Abstract
Polycystic ovary syndrome (PCOS) is an endocrine disorder that affects women of childbearing age, resulting in reproductive dysfunction, hyperinsulinemia, and obesity. While several drugs are currently approved for use in these patients, their relative effectiveness remains controversial. The purpose of this meta-analysis was to evaluate the reproductive efficacy and safety of exenatide, a glucagon-like peptide-1 receptor agonist, versus metformin, an insulin sensitizer, in the treatment of patients with PCOS. Nine randomized controlled trials (RCTs) were included, comprising 785 PCOS patients, of whom 385 received exenatide and 400 received metformin. Compared with metformin, exenatide was significantly more effective in treating these patients, as demonstrated by increased pregnancy rate (relative risk (RR) = 1.93, 95% confidence interval (CI) 1.28 to 2.92, P = 0.002), greater ovulation rate (RR = 1.41, 95% CI 1.11 to 1.80, P = 0.004), decreased body mass index (mean difference = − 1.72 kg/m2, 95% CI − 2.27 to − 1.18, P = 0.00001), and improved insulin resistance (standard mean difference = − 0.62, 95% CI − 0.91 to − 0.33, P < 0.0001). There was no significant difference in the occurrence of adverse events (gastrointestinal reactions, hypoglycemia, etc.) between the two therapies. However, given the moderate to high quality and possible bias of the included studies, the available evidence is inconclusive. More high-quality studies are needed to assess the effects of exenatide in order to provide stronger evidence for its use in this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is an endocrine disease that is mainly characterized by androgen excess, as well as reproductive and metabolic dysfunction [1]. It affects approximately 6%–10% of pre-menopausal women worldwide [2,3,4]. PCOS typically manifests as impaired ovulation and hyperandrogenism, as the ovaries of PCOS patients show pronounced over-synthesis of steroid hormones compared with normal follicular membrane cells. The most prominent clinical features include reproductive dysfunction, anovulation, and disrupted menstruation [5, 6]. Additional clinical features include hyperinsulinemia, marked insulin resistance [7] and obesity, which interact with each other to aggravate disease progression, as well as hirsutism and/or acne [8]. PCOS not only presents with infertility, but also increases the risk of spontaneous abortion, congenital fetal disease, and obstetric complications in patients who do become pregnant [9]. Furthermore, PCOS has lasting impacts far beyond childbearing age and can influence many aspects of women’s overall health, as it is associated with an increased risk of developing metabolic syndrome, anxiety and depression, and endometrial cancer [10,11,12].
The core objectives when treating patients with PCOS include improving reproductive system function, decreasing insulin resistance, treating symptoms caused by androgen excess, reducing the risk of cardiovascular complications, and promoting weight loss. Because the pathogenesis of PCOS is still unclear, lifestyle intervention is the first choice for treatment, and the main focus is weight loss, as this is a crucial factor affecting pregnancy outcomes [13]. As many as 74% of patients with PCOS are classified as obese [14]. This obesity is usually associated with hyperinsulinemia, followed by increased ovarian androgen secretion [15], which in turn causes visceral fat deposition, aggravating insulin resistance and further increasing androgen secretion due to elevated insulin levels [16]. These changes are also important causes of ovulation disorders and abnormal menstruation.
In the past, metformin (MET) has been recommended as the first choice for weight loss in patients with PCOS [17]. MET is an insulin sensitizer that reduces insulin levels and improves insulin receptor activity [18], resulting in decreased insulin resistance, lower androgen levels, and improved weight control [19, 20]. In addition to these effects, MET significantly increases ovulation rate compared with placebo [21, 22]. However, evidence regarding its efficacy in optimizing both fertility and pregnancy outcomes is inconclusive [23], and treatment with MET may not be sufficient for addressing reproductive dysfunction in patients with PCOS [24]. Indeed, a recent meta-analysis showed that treatment with MET resulted in very limited improvement in pregnancy and live birth rates compared with placebo [25]. In addition, there are many contraindications to the use of MET, with the most serious potential adverse reaction being lactic acidosis. Thus, treatment of PCOS with MET remains controversial.
In recent years, glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have attracted attention as a new option for PCOS treatment, and are currently recommended by the European Society of Endocrinology for treating this patient population [26]. Among the commercially available GLP-1 RAs, only exenatide (EX) and liraglutide have been recommended for PCOS [27], and so far few randomized controlled trials (RCTs) have shown that liraglutide improves ovulation or pregnancy outcomes, making EX more promising for clinical application. EX, a gut-derived incretin hormone that enhances insulin sensitivity, reduces blood glucose and insulin levels. Moreover, it can inhibit gastric emptying, thus reducing appetite and body weight [28, 29]. In addition, a study performed in rats showed that EX significantly improved endocrine and reproductive status; androgen secretion, body weight, and HOMA-IR were significantly decreased in the rats treated with EX compared with the control group, which may have been related to the increased expression of AMPKα and SIRT11 [30].
Recent studies have suggested that EX provides more adequate control of PCOS symptoms than MET [26, 27, 31, 32], although whether EX improves ovulation and pregnancy rates, as well as whether it has a similar safety profile to MET, is still controversial. While several RCTs have been carried out to answer these important questions, most of them were underpowered to provide a robust and clinically applicable conclusion. Therefore, the aim of this study was to perform a meta-analysis of RCTs to evaluate the efficacy and safety of EX versus MET in the treatment of patients with PCOS.
Materials and Methods
Search Strategy
Electronic databases (PubMed, Embase, Cochrane Library, CNKI, ChinaInfo, and VIP) were searched for RCTs of EX in the treatment of women with PCOS, from the time the databases were established to August 2022. The search terms used were as follows: polycystic ovary syndrome, Stein-Leventhal, ovarian degeneration, sclerocystic ovary, endocrine sexual disorders, exenatide, GLP-1, glucagon-like peptide-1, Byetta, Bydureon, AC 2993, Exendin 4. No language restrictions were applied.
Our study is registered with the International Prospective Register of Systematic Reviews (PROSPERO, CRD42022337219).
Study Selection
Inclusion Criteria
Studies were included if they fulfilled the following criteria: (1) Participants: All patients with PCOS diagnosed with the Rotterdam criteria (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004). All patients were women of childbearing age, and with no limit in terms of country, disease course, disease degree, and whether or not PCOS was combined with a glucose metabolism disorder; (2) Intervention: EX; (3) Comparison: MET; (4) Main outcomes: pregnancy rate, ovulation rate, body mass index (BMI), homeostasis model assessment of insulin resistance (HOMA-IR), adverse events; (5) Study design: RCT.
Exclusion Criteria
Exclusion criteria included duplicate publications; retrospective studies; non-RCTs; non-human models; conference literature; no full-text available.
Data Extraction
Two authors (ZRY and SHW) independently screened the abstracts and full texts of potentially eligible articles, and extracted the data, including:
-
(1)
Title, author, and publication year;
-
(2)
Basic characteristics of the participants: number of samples, intervention measures, age, and BMI;
-
(3)
Main outcomes: pregnancy rate, ovulation rate (as determined by measuring serum progesterone levels), BMI, HOMA-IR (according to the formula: fasting insulin (µU/L) × fasting glucose (nmol/L)/22.5), adverse events;
-
(4)
Secondary outcomes: body weight, waist circumference (WC), abdominal girth (AG), waist-to-hip ratio (WHR), sex hormone-binding globulin (SHBG), serum total testosterone (TT), menstrual frequency, androstenedione (AD), dehydroepiandrosterone sulphate (DHEA-S), free androgen index (FAI), luteinizing hormone (LH), follicle-stimulating hormone (FSH), triglyceride (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), hypersensitive C-reactive protein (hs-CRP), fasting plasma glucose (FPG), 2 h postprandial blood glucose (2hPBG), fasting insulin (FINS), and 2-h insulin (2hINS).
If any data were missing from a published paper, the lead author was contacted to request the data. If there were any discrepancies in the extracted data, then a third author (CQY) was consulted to resolve the differences.
Evaluation of Study Quality
The risk of bias in each study was assessed according to Cochrane review criteria [33]. Two authors (ZRY and SHW) separately evaluated the quality of each study in seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases, and each study was then classified as being at “low risk,” “unclear,” or “high risk” for bias. A third author (CQY) was consulted to resolve any discrepancies in classification between ZRY and SHW.
Statistical Analysis
Statistical analysis was performed using RevMan5.4 software. The standard mean difference (SMD) or mean difference (MD) was used to evaluate continuous data, with a 95% confidence interval (CI). MD was used when continuous data were measured using the same scale. SMD was used to pool estimates from trials that measured data using different scales [34]. Dichotomous variables were assessed, and the results are expressed as relative risk (RR), with a 95% CI.
I2 and Q tests were used to analyze the heterogeneity of the studies. An I2 value > 50% or a P value < 0.1 indicated statistically significant heterogeneity, and these studies were then analyzed using a random-effects model. If the I2 value was still > 50%, sensitivity analyses and subgroup analyses were performed.
Z tests were performed to assess the overall effect, with a Z score of > 1.96 indicating a significant effect at a 95% value of significance.
Funnel plots were not used to present publication bias, as too few studies were included to generate such plots [33].
Results
Literature Search Results and Study Screening
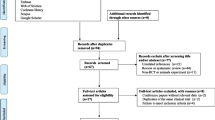
A total of 679 articles were initially retrieved from the database searches. After duplicate articles were removed, the articles were then screened to ensure that they matched the inclusion criteria. Next, the authors of articles with missing data were contacted, and any articles for which the missing data could not be obtained were excluded. Ultimately, nine RCTs comparing EX with MET were included in the meta-analysis (Fig. 1).
Included Studies
Nine studies comprising 785 patients were included in the meta-analysis [35,36,37,38,39,40,41,42,43]. For all of the included studies, the intervention group was treated with EX only (385 patients), and the control group was treated with MET only (400 patients). The patients were all women of childbearing age. The characteristics of the included studies are summarized in Table 1.
Assessment of the Risk of Study Bias
Next, we evaluated the risk of bias in each of the nine included studies (Fig. 2 and Supp. Figure 1). Three studies [35, 39, 41] did not state clearly whether group allocation was performed by random sequence generation, whereas the other six studies did state that they used this method. Only one study [38] clearly stated that allocation concealment was applied. Two studies [38, 40] did not involve blinding of the participants and study personnel, and it was unclear if blinding was applied in the other seven studies. All studies were classified as “low risk” in terms of detection bias, attrition bias, and reporting bias. Only one study exhibited “other bias,” in that it was performed in a high-altitude geographical area.
Meta-analysis Results
Primary Outcomes
Four articles [36, 38, 40, 41] were included in the assessment of pregnancy rate. The results showed that the pregnancy rate of patients treated with EX was significantly higher than that of patients treated with MET (RR = 1.93, 95% CI 1.28 to 2.92, Z = 3.12, P = 0.002) (Fig. 3A).
Four articles [35, 36, 38, 40] were included in the assessment of ovulation rate. The results showed that the ovulation rate of patients treated with EX was significantly higher than that of patients treated with MET (RR = 1.41, 95% CI 1.11 to 1.80, Z = 2.85, P = 0.004) (Fig. 3B).
Eight articles [36,37,38,39,40,41,42,43] were included in the assessment of BMI. The results showed that the BMI of patients treated with EX was significantly lower than that of patients treated with MET (MD = − 1.72 kg/m2, 95% CI − 2.27 to − 1.18, Z = 6.18, P = 0.00001) (Fig. 3C).
Seven articles [35,36,37, 39,40,41,42] were included in the assessment of HOMA-IR. The results showed that the HOMA-IR of PCOS patients treated with EX group was significantly lower than that of patients treated with MET (SMD = − 0.62, 95% CI − 0.91 to − 0.33, Z = 4.18, P < 0.0001) (Fig. 3D).
Adverse Reactions
Six articles [35, 38, 40,41,42,43] were included in the assessment of gastrointestinal reactions (nausea, diarrhea, vomiting, etc.). The results showed that there was no significant difference in the rate of gastrointestinal reactions between the two groups (RR = 0.83, 95% CI 0.61 to 1.13, Z = 1.16, P = 0.25) (Fig. 4A).
Four articles [35, 36, 38, 39] were included in the assessment of hypoglycemic events. The results showed that there was no significant difference in the rate of hypoglycemic events between the two groups (RR = 1.07, 95% CI 0.19 to 6.11, Z = 0.08, P = 0.94) (Fig. 4B).
Four articles [40,41,42,43] were included in the assessment of other adverse reactions (headache, dizziness, fatigue, etc.). The results showed that there was no significant difference in the rate of other adverse reactions between the two groups (RR = 1.45, 95% CI 0.20 to 10.60, Z = 0.37, P = 0.71) (Fig. 4C).
Thus, there was no increase in the rate of adverse events associated with EX compared with MET.
Subgroup Analysis
There was substantial heterogeneity in HOMA-IR among the seven articles included in the assessment of this outcome [35,36,37, 39,40,41,42]. Sensitivity analyses were performed but did not resolve the heterogeneity. In four studies [36, 39, 41, 42] the intervention lasted for 12 weeks, whereas in two studies [37, 40] it lasted for 24 weeks, and in one study [35] it lasted for 25 weeks. The studies were therefore divided into two groups based on the duration of the intervention: group A included studies that lasted less than 24 weeks, and group B included studies that lasted 24 weeks or more. Subgroup analysis showed that the source of heterogeneity was the duration of the intervention. The level of heterogeneity in each subgroup was acceptable. Further analysis showed that EX was more effective than MET at decreasing HOMA-IR in each of the two subgroups (Fig. 5).
Secondary Outcomes
The results from the meta-analysis showed that EX was more effective than MET at reducing body weight, WC, AG, WHR, FPG, 2hPBG, FINS, 2hINS, hs-CRP, and DHEA-S. In addition, EX was more effective than MET at increasing FSH and SHBG.
There was no difference in menstrual frequency, LH, FAI, TT, AD, TC, TG, HDL-C, or LDL-C between EX and MET (Table 2).
Discussion
The aim of this meta-analysis was to determine the reproductive efficacy and safety of EX compared with MET in patients with PCOS. We found that EX was more effective than MET in this patient population in terms of improving reproductive outcomes, promoting weight loss, and improving insulin resistance. There was no significant difference between EX and MET in terms of gastrointestinal reactions, hypoglycemia, and other adverse events.
Our meta-analysis revealed that EX is more effective than MET at improving reproductive outcomes in patients with PCOS, including pregnancy rates, ovulation rates, and sex hormone levels. In women with PCOS, ovarian follicle development is perturbed due to ovarian hyperandrogenism, hyperinsulinemia from insulin resistance, and altered intrafollicular paracrine signaling, resulting in polycystic ovarian morphology, ovulatory dysfunction, and infertility [44]. Hyperinsulinemia directly increases androgen secretion, but also increases the level of serum free testosterone by reducing the production of SHBG, which causes infertility [45]. A study performed in a DHEA-treated rat model of PCOS indicated that EX improves several aspects of follicle morphology, such as the number of cystic follicles and granule cell layers [46]. Our study showed that EX was more significantly more effective than MET at increasing SHBG and FSH and decreasing DHEA-S, although the two drugs had similar effects on other sex hormone indices (TT, LH, FAI, and AD). This suggests that the superior effectiveness of EX in this patient population in terms of improving reproductive function may be due to its effects on SHBG, FSH, and DHEA-S levels, potentially indirectly by lowering insulin resistance.
Our results also showed that EX is more effective than MET at treating features of PCOS other than reproductive function, including obesity, insulin resistance, and inflammation. Adipose tissue represents an intracrine source of androgen synthesis and may give rise to functional hyperandrogenism. EX inhibits gastric emptying mediated by the gastric vagus nerve and plays an important role in the brainstem and hypothalamic nuclei to regulate homeostatic feeding, prolong the digestive cycle, and reduce active feeding, resulting in significant weight loss [47, 48]; in keeping with this, the current meta-analysis revealed that treatment with EX resulted in significant reductions in body weight, BMI, WC, AG, and WHR compared with treatment with MET. While multiple mechanisms have been proposed for the insulin resistance seen in patients with PCOS, such as decreases in kinase activity, phosphorylation of insulin-receptor substrate, PI3K activity, and glucose transporter translocation [49, 50], impairment of downstream metabolic insulin signaling [51], and increased androgen production in the ovary [52, 53], this process is still poorly understood. Our analysis showed that EX significantly improved HOMA-IR, FPG, 2hPBG, FINS, and 2hINS compared with treatment with MET, reinforcing the importance of insulin sensitivity in these patients. Furthermore, we found that the hs-CRP level was significantly lower in patients treated with EX than in patients treated with MET, suggesting that EX could help reduce inflammation in patients with PCOS. Indeed, previous studies have shown that EX, as a GLP-1 RA, may have anti-inflammatory effects [54], and that EX can inhibit the expression of inflammatory mediators [55], although the underlying mechanism remains unclear. Taken together, these findings imply that EX is more effective than MET at treating patients with PCOS due to its enhanced ability to promote weight loss, increase insulin sensitivity, and decrease inflammation.
Interestingly, while our meta-analysis found that EX was more effective than MET in treating key symptoms of PCOS, it also showed that the adverse reactions to both drugs were comparable, as there was a similar incidence of gastrointestinal reactions, hypoglycemia, and other adverse events between the two treatment groups. A previous study showed that the main adverse reactions seen with EX are gastrointestinal reactions (usually nausea, vomiting, diarrhea, etc.), most of which resolve spontaneously without any intervention [56]. We speculate that the gastrointestinal reactions associated with EX are closely related to the regulatory effects of GLP-1 on the feeding center. Our findings suggest that EX is just as safe as MET for the treatment of PCOS, in addition to being more effective.
The main strength of our study is that pregnancy and ovulation were selected as the main outcomes to analyze the effectiveness of PCOS treatment, as these outcomes have practical significance for treatment decision-making. As an insulin sensitizer, MET is beneficial for treating metabolic abnormalities, but it is less effective at addressing problems with reproductive function; therefore, studies such as ours exploring the effectiveness of other drugs will increase the number of viable treatment options available for this condition. There were, however, some limitations to this study. First, most of the participants were overweight or obese, and therefore at higher risk for metabolic disorders, so we were unable to determine whether the beneficial effects of EX on fertility were mediated directly by its effects on the reproductive system or indirectly by promoting weight loss and improving insulin resistance; this should be investigated in future studies. Second, some of the included studies were not blinded or did not describe the blinding method, which may have biased the reliability of the results. Finally, the sample size used for the meta-analysis was relatively small; the efficacy and safety of EX should be examined in a controlled, multi-center clinical study with a larger sample size to provide stronger evidence for its use in patients with PCOS.
Given that new GLP1-RAs have been used in clinical practice, it would also be beneficial for future studies to investigate the efficacy of these new treatments compared with drugs in current use. For example, several studies have compared the efficacy of multiple GLP1-RAs, including EX, liraglutide, and semaglutide, in PCOS and found that they generally tend to promote weight loss, reduce the risk of cardiovascular disease, improve insulin sensitivity, improve hormone parameters, increase fertility, and enhance ovulation and pregnancy [27, 31, 57]. In addition, a recent review summarized the evidence for the broad cardiovascular and metabolic benefits of GLP1-RAs (lixisenatide, exenatide, liraglutide, semaglutide, albiglutide, and dulaglutide) in nondiabetic patients with a variety of conditions, including PCOS [58]. Semaglutide in particular has been shown to significantly delay gastric emptying in obese women with PCOS [59], which could have important implications for weight loss in this population. Based on our finding that EX is more effective than MET at treating key symptoms of PCOS, it is likely that some of the newer GLP1-RAs could be even more effective, and this possibility should be investigated in future studies.
Conclusions
In summary, our meta-analysis found moderate to high quality evidence that EX is more effective than MET at improving reproductive function, and that there is no significant difference in adverse events between the two drugs. In addition, we found that the beneficial effects of EX on fertility might be related to improvements in insulin resistance and weight control. More high-quality RCTs need to be conducted to assess the long-term effects of EX, as well as the effectiveness of more potent GLP1-RAs, in patients with POCS.
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- AD:
-
Androstenedione
- AG:
-
Abdominal girth
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CNKI:
-
China National Knowledge Infrastructure
- DHEA-S:
-
Dehydroepiandrosterone sulfate
- EX:
-
Exenatide
- FAI:
-
Free androgen index
- FINS:
-
Fasting insulin
- FPG:
-
Fasting plasma glucose
- FSH:
-
Follicle stimulating hormone
- GLP-1 RAs:
-
Glucagon-like peptide-1 receptor agonists
- HDL-C:
-
High density lipoprotein Cholesterol
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- hs-CRP:
-
Hypersensitive C-reactive protein
- IR:
-
Insulin resistance
- LDL-C:
-
Low density lipoprotein cholesterol
- LH:
-
Luteinizing hormone
- MD:
-
Mean difference
- MET:
-
Metformin
- PCOS:
-
Polycystic Ovary Syndrome
- RCT:
-
Randomized controlled trial
- RR:
-
Relative risk
- SHBG:
-
Sex hormone-binding globulin
- SMD:
-
Standard mean difference
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- TT:
-
Total testosterone
- VIP:
-
China Science and Technology Journal Database
- WC:
-
Waist circumference
- WHR:
-
Waist-to-hip ratio
- 2hINS:
-
2-H insulin
- 2hPBG:
-
2-Hour postprandial blood glucose
References
Ibáñez L, Oberfield SE, Witchel S, Auchus RJ, Chang RJ, Codner E, Dabadghao P, Darendeliler F, Elbarbary NS, Gambineri A, Garcia Rudaz C, Hoeger KM, López-Bermejo A, Ong K, Peña AS, Reinehr T, Santoro N, Tena-Sempere M, Tao R, Yildiz BO, Alkhayyat H, Deeb A, Joel D, Horikawa R, de Zegher F, Lee PA. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolescence. Horm Res Paediatr. 2017;88(6):371–95. https://doi.org/10.1159/000479371.
Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. 2012;27(10):3067–73. https://doi.org/10.1093/humrep/des232.
Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–9. https://doi.org/10.1210/jc.2003-032046.
Dadachanji R, Shaikh N, Mukherjee S. Genetic Variants Associated with Hyperandrogenemia in PCOS Pathophysiology. Genet Res Int. 2018;18(2018):7624932. https://doi.org/10.1155/2018/7624932.
Jiang NX, Li XL. The Disorders of Endometrial Receptivity in PCOS and Its Mechanisms. Reprod Sci. 2022;29(9):2465–76. https://doi.org/10.1007/s43032-021-00629-9.
Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, Jacobs HS. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod. 1995;10(8):2107–11. https://doi.org/10.1093/oxfordjournals.humrep.a136243.
DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril. 2005;83(5):1454–60. https://doi.org/10.1016/j.fertnstert.2004.11.070.
Zeng X, Xie YJ, Liu YT, Long SL, Mo ZC. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta. 2020;502:214–21. https://doi.org/10.1016/j.cca.2019.11.003.
Liu Q, Wang J, Xu Q, Kong L, Wang J. A retrospective cohort study of obstetric complications and birth outcomes in women with polycystic ovarian syndrome. J Obstet Gynaecol. 2022;42(4):574–9. https://doi.org/10.1080/01443615.2021.1931066.
Nandi A, Chen Z, Patel R, Poretsky L. Polycystic ovary syndrome. Endocrinol Metab Clin North Am. 2014;43(1):123–47. https://doi.org/10.1016/j.ecl.2013.10.003.
Jedel E, Waern M, Gustafson D, Landén M, Eriksson E, Holm G, Nilsson L, Lind AK, Janson PO, Stener-Victorin E. Anxiety and depression symptoms in women with polycystic ovary syndrome compared with controls matched for body mass index. Hum Reprod. 2010;25(2):450–6. https://doi.org/10.1093/humrep/dep384.
Puurunen J, Piltonen T, Morin-Papunen L, Perheentupa A, Järvelä I, Ruokonen A, Tapanainen JS. Unfavorable hormonal, metabolic, and inflammatory alterations persist after menopause in women with PCOS. J Clin Endocrinol Metab. 2011;96(6):1827–34. https://doi.org/10.1210/jc.2011-0039.
Cena H, Chiovato L, Nappi RE. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J Clin Endocrinol Metab. 2020;105(8):e2695–709. https://doi.org/10.1210/clinem/dgaa285.
Yildiz BO, Knochenhauer ES, Azziz R. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(1):162–8. https://doi.org/10.1210/jc.2007-1834.
Rachoń D, Teede H. Ovarian function and obesity–interrelationship, impact on women’s reproductive lifespan and treatment options. Mol Cell Endocrinol. 2010;316(2):172–9. https://doi.org/10.1016/j.mce.2009.09.026.
Al-Jefout M, Alnawaiseh N, Al-Qtaitat A. Insulin resistance and obesity among infertile women with different polycystic ovary syndrome phenotypes. Sci Rep. 2017;7(1):5339. https://doi.org/10.1038/s41598-017-05717-y.
Nathan N, Sullivan SD. The utility of metformin therapy in reproductive-aged women with polycystic ovary syndrome (PCOS). Curr Pharm Biotechnol. 2014;15(1):70–83. https://doi.org/10.2174/1389201015666140330195142.
Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–1585. https://doi.org/10.1007/s00125-017-4342-z.
Diamanti-Kandarakis E, Christakou CD, Kandaraki E, Economou FN. Metformin: an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur J Endocrinol. 2010;162(2):193–212. https://doi.org/10.1530/EJE-09-0733.
Zhao H, Xing C, Zhang J, He B. Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: a network meta-analysis. Reprod Health. 2021;18(1):171. https://doi.org/10.1186/s12978-021-01207-7.
Oppelt PG, Mueller A, Jentsch K, Kronawitter D, Reissmann C, Dittrich R, Beckmann MW, Cupisti S. The effect of metformin treatment for 2 years without caloric restriction on endocrine and metabolic parameters in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2010;118(9):633–7. https://doi.org/10.1055/s-0029-1237705.
Hadžiomerović-Pekić D, Wildt L, Weiss JM, Moeller K, Mattle V, Seeber BE. Metformin, naltrexone, or the combination of prednisolone and antiandrogenic oral contraceptives as first-line therapy in hyperinsulinemic women with polycystic ovary syndrome. Fertil Steril. 2010;94(6):2385–8. https://doi.org/10.1016/j.fertnstert.2010.02.041.
Raperport C, Chronopoulou E, Homburg R. Effects of metformin treatment on pregnancy outcomes in patients with polycystic ovary syndrome. Expert Rev Endocrinol Metab. 2021;16(2):37–47. https://doi.org/10.1080/17446651.2021.1889366.
Duranteau L, Lefevre P, Jeandidier N, Simon T, Christin-Maitre S. Should physicians prescribe metformin to women with polycystic ovary syndrome PCOS? Ann Endocrinol (Paris). 2010;71(1):25–7. https://doi.org/10.1016/j.ando.2009.12.005.
Magzoub R, Kheirelseid EAH, Perks C, Lewis S. Does metformin improve reproduction outcomes for non-obese, infertile women with polycystic ovary syndrome? Meta-analysis and systematic review. Eur J Obstet Gynecol Reprod Biol. 2022;271:38–62. https://doi.org/10.1016/j.ejogrb.2022.01.025.
Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, Kelestimur F, Macut D, Micic D, Pasquali R, Pfeifer M, Pignatelli D, Pugeat M, Yildiz BO, on behalf of the ESE PCOS Special Interest Group. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171(4):P1–29. https://doi.org/10.1530/EJE-14-0253.
Lamos EM, Malek R, Davis SN. GLP-1 receptor agonists in the treatment of polycystic ovary syndrome. Expert Rev Clin Pharmacol. 2017;10(4):401–8. https://doi.org/10.1080/17512433.2017.1292125.
Gentilella R, Bianchi C, Rossi A, Rotella CM. Exenatide: a review from pharmacology to clinical practice. Diabetes Obes Metab. 2009;11(6):544–56. https://doi.org/10.1111/j.1463-1326.2008.01018.x.
Shaefer CF Jr, Kushner P, Aguilar R. User’s guide to mechanism of action and clinical use of GLP-1 receptor agonists. Postgrad Med. 2015;127(8):818–26. https://doi.org/10.1080/00325481.2015.1090295.
Tao X, Cai L, Chen L, Ge S, Deng X. Effects of metformin and Exenatide on insulin resistance and AMPKα-SIRT1 molecular pathway in PCOS rats. J Ovarian Res. 2019;12(1):86. https://doi.org/10.1186/s13048-019-0555-8.
Siamashvili M, Davis SN. Update on the effects of GLP-1 receptor agonists for the treatment of polycystic ovary syndrome. Expert Rev Clin Pharmacol. 2021;14(9):1081–9. https://doi.org/10.1080/17512433.2021.1933433.
Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metab. 2021;106(3):e1071–83. https://doi.org/10.1210/clinem/dgaa839.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JPT, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. https://doi.org/10.1002/14651858.ED000142.
Si XN, Ma J. Effect of exenatide on endometrial insulin resistance and endocrine metabolism in patients with polycystic ovary syndrome. Matern Child Health Care China. 2019;34:547–9. https://doi.org/10.7620/zgfybj.j.issn.1001-4411.2019.03.22.
Li C, Zheng LL. The efficacy and safety of exenatide combined with metformin for polycystic ovary syndrome in obese women of reproductive age. Zhengzhou University 2017. https://scholar.google.com/scholar?hl=zh-CN&as_sdt=0%2C5&q=%E8%89%BE%E5%A1%9E%E9%82%A3%E8%82%BD%E8%81%94%E5%90%88%E4%BA%8C%E7%94%B2%E5%8F%8C%E8%83%8D%E5%AF%B9%E5%A4%9A%E5%9B%8A%E5%8D%B5%E5%B7%A2%E7%BB%BC%E5%90%88%E5%BE%81%E7%9A%84%E5%B9%B2%E9%A2%84%E4%BD%9C%E7%94%A8&btnG=#d=gs_qabs&t=1680161844634&u=%23p%3DwTDMRiFHjHYJ.
Yuan Y, Huang ZS. Effects of exenatide on insulin resistance and epicardial fat thickness in obese polycystic ovary syndrome patients. Xiamen University 2018. https://scholar.google.com/scholar?hl=zh-CN&as_sdt=0%2C5&q=%E8%89%BE%E5%A1%9E%E9%82%A3%E8%82%BD%E5%AF%B9%E8%82%A5%E8%83%96%E5%9E%8B%E5%A4%9A%E5%9B%8A%E5%8D%B5%E5%B7%A2%E7%BB%BC%E5%90%88%E5%BE%81%E6%82%A3%E8%80%85%E8%83%B0%E5%B2%9B%E7%B4%A0%E6%8A%B5%E6%8A%97%E5%8F%8A%E5%BF%83%E5%A4%96%E8%86%9C%E8%84%82%E8%82%AA%E5%8E%9A%E5%BA%A6%E7%9A%84%E5%BD%B1%E5%93%8D&btnG=#d=gs_qabs&t=1680162146223&u=%23p%3Dvh7_UCe9Gu8J.
Lin WH, Zheng ZQ. Comparison of treatment with exenatide and metformin in overweight women with polycystic ovary syndrome. Fujian Medical University 2015. https://d.wanfangdata.com.cn/thesis/ChJUaGVzaXNOZXdTMjAyMzAxMTISB0Q3MzgxODEaCDJ0emdtZ2hr.
Fan XX, Hu YL, Hu YJ, et al. Effect study of Exenatide on patients with polycystic ovary syndrome and type 2 diabetes in plateau area. Chin J Diabetes. 2017;25:241–4. https://doi.org/10.3969/j.issn.1006-6187.2017.03.010.
Elkind-Hirsch K, Marrioneaux O, Bhushan M, Vernor D, Bhushan R. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(7):2670–8. https://doi.org/10.1210/jc.2008-0115.
Liu X, Zhang Y, Zheng SY, Lin R, Xie YJ, Chen H, Zheng YX, Liu E, Chen L, Yan JH, Xu W, Mai TT, Gong Y. Efficacy of exenatide on weight loss, metabolic parameters and pregnancy in overweight/obese polycystic ovary syndrome. Clin Endocrinol (Oxf). 2017;87(6):767–74. https://doi.org/10.1111/cen.13454.
Zheng SY, Zhang Y, Long T, et al. Short term monotherapy with exenatide is superior to metformin in weight loss, improving insulin resistance and inflammation in Chinese overweight/obese PCOS women. Obes Med. 2017;7:15–20. https://doi.org/10.1016/j.obmed.2017.06.003.
Tao T, Zhang Y, Zhu YC, Fu JR, Wang YY, Cai J, Ma JY, Xu Y, Gao YN, Sun Y, Fan W, Liu W. Exenatide, Metformin, or Both for Prediabetes in PCOS: A Randomized, Open-label, Parallel-group Controlled Study. J Clin Endocrinol Metab. 2021;106(3):e1420–32. https://doi.org/10.1210/clinem/dgaa692.
Dumesic DA, Richards JS. Ontogeny of the ovary in polycystic ovary syndrome. Fertil Steril. 2013;100(1):23–38. https://doi.org/10.1016/j.fertnstert.2013.02.011.
Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): The hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467–520. https://doi.org/10.1210/er.2015-1104.
Sun L, Ji C, Jin L, Bi Y, Feng W, Li P, Shen S, Zhu D. Effects of Exenatide on Metabolic Changes, Sexual Hormones, Inflammatory Cytokines, Adipokines, and Weight Change in a DHEA-Treated Rat Model. Reprod Sci. 2016;23(9):1242–9. https://doi.org/10.1177/1933719116635278.
Egan JM, Clocquet AR, Elahi D. The insulinotropic effect of acute exendin-4 administered to humans: comparison of nondiabetic state to type 2 diabetes. J Clin Endocrinol Metab. 2002;87(3):1282–90. https://doi.org/10.1210/jcem.87.3.8337.
Smith NK, Hackett TA, Galli A, Flynn CR. GLP-1: Molecular mechanisms and outcomes of a complex signaling system. Neurochem Int. 2019;128:94–105. https://doi.org/10.1016/j.neuint.2019.04.010.
Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. https://doi.org/10.1038/414799a.
Moghetti P. Insulin Resistance and Polycystic Ovary Syndrome. Curr Pharm Des. 2016;22(36):5526–34. https://doi.org/10.2174/1381612822666160720155855.
Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. https://doi.org/10.1210/er.2011-1034.
Nelson-Degrave VL, Wickenheisser JK, Hendricks KL, Asano T, Fujishiro M, Legro RS, Kimball SR, Strauss JF 3rd, McAllister JM. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol Endocrinol. 2005;19(2):379–90. https://doi.org/10.1210/me.2004-0178.
Hansen SL, Svendsen PF, Jeppesen JF, Hoeg LD, Andersen NR, Kristensen JM, Nilas L, Lundsgaard AM, Wojtaszewski JFP, Madsbad S, Kiens B. Molecular Mechanisms in Skeletal Muscle Underlying Insulin Resistance in Women Who Are Lean With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2019;104(5):1841–54. https://doi.org/10.1210/jc.2018-01771.
Lee YS, Jun HS. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016:3094642. https://doi.org/10.1155/2016/3094642.
Chaudhuri A, Ghanim H, Vora M, Sia CL, Korzeniewski K, Dhindsa S, Makdissi A, Dandona P. Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab. 2012;97(1):198–207. https://doi.org/10.1210/jc.2011-1508.
Anderson SL, Trujillo JM, McDermott M, Saseen JJ. Determining predictors of response to exenatide in type 2 diabetes. J Am Pharm Assoc. 2012;52(4):466–71. https://doi.org/10.1331/JAPhA.2012.10217.
Abdalla MA, Deshmukh H, Atkin S, Sathyapalan T. The potential role of incretin-based therapies for polycystic ovary syndrome: a narrative review of the current evidence. Ther Adv Endocrinol Metab. 2021;27(12):2042018821989238. https://doi.org/10.1177/2042018821989238.
Xu D, Nair A, Sigston C, Ho C, Li J, Yang D, Liao X, Chen W, Kuang M, Li Y, Reid C, Xiao H. Potential Roles of Glucagon-Like Peptide 1 Receptor Agonists (GLP-1 RAs) in Nondiabetic Populations. Cardiovasc Ther. 2022;16(2022):6820377. https://doi.org/10.1155/2022/6820377.
Jensterle M, Ferjan S, Ležaič L, Sočan A, Goričar K, Zaletel K, Janez A. Semaglutide delays 4-hour gastric emptying in women with polycystic ovary syndrome and obesity. Diabetes Obes Metab. 2022;25(4):975–84. https://doi.org/10.1111/dom.14944.
Funding
This work was funded by the Natural Science Foundation of Hunan Province, China (S2021JJSSLH0100).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
This study did not involve any human participants or animals.
Conflict of Interest
The authors report no financial or commercial conflicts of interest.
Consent for Publication
Not applicable.
Consent to Participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ye, ZR., Yan, CQ., Liao, N. et al. The Effectiveness and Safety of Exenatide Versus Metformin in Patients with Polycystic Ovary Syndrome: A Meta-Analysis of Randomized Controlled Trials. Reprod. Sci. 30, 2349–2361 (2023). https://doi.org/10.1007/s43032-023-01222-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01222-y