Abstract
Background
Autoantibodies develop in autoimmune diseases, cancer, diabetes mellitus (DM), and atherosclerosis-related diseases. However, autoantibody biomarkers have not been successfully examined for diagnosis and therapy.
Methods
Serological identification of antigens through recombinant cDNA expression cloning (SEREX) was used for primary screening of antigens. The cDNA product was expressed in bacteria and purified. Amplified luminescent proximity homogeneous assay-linked immunosorbent assay (AlphaLISA) was used to evaluate antibody levels in serum samples.
Results
Phosphoenolpyruvate carboxykinase 1 (PCK1) was recognized as an antigen by serum IgG antibodies in the sera of patients with atherosclerosis. AlphaLISA showed significantly higher serum antibody levels against recombinant PCK1 protein in patients with DM and cardiovascular disease than in healthy donors, but not in those with acute ischemic stroke, transient ischemic attack, or obstructive sleep apnea syndrome. The area under the receiver operating characteristic curve for anti-PCK1 antibodies was 0.7024 for DM. The serum anti-PCK1 antibody levels were associated with age, platelet count, and blood pressure. Anti-PCK1-antibody-positive patients showed significantly lower overall survival than the negative patients.
Conclusions
Serum anti-PCK1 antibody levels were found to be associated with DM. The anti-PCK1 antibody marker is useful for predicting the overall survival of patients with DM.
Similar content being viewed by others
Background
The number of patients with diabetes mellitus (DM) has considerably increased worldwide, which is now referred to as the diabetes pandemic [1]. DM is a disease in which blood glucose levels increase owing to decreased insulin secretion or increased resistance. Prolonged high blood glucose levels increase the risk of diabetic complications such as acute ischemic stroke (AIS) and cardiovascular disease (CVD), which are mainly caused by the development of atherosclerosis [2]. Early treatment of DM is important, not only to prevent diabetic complications, but also to prolong the life span of patients with DM [3].
We screened autoantibodies in the sera of patients with atherosclerosis using serological analysis of recombinant cDNA expression libraries (SEREX) and protein array methods. The following autoantibodies have been identified as biomarkers for atherosclerotic diseases including AIS [4, 5] and CVD [6,7,8]: matrix metalloproteinase 1 [9], adaptor-related protein complex 3 subunit delta 1 [10], forkhead box J2 [11], and bone morphogenetic protein 1 [12]. It is not surprising that these autoantibodies are also associated with DM, as there is a strong association between atherosclerosis and DM [13].
In this study, we identified phosphoenolpyruvate carboxykinase 1 (PCK1, also known as PEPCK) by SEREX screening using sera from patients with atherosclerosis and found that its autoantibody levels were specifically elevated in patients with DM. The anti-PCK1 antibody marker is useful for predicting the overall survival of patients with DM. Furthermore, the possible relevance of PCK1 activity in DM development is discussed.
Methods
Patient and control sera
The protocols of this study complied with the 1975 Declaration of Helsinki and were approved by the Local Ethical Review Board of Chiba University, Graduate School of Medicine in Chiba, Japan (No. 2017–251, 2018–320, 2020–1129), as well as by the Review Boards of the participating hospitals. Sera were collected from patients who provided written, informed consent. Each serum sample was centrifuged at 3000 × g for 10 min, and the supernatant was stored at − 80 °C until use, avoiding repeated freezing/thawing of samples.
Serum samples from 275 patients with DM, 85 patients with CVD, and 86 patients with obstructive sleep apnea syndrome (OSAS) were obtained from the Chiba University Hospital. Samples collected from 228 patients with AIS and 44 with transient ischemic attack (TIA) were obtained from Chiba Prefectural Sawara Hospital. Serum samples from patients with AIS, TIA, and CVD were obtained within 2 weeks of disease onset. Serum samples from healthy donors (HDs) were obtained from the Port Square Kashiwado Clinic and Chiba Prefectural Sawara Hospital. These HD participants were selected from those that exhibited no abnormalities on cranial magnetic resonance imaging for comparison with TIA and AIS. We compared patients with DM to 81 HDs, patients with OSAS and CVD to 76 HDs, and patients with TIA or AIS to 138 HDs. HDs selected for comparison had blood samples collected at approximately the same time as the samples from each respective disease group.
The patients were randomly selected, and the definition of each disease is as follows. (1) DM was diagnosed according to the diagnostic criteria of the Japanese Diabetes Society [14]. Moreover, patients who had already been prescribed oral hypoglycemia agents and/or insulin injections were considered patients with DM. (2) Patients with CVD were those who visited the emergency medical department due to acute myocardial infarction or unstable angina pectoris. (3) OSAS was diagnosed by polysomnography (PSG) [15]. (4) For AIS, TIA, the stroke subtype of each patient was also determined according to the criteria of the Trial of Org 10,172 in the Acute Stroke Treatment classification system [16]. In this analysis, large-artery atherosclerosis or small-artery occlusion (lacune) were considered AIS or cerebral infarction [11].
The patients with diabetes were followed up for 100 months. Each patient’s status was checked on the electric medical record on all hospital visits.
SEREX screening
To select the antigens recognized by serum IgG antibodies, SEREX screening was performed using sera from patients with atherosclerosis and a human aortic endothelial cell cDNA phage library (Uni-ZAP XR Premade Library, Stratagene, La Jolla, CA), as described previously [17]. The SEREX process is a well-established technique for pinpointing antigenic proteins. SEREX merges molecular cloning via phage expression libraries with serological typing, making it a highly efficient and user-friendly approach for identifying antigenic markers across the human genome. This method has successfully uncovered over 1000 novel tumor antigens and is widely regarded as a powerful tool for identifying potential targets in various forms of malignant tumors. We searched for antibody markers supposedly associated with atherosclerosis using SEREX. We identified approximately 100 different antibodies that might be related to atherosclerotic vascular disease, some of which had been already reported. PCK1-Ab was identified through this screening. As diabetes seems to be closely related to the development and progression of atherosclerotic vascular disease, we analyzed the relationship between PCK1-Ab and DM.
Preparation of recombinant PCK1 protein
We cloned the human PCK1 cDNA sequence (accession number: NP_001284695.1) into the EcoRI/XhoI site of pGEX-4 T-1 (Cytiva, Pittsburgh, PA, USA). We induced the expression of the cDNA product by treating Escherichia coli BL-21 cells harboring the pGEX-4 T-1-PCK1 with 0.1 mM of isopropyl-β-D-thiogalactoside (Wako Pure Chemicals, Osaka, Japan) at 37 °C for 3 h. Cells were lysed by sonication in the BugBuster Master Mix (Merck Millipore, Darmstadt, Germany). Glutathione S-transferase (GST)-fused PCK1 protein was purified using Glutathione-Sepharose 4 Fast Flow medium (Cytiva) and then concentrated to 1.56 mg/mL in phosphate-buffered saline as described previously [9, 10].
The GST-fused full-length protein was expressed in bacteria and purified by affinity chromatography, as previously described [9].
Amplified luminescence proximity homogeneous assay-linked immunosorbent assay (AlphaLISA)
AlphaLISA was performed in 384-well microtiter plates (white opaque OptiPlate™, Perkin Elmer, Waltham, MA, USA), containing either 2.5 µL of 1:100-diluted serum with 2.5 µL of GST or GST-PCK1 proteins (10 µg/mL) in AlphaLISA buffer [25 mM N-(2-hydroxyethyl) piperazine-Nʹ-2-ethane sulfonic acid, pH 7.4, 0.1% casein, 0.5% Triton X-100, 1 mg/mL dextran-500, and 0.05% ProClin-300]. We incubated the reaction mixture at room temperature for 6–8 h, and then added anti-human IgG-conjugated acceptor beads (2.5 µL at 40 µg/mL) and glutathione-conjugated donor beads (2.5 µL at 40 µg/mL). The mixture was then incubated at room temperature in the dark for 7–21 days. We measured the chemical emissions using an EnSpire Alpha microplate reader (PerkinElmer), as described previously [8, 9, 17, 18]. We calculated the specific reactions by subtracting the emission photon counts of the GST control from the counts of the GST-fused PCK1 protein.
AlphaLISA is a novel, recently developed method. After examining suitable AlphaLISA conditions in this study, we concluded that incubation for 7–21 days is the best option to obtain specific antigen-Ab reaction as well as to reduce background noise.
Statistical analysis
We employed the Mann–Whitney U test to determine the significant differences between the two groups, and the Kruskal–Wallis test (Mann–Whitney U test with Bonferroni correction applied) to evaluate the differences among three groups. Correlations were calculated using Spearman’s correlation. All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). We assessed the predictive values of the putative disease markers via receiver operating characteristic (ROC) curve analysis and set the cutoff values of the Youden index, which maximizes the sum of sensitivity and specificity. We evaluated patient survival using the Kaplan–Meier method and compared them using the log-rank test. All tests were 2-tailed, and P values < 0.05 were considered to indicate statistically significant differences.
Results
Recognition of PCK1 by serum antibodies in patients with atherosclerosis
We performed SEREX screening and identified PCK1 (Accession Number: NM_002591.4) as the antigen recognized by antibodies in the sera of patients with atherosclerosis. Subsequently, the GST-fused full-length PCK1 protein and control GST protein were expressed in bacteria and purified by affinity chromatography. The results of sodium dodecyl-sulfate–polyacrylamide gel electrophoresis showed that the purity of both GST-PCK1 and GST was higher than 95% (Supplementary Figure S1).
Elevated levels of serum anti-PCK1 antibodies (s-PCK1-Abs) in patients with DM
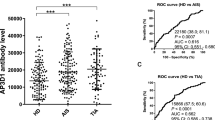
Next, we examined s-PCK1-Ab levels in patients with DM using GST-PCK1 as an antigen. Serum samples from 81 HDs and 275 patients with DM were obtained from Port Square Kashiwado Clinic and Chiba University Hospital, respectively. The s-PCK1-Ab levels were significantly higher in patients with DM than in HDs (Fig. 1a). Using the cutoff values of the average plus two standard deviations (SDs) of the HD values, the positive rates of s-PCK1-Abs in HDs and patients with DM were 2.5% and 29.5%, respectively (Table 1). We performed ROC analysis to evaluate the ability of the s-PCK1-Ab marker to indicate the presence of DM. The area under the ROC curve (AUC) for s-PCK1-Abs was 0.7024, yielding sensitivity and specificity of 36.73% and 95.06%, respectively (Fig. 1b).
Comparison of s-PCK1-Ab levels between healthy donors (HDs) and patients with diabetes mellitus (DM). a The serum anti-PCK1 antibody (s-PCK1-Ab) levels of HDs and patients with DM were examined by AlphaLISA using GST-PCK12-302 protein as the antigen, followed by subtraction of the levels against control GST. A scatter dot plot of the s-PCK1-Ab levels is shown. The bars represent the average and the average ± SD. P values were calculated using the Kruskal–Wallis test. *** P < 0.001 vs. HD specimens. The total (male/female) numbers, average ages ± standard deviations (SDs), average antibody levels ± SDs, cutoff values, positive numbers, positive rates (%), and P values versus HDs are summarized and shown in Table 1. b Receiver operating characteristic (ROC) curve analysis. The ability of s-PCK1-Abs to detect DM was evaluated using ROC curve analysis. Numbers in the figures indicate the areas under the ROC curve (AUC), cutoff values for antibody levels, sensitivity, specificity, and 95% confidence interval (95% CI). c Comparison of the overall survival in patients with DM between the positive and negative groups of s-PCK1-Abs. Kaplan–Meier with the log-rank test is shown
The overall survival during the follow-up period of 100 months was compared between the s-PCK1-Ab-positive and -negative DM groups with a cutoff value of the Youden index. Thirty-three deaths were confirmed (99.6% of the cases were followed up). Two patients died owing to myocardial infarction, one owing to cerebral infarctions, and 12 owing to cancer. The causes of death were unknown for 18 cases.
The s-PCK1-Ab-positive group showed a more unfavorable prognosis than the negative group (P = 0.022) (Fig. 1c). It should be noted that the difference was more evident in the late stage (after 70 months) than in the early stage (before 60 months).
Levels of s-PCK1-Abs in the patients with atherosclerosis-related diseases
PCK1 was screened by SEREX using sera from patients with atherosclerosis, so the antibody levels in other atherosclerosis-related diseases were also examined. We first examined the s-PCK1-Ab levels in patients with AIS or TIA. Sera from HDs and patients with AIS and TIA were obtained from Chiba Prefectural Sawara Hospital. AlphaLISA results revealed that s-PCK1-Ab levels were not significantly different between patients with AIS or TIA and HDs (Fig. 2a). At a cutoff value equivalent to the average plus two SDs of the HD values, the s-PCK1-Ab-positive rates for the HDs, patients with AIS, and those with TIA were 2.9%, 15.9%, and 8.8%, respectively (Table 2). Thus, a slight increase in positivity was observed in AIS and TIA.
Comparison of serum s-PCK1-Ab levels between healthy donors (HDs) and patients. The s-PCK1-Ab levels of HDs and patients with transient ischemic attack (TIA) or acute ischemic stroke (AIS) (a) and cardiovascular disease (CVD) or obstructive sleep apnea syndrome (OSAS), (b) were examined by AlphaLISA using glutathione S-transferase (GST)-PCK12-302 protein as the antigen, followed by subtraction of the levels against control GST. A scatter dot plot of the s-PCK1-Ab levels is shown as described in the legends of Fig. 1. The bars represent the average and average ± SD. P values were calculated using the Kruskal–Wallis test. *, P < 0.05 vs. HD specimens. ns, not significant. The total (male/female) numbers, average ages ± standard deviations (SDs), average antibody levels ± SDs, cutoff values, positive numbers, positive rates (%), and P values versus HDs are summarized and shown in Tables 2 and 3
Atherosclerosis is a major risk factor for CVD, and OSAS is frequently accompanied by hypertension leading to atherosclerosis [6, 7, 16]. We examined antibody levels in serum samples from patients with CVD or OSAS obtained from Chiba University Hospital. The s-PCK1-Ab levels were slightly but significantly higher in the patients with CVD, but not in those with OSAS, as compared with those in HDs (Fig. 2b). At a cutoff value of the average plus two SDs of the HD samples, the positivity rates of HDs and patients with CVD and OSAS were 5.3%, 4.7%, and 9.3%, respectively (Table 3). Thus, c-PCK1-Abs may not be closely associated with CVD or OSAS.
Correlation analysis
We performed a relation analysis between s-PCK1-Ab levels and participant data using 275 specimens from the DM cohort at Chiba University Hospital. In this analysis, we employed the Mann–Whitney U test to compare s-PCK1-Ab levels between male and female participants, type-1 and type-2 DM, with or without obesity (body mass index [BMI] ≥ 25), hypertension, CVD, dyslipidemia, and smoking or alcohol intake habits. We observed that s-PCK1-Ab levels were significantly higher in patients with hypertension than in those without (Table 4).
We performed Spearman's correlation analysis to determine the correlation between s-PCK1-Ab levels and the continuous variables of participant parameters such as age, height, weight, BMI, blood test data, and lifestyle factors such as smoking duration (years) and alcohol intake frequency (times/week). The results showed a significant correlation between s-PCK1-Ab levels and age, calcium level, creatine phosphokinase level, platelet number, and blood pressure (Table 5).
Discussion
During our analysis, the initial SEREX screening identified PCK1 as an antigen as recognized by serum IgG in patients with atherosclerosis, and subsequently, recombinant GST-tagged PCK1 protein of 301 amino acids was purified. Using recombinant PCK1 protein as an antigen, we examined serum antibody levels using AlphaLISA. The results showed that significantly higher s-PCK1-Ab levels were observed exclusively in patients with DM, but not in those with AIS, TIA, and OSAS, compared with those in HDs (Figs. 1a, 2a, b, Tables 1, 2, 3 and 4). Patients with CVD showed a minimal significant difference from HDs. The AUC value of s-PCK1-Abs versus DM was 0.7024 (Fig. 1b). This DM-specific association of s-PCK1-Ab is distinct from previous results that showed that most of the SEREX autoantibodies screened using sera from patients with atherosclerosis were associated with multiple atherosclerosis-related diseases such as AIS and CVD [17, 18]. Our analysis shows that s-PCK1-Abs indicate the presence of DM with a sensitivity and specificity of 36.73% and 95.06%, respectively, by ROC curve analysis. A sensitivity of 36.73% was considered high because the specificity was 95.06%. When the specificity was set to 60%, the sensitivity increased to approximately 65% and 95.06%, respectively.
The comparison of patients' data with the Mann–Whitney U test and Spearman's correlation analysis showed a close correlation between s-PCK1-Ab levels and hypertension (Tables 4 and 5). Antibody levels did not significantly correlate with blood sugar (BS) (P = 0.4358), HbA1c (P = 0.4515), or glycoalbumin (P = 0.1098), which are typical DM markers (Table 5).
PCK1 is an enzyme that converts oxaloacetic acid to phosphoenolpyruvate; there are two isozymes in humans, mitochondrial (PCK 2) and cytosolic (PCK1) PCK [19,20,21]. PCK1 is involved in gluconeogenesis and is a crucial enzyme in glucose metabolism in the body [22]. Indeed, PCK1-knockout mice die early after birth with profound hypoglycemia [23], which was partially rescued by the overexpression of PCK1 in the liver [24]. Therefore, gluconeogenesis under the control of PCK1 in the liver is crucial to avoid hypoglycemia. In contrast, the overexpression of PCK1 in mice leads to diabetes [25]. It has also been reported that a − 232C/G containing promoter of PCK1 showed 5- to 100-fold increased basal expression of PCK1 compared with –232C, and − 232C/G polymorphism of PCK1 has been associated with an increased risk of type 2 DM [26,27,28]. Glucagon, glucocorticoid, and retinoic acid increased the expression of PCK1, whereas insulin inhibited its expression [29]. Therefore, PCK1 expression increases in the presence of insulin resistance.
Our results showed that s-PCK1-Ab levels were higher in patients with DM than in HDs. Since PCK1 expression seems to increase under diabetic conditions, it might make sense that s-PCK1-Ab levels were higher among patients with DM. One of the major reasons for the development of autoantibodies could be the destruction of lesion tissue, followed by the leakage of intracellular antigenic proteins. Although the leaked proteins may rapidly degrade, repeated leaking and exposure to antigens can tremendously elevate antibody levels. Autoantibody markers are more sensitive than antigen markers. This means that antibody markers can detect very early stages of disease progression [11]. Thus, it is not surprising that antibody markers predict the fate of the disease several years later. Since patients with DM, especially those with insulin resistance, often complicate nonalcoholic fatty liver disease and nonalcoholic steatohepatitis, there is a high chance of PCK1 leaking from live cells, which might produce antibodies against PCK1 [30]. Insulin resistance develops not only in atherosclerotic vascular disease but also in hypertension; therefore, s-PCK1-Ab levels are related to CVD and hypertension.
Apart from the complications and comorbidities of DM, it is intriguing that s-PCK1-Ab levels are related to prognosis. Since the mortality rate of DM is much higher than that of HDs, prognostic markers can be used to detect high-risk patients with poor prognosis among patients with DM. Thus, s-PCK1-Ab may be a useful marker. However, we still do not know why high s-PCK1-Ab levels are related to poor prognosis. One reason for this may be the high incidence of CVD. Indeed, it has been reported that a functional promoter polymorphism in PCK1 is associated with carotid wall thickness [31], which is associated with CVD. PCK1 and PCK2 have also been reported to be critical for the growth of certain cancers [32]. Hence, high levels of s-PCK1-Ab may be related to a high incidence of cancer. The reason for death has not been investigated in all patients with DM who were followed up in this study. Therefore, in future studies, we will identify why high s-PCK1-Ab levels are related to poor prognosis.
This study had some limitations. First, s-PCK1-Abs were measured among the specimens collected at a Japanese university and hospital, which may have biased the specimen population. Second, we do not know the mechanism by which s-PCK1-Abs were increased in patients with DM and hypertension and were related to poor prognosis. Third, the number of patients is small. Therefore, a study with a larger sample and longer duration should be performed in the future to confirm our current findings.
Conclusions
s-PCK1-Abs are highly sensitive and specific for DM and could be a novel prognostic marker for patients with DM.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
95% Confidence interval
- AIS:
-
Acute ischemic stroke
- AlphaLISA:
-
Amplified luminescence proximity homogeneous assay-linked immunosorbent assay
- AUC:
-
Area under the ROC curve
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- DM:
-
Diabetes mellitus
- GST:
-
Glutathione S-transferase
- HD:
-
Healthy donor
- OSAS:
-
Obstructive sleep apnea syndrome
- PCK:
-
Phosphoenolpyruvate carboxykinase 1
- ROC:
-
Receiver operating characteristic
- s-PCK1-Ab:
-
Serum anti-PCK1 antibody
- SD:
-
Standard deviation
- SEREX:
-
Serological identification of antigens by recombinant cDNA expression cloning
- TIA:
-
Transient ischemic attack
References
Ali MK, Siegel KR, Chandrasekar E, Tandon N, Montoya PA, Mbanya JC, et al. Diabetes: an update on the pandemic and potential solutions. In: Prabhakaran D, Anand S, Gaziano TA, Mbanya JC, Wu Y, Nugent R, editors., et al., Cardiovascular, respiratory, and related disorders. 3rd ed. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017.
Glovaci D, Fan W, Wong ND. Epidemiology of diabetes mellitus and cardiovascular disease. Curr Cardiol Rep. 2019;21:21. https://doi.org/10.1007/s11886-019-1107-y.
Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. adults, 1999-2018. N Engl J Med. 2021;384:2219–28. https://doi.org/10.1056/NEJMsa2032271.
Yoshida Y, Hiwasa T, Machida T, Kobayashi E, Mine S, Matsushima J, et al. Elevation of autoantibody in patients with ischemic stroke. Neurol Med Chir (Tokyo). 2018;58:303–10. https://doi.org/10.2176/nmc.ra.2018-0022.
Yoshida Y, Wang H, Hiwasa T, Machida T, Kobayashi E, Mine S, et al. Elevation of autoantibody level against PDCD11 in patients with transient ischemic attack. Oncotarget. 2018;9:8836–48. https://doi.org/10.18632/oncotarget.23653.
Matsumura T, Terada J, Kinoshita T, Sakurai Y, Yahaba M, Tsushima K, et al. Circulating autoantibodies against neuroblastoma suppressor of tumorigenicity 1 (NBL1): A potential biomarker for coronary artery disease in patients with obstructive sleep apnea. PLoS ONE. 2018;13:e0195015. https://doi.org/10.1371/journal.pone.0195015.
Katsumata Y, Terada J, Matsumura T, Koshikawa K, Sakao S, Tomiyoshi G, et al. Circulating anti-sorting nexins 16 antibodies as an emerging biomarker of coronary artery disease in patients with obstructive sleep apnea. Diagnostics (Basel). 2020;10:71. https://doi.org/10.3390/diagnostics10020071.
Yoshida Y, Zhang XM, Wang H, Machida T, Mine S, Kobayashi E, et al. Elevated levels of autoantibodies against DNAJC2 in sera of patients with atherosclerotic diseases. Heliyon. 2020;6:e04661. https://doi.org/10.1016/j.heliyon.2020.e04661.
Wang H, Zhang XM, Tomiyoshi G, Nakamura R, Shinmen N, Kuroda H, et al. Association of serum levels of antibodies against MMP1, CBX1, and CBX5 with transient ischemic attack and cerebral infarction. Oncotarget. 2018;9:5600–13. https://doi.org/10.18632/oncotarget.23789.
Li SY, Yoshida Y, Kobayashi E, Kubota M, Matsutani T, Mine S, et al. Serum anti-AP3D1 antibodies are risk factors for acute ischemic stroke related with atherosclerosis. Sci Rep. 2021;11:13450. https://doi.org/10.1038/s41598-021-92786-9.
Hiwasa T, Wang H, Goto KI, Mine S, Machida T, Kobayashi E, et al. Serum anti-DIDO1, anti-CPSF2, and anti-FOXJ2 antibodies as predictive risk markers for acute ischemic stroke. BMC Med. 2021;19:131. https://doi.org/10.1186/s12916-021-02001-9.
Machida T, Kubota M, Kobayashi E, Iwadate Y, Saeki N, Yamaura A, et al. Identification of stroke-associated-antigens via screening of recombinant proteins from the human expression cDNA library (SEREX). J Transl Med. 2015;13:71. https://doi.org/10.1186/s12967-015-0393-4.
Huang H, Liu J, Liang X, Fang L, Yang C, Ke K, et al. Trends in the prevalence of elevated cardiovascular risk and the control of its risk factors among US adults, 2001–2020. Front Cardiovasc Med. 2023;10:1153926. https://doi.org/10.3389/fcvm.2023.1153926.
Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010;1:212–28. https://doi.org/10.1111/j.2040-1124.2010.00074.x.
Matsumura T, Terada J, Kinoshita T, Sakurai Y, Yahaba M, Ema R, et al. Circulating anti-coatomer protein complex subunit epsilon (COPE) autoantibodies as a potential biomarker for cardiovascular and cerebrovascular events in patients with obstructive sleep apnea. J Clin Sleep Med. 2017;13:393–400. https://doi.org/10.5664/jcsm.6488.
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. https://doi.org/10.1161/01.str.24.1.35.
Li SY, Yoshida Y, Kobayashi E, Adachi A, Hirono S, Matsutani T, et al. Association between serum anti-ASXL2 antibody levels and acute ischemic stroke, acute myocardial infarction, diabetes mellitus, chronic kidney disease and digestive organ cancer, and their possible association with atherosclerosis and hypertension. Int J Mol Med. 2020;46:1274–88. https://doi.org/10.3892/ijmm.2020.4690.
Sumazaki M, Shimada H, Ito M, Shiratori F, Kobayashi E, Yoshida Y, et al. Serum anti-LRPAP1 is a common biomarker for digestive organ cancers and atherosclerotic diseases. Cancer Sci. 2020;111:4453–64. https://doi.org/10.1111/cas.14652.
Pilz AJ, Willer E, Povey S, Abbott CM. The genes coding for phosphoenolpyruvate carboxykinase-1 (PCK1) and neuronal nicotinic acetylcholine receptor alpha 4 subunit (CHRNA4) map to human chromosome 20, extending the known region of homology with mouse chromosome 2. Ann Hum Genet. 1992;56:289–93. https://doi.org/10.1111/j.1469-1809.1992.tb01155.x.
Modaressi S, Brechtel K, Christ B, Jungermann K. Human mitochondrial phosphoenolpyruvate carboxykinase 2 gene. Structure, chromosomal localization and tissue-specific expression. Biochem J. 1998;333:359–66. https://doi.org/10.1042/bj3330359.
Suzuki M, Yamasaki T, Shinohata R, Hata M, Nakajima H, Kono N. Cloning and reporter analysis of human mitochondrial phosphoenolpyruvate carboxykinase gene promoter. Gene. 2004;338:157–62. https://doi.org/10.1016/j.gene.2004.06.005.
Yang J, Kalhan SC, Hanson RW. What is the metabolic role of phosphoenolpyruvate carboxykinase? J Biol Chem. 2009;284:27025–9. https://doi.org/10.1074/jbc.R109.040543.
Hakimi P, Johnson MT, Yang J, Lepage DF, Conlon RA, Kalhan SC, et al. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutr Metab (Lond). 2005;2:33. https://doi.org/10.1186/1743-7075-2-33.
Semakova J, Hyrossova P, Mendez-Lucas A, Cutz E, Bermudez J, Burgess S, et al. PEPCK-C reexpression in the liver counters neonatal hypoglycemia in Pck1 (del/del) mice, unmasking role in non-gluconeogenic tissues. J Physiol Biochem. 2017;73:89–98. https://doi.org/10.1007/s13105-016-0528-y.
Franckhauser S, Munoz S, Elias I, Ferre T, Bosch F. Adipose overexpression of phosphoenolpyruvate carboxykinase leads to high susceptibility to diet-induced insulin resistance and obesity. Diabetes. 2006;55:273–80. https://doi.org/10.2337/diabetes.55.02.06.db05-0482.
Cao H, van der Veer E, Ban MR, Hanley AJ, Zinman B, Harris SB, et al. Promoter polymorphism in PCK1 (phosphoenolpyruvate carboxykinase gene) associated with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2004;89:898–903. https://doi.org/10.1210/jc.2003-031361.
Gouni-Berthold I, Giannakidou E, Faust M, Berthold HK, Krone W. Association of the promoter polymorphism -232C/G of the phosphoenolpyruvate carboxykinase gene (PCK1) with type 2 diabetes mellitus. Diabet Med. 2006;23:419–25. https://doi.org/10.1111/j.1464-5491.2006.01819.x.
Rees SD, Britten AC, Bellary S, O’Hare JP, Kumar S, Barnett AH, et al. The promoter polymorphism -232C/G of the PCK1 gene is associated with type 2 diabetes in a UK-resident South Asian population. BMC Med Genet. 2009;10:83. https://doi.org/10.1186/1471-2350-10-83.
Yabaluri N, Bashyam MD. Hormonal regulation of gluconeogenic gene transcription in the liver. J Biosci. 2010;35:473–84. https://doi.org/10.1007/s12038-010-0052-0.
Ciardullo S, Perseghin G. Trends in prevalence of probable fibrotic nonalcoholic steatohepatitis in the United States, 1999–2016. Liver Int. 2022;43:340–4. https://doi.org/10.1111/liv.15503.
Hegele RA, Al-Shali KZ, House AA, Hanley AJ, Harris SB, Mamakeesick M, et al. Disparate associations of a functional promoter polymorphism in PCK1 with carotid wall ultrasound traits. Stroke. 2005;36:2566–70. https://doi.org/10.1161/01.STR.0000190833.43791.be.
Grasmann G, Smolle E, Olschewski H, Leithner K. Gluconeogenesis in cancer cells - Repurposing of a starvation-induced metabolic pathway? Biochim Biophys Acta Rev Cancer. 2019;1872:24–36. https://doi.org/10.1016/j.bbcan.2019.05.006.
Acknowledgements
The authors would like to thank Prof. Hao Wang (Jinan University) and Dr. Xiao-Meng Zhang (Chiba University) for supporting this research and Ms. Masae Suzuki, Risa Kimura, and Akiko Kimura for technical assistance.
Funding
This work was supported, in part, by research grants from the Japan Science and Technology Agency (JST) and JSPS KAKENHI Grant Numbers 20K17953, 19K09451, 17K19810, 19K08596, 20K07810, 16K10520, 20K07810, 21K07395, and 15K10117.
Author information
Authors and Affiliations
Contributions
T.N. Investigation, Writing—original draft, M.T. Writing—review & editing, A.H., H.Y., T.I., K.Y., Investigation, S.Y.L. Investigation, Writing—original draft, M.K., B.S.Z. Investigation, Y.Y. Writing—original draft. T.M., S.M., T.M., Y.K., J.T., A.N., K.T., H.T., R.N. Investigation, H.K. Project administration Y.I. Conceptualization, Writing—review & editing, T.H. Conceptualization, Project administration, Investigation, Writing—original draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocols of this study complied with the 1975 Declaration of Helsinki and were approved by the Local Ethical Review Board of Chiba University, Graduate School of Medicine in Chiba, Japan (No. 2017–251, 2018–320, 2020–1129), as well as by the Review Boards of the participating hospitals. Sera were collected from patients who provided written, informed consent.
Consent for publication
Not applicable.
Competing interests
The present study was performed in collaboration with Fujikura Kasei Co. Ltd. RN and HK are employees of Fujikura Kasei Co. Ltd. All other authors do not have any competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Figure S1.
Sodium dodecyl-sulfate (SDS)–polyacrylamide gel electrophoresis of purified proteins. Purified GST (control) and GST-PCK1 proteins (1 μg) were electrophoresed using SDS–polyacrylamide (10%) gel, followed by staining with Coomassie Brilliant Blue (NacalaiTesque, Kyoto, Japan). The molecular weights of the size markers (Protein Ladder One Plus, NacalaiTesque) are presented on the left. Arrows indicate protein positions: GST: 26 kDa, GST-PCK1: 94 kDa.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Namiki, T., Takemoto, M., Hayashi, A. et al. Serum anti-PCK1 antibody levels are a prognostic factor for patients with diabetes mellitus. BMC Endocr Disord 23, 239 (2023). https://doi.org/10.1186/s12902-023-01491-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01491-3