, and Discontinuation Predictors in a Real-world Setting. Clin Ther.42(9):1738–49 e1. https://doi.org/10.1016/j.clinthera.2020.07.002.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1 Introduction—GRADE evidence profiles and summary of findings tables. J Clinical Epidemiol. 2011;64(4):383–94. https://doi.org/10.1016/j.jclinepi.2010.04.026.
Craig C, Lerner K, Lamendola C, Liu LF, Schwartz H, et al. Effect of Exenatide on Menstrual Cyclicity, Weight, and Metabolic Profile in Insulin-Resistant Women with PCOS. Diabetes. 2014;63:1701.
Chen L, Long T, Zheng S, Liu X, Yan J, Mai T et al. 2017 The effect of exenatide on metabolic syndrome with polycystic ovary syndrome. Diabetes/Metabolism Research and Reviews.33. https://doi.org/10.1002/dmrr.2948.
Dawson AJ, Sathyapalan T, Vince R, Coady AM, Ajjan RA, Kilpatrick ES et al. 2019 The Effect of Exenatide on Cardiovascular Risk Markers in Women With Polycystic Ovary Syndrome. Front Endocrinol (Lausanne).10:189. https://doi.org/10.3389/fendo.2019.00189.
Elkind-Hirsch KE, Chappell N, Seidemann E, Storment J, Bellanger D. Exenatide, Dapagliflozin, or Phentermine/Topiramate Differentially Affect Metabolic Profiles in Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2021;106(10):3019–33. https://doi.org/10.1210/clinem/dgab408.
Tang L, Yuan L, Yang G, Wang F, Fu M, Chen M, et al. Changes in whole metabolites after exenatide treatment in overweight/obese polycystic ovary syndrome patients. Clin Endocrinol (Oxf). 2019;91(4):508–16. https://doi.org/10.1111/cen.14056.
Moreno JL, Willett KC, Desilets AR. Exenatide as a novel weight loss modality in patients without diabetes. Ann Pharmacother. 2012;46(12):1700–6. https://doi.org/10.1345/aph.1R372.
Liu J, Hu Y, Xu Y, Jia Y, Miao L, Wang G. Comparison of Exenatide and Metformin Monotherapy in Overweight/Obese Patients with Newly Diagnosed Type 2 Diabetes. Int J Endocrinol. 2017;2017:9401606. https://doi.org/10.1155/2017/9401606.
Elkind-Hirsch K, Marrioneaux O, Bhushan M, Vernor D, Bhushan R. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(7):2670–8. https://doi.org/10.1210/jc.2008-0115.
Tao T, Zhang Y, Zhu YC, Fu JR, Wang YY, Cai J, et al. Exenatide, Metformin, or Both for Prediabetes in PCOS: A Randomized, Open-label, Parallel-group Controlled Study. J Clin Endocrinol Metab. 2021;106(3):e1420–32. https://doi.org/10.1210/clinem/dgaa692.
Wang JRX, Jin F, Sun Y, Wang J. Effects of exenatide combined with clomifene citrate on insulin resistance and angiotensin II/Angiotensin-(1–7) in peripheral blood in patients with polycystic ovary syndrome. Biomedical Research (India). 2017;27(12):3569–76.
Ma RL, Deng Y, Wang YF, Zhu SY, Ding XS, Sun AJ. Short-term combined treatment with exenatide and metformin for overweight/obese women with polycystic ovary syndrome. Chin Med J. 2021;134(23):2882–9. https://doi.org/10.1097/CM9.0000000000001712.
van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 2014;221(1):T1-16. https://doi.org/10.1530/JOE-13-0414.
Amer SA, Smith J, Mahran A, Fox P, Fakis A. Double-blind randomized controlled trial of letrozole versus clomiphene citrate in subfertile women with polycystic ovarian syndrome. Hum Reprod. 2017;32(8):1631–8. https://doi.org/10.1093/humrep/dex227.
Hsueh AJ, Kawamura K, Cheng Y, Fauser BC. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36(1):1–24. https://doi.org/10.1210/er.2014-1020.
Cena H, Chiovato L, Nappi RE. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J Clin Endocrinol Metab. 2020;105(8):e2695–709. https://doi.org/10.1210/clinem/dgaa285.
O'Reilly M, Gathercole L, Capper F, Arlt W, Tomlinson J. 2015 Effect of insulin on AKR1C3 expression in female adipose tissue: in-vivo and in-vitro study of adipose androgen generation in polycystic ovary syndrome. The Lancet.385. https://doi.org/10.1016/s0140-6736(15)60331-2.
Han Y, Li Y, He B. GLP-1 receptor agonists versus metformin in PCOS: a systematic review and meta-analysis. Reprod Biomed Online. 2019;39(2):332–42. https://doi.org/10.1016/j.rbmo.2019.04.017.
Li R, Yu G, Yang D, Li S, Lu S, Wu X et al. 2014 Prevalence and predictors of metabolic abnormalities in Chinese women with PCOS: a cross- sectional study. Prevalence and predictors of metabolic abnormalities in Chinese women with PCOS: a cross- sectional study.14:76. https://doi.org/10.1186/1472-6823-14-76.
Lim SS, Kakoly NS, Tan JWJ, Fitzgerald G, Bahri Khomami M, Joham AE, et al. Metabolic syndrome in polycystic ovary syndrome: a systematic review, meta-analysis and meta-regression. Obes Rev. 2019;20(2):339–52. https://doi.org/10.1111/obr.12762.
Joham AE, Norman RJ, Stener-Victorin E, Legro RS, Franks S, Moran LJ, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10(9):668–80. https://doi.org/10.1016/s2213-8587(22)00163-2.
Hanssen R, Kretschmer AC, Rigoux L, Albus K, Edwin Thanarajah S, Sitnikow T, et al. GLP-1 and hunger modulate incentive motivation depending on insulin sensitivity in humans. Mol Metab. 2021;45:101163. https://doi.org/10.1016/j.molmet.2021.101163.
Palomba S, Piltonen TT, Giudice LC. Endometrial function in women with polycystic ovary syndrome: a comprehensive review. Hum Reprod Update. 2021;27(3):584–618. https://doi.org/10.1093/humupd/dmaa051.
Salamun V, Jensterle M, Janez A, Vrtacnik BE. Liraglutide increases IVF pregnancy rates in obese PCOS women with poor response to first-line reproductive treatments: a pilot randomized study. Eur J Endocrinol. 2018;179(1):1–11. https://doi.org/10.1530/EJE-18-0175.
Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14(2):95–109. https://doi.org/10.1111/j.1467-789X.2012.01053.x.
Luke B. Adverse effects of female obesity and interaction with race on reproductive potential. Fertil Steril. 2017;107(4):868–77. https://doi.org/10.1016/j.fertnstert.2017.02.114.
Luke B, Brown MB, Missmer SA, Bukulmez O, Leach R, Stern JE, et al. The effect of increasing obesity on the response to and outcome of assisted reproductive technology: a national study. Fertil Steril. 2011;96(4):820–5. https://doi.org/10.1016/j.fertnstert.2011.07.1100.
Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171(4):P1–29. https://doi.org/10.1530/eje-14-0253.
Acknowledgements
We would like to thank all the authors of the reviewed studies included in this meta-analysis who contributed by sharing the relevant data on request.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
GY Y and HY were responsible for study design and manuscript writing. XS, SH, YM, MY, XW, and KZ take charge of articles searching, data collection and analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
PRISMA 2009 Checklist.
Additional file 2:
Table S1. The search history on PubMed. Table S2. RCT Risk of Bias Assessment. Fig S1. Effects on HOMA-IR, FBG and FINS between exenatide and metformin. Fig S2. Effects on Weight, BMI, WC, WHR between exenatide and metformin. Fig S3. Sensitivity analysis of TT and FAI. Fig S4. subgroup analyses of FBG. Fig S5. Subgroup analyses of BMI. Fig S6. Putative mechanism of beneficial effects of exenatide.
Additional file 3.
Table for excluded literature in meta-analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, Y., Song, X., Hamiti, S. et al. Comparison of exenatide alone or combined with metformin versus metformin in the treatment of polycystic ovaries: a systematic review and meta-analysis. BMC Endocr Disord 23, 250 (2023). https://doi.org/10.1186/s12902-023-01497-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01497-x