Abstract
Polycystic ovary syndrome (PCOS) is a common condition affecting reproductive-aged women with reproductive, metabolic and psychological consequences. Weight and lifestyle (diet, physical activity and behavioural) management are first-line therapy in international evidence-based guidelines for PCOS. While these recommend following population-level diet and physical activity guidelines, there is ongoing interest and research in the potential benefit of including psychological and sleep interventions, as well as a range of traditional, complimentary and integrative medicine (TCIM) approaches, for optimal management of PCOS. There is limited evidence to recommend a specific diet composition for PCOS with approaches including modifying protein, carbohydrate or fat quality or quantity generally having similar effects on the presentations of PCOS. With regards to physical activity, promising evidence supports the provision of vigorous aerobic exercise, which has been shown to improve body composition, cardiorespiratory fitness and insulin resistance. Psychological and sleep interventions are also important considerations, with women displaying poor emotional wellbeing and higher rates of clinical and subclinical sleep disturbance, potentially limiting their ability to make positive lifestyle change. While optimising sleep and emotional wellbeing may aid symptom management in PCOS, research exploring the efficacy of clinical interventions is lacking. Uptake of TCIM approaches, in particular supplement and herbal medicine use, by women with PCOS is growing. However, there is currently insufficient evidence to support integration into routine clinical practice. Research investigating inositol supplementation have produced the most promising findings, showing improved metabolic profiles and reduced hyperandrogenism. Findings for other supplements, herbal medicines, acupuncture and yoga is so far inconsistent, and to reduce heterogeneity more research in specific PCOS populations, (e.g. defined age and BMI ranges) and consistent approaches to intervention delivery, duration and comparators are needed. While there are a range of lifestyle components in addition to population-recommendations for diet and physical activity of potential benefit in PCOS, robust clinical trials are warranted to expand the relatively limited evidence-base regarding holistic lifestyle management. With consumer interest in holistic healthcare rising, healthcare providers will be required to broaden their knowledge pertaining to how these therapies can be safely and appropriately utilised as adjuncts to conventional medical management.
Similar content being viewed by others
Introduction
Polycystic ovary syndrome (PCOS) is a common condition affecting up to 13% of reproductive-aged women [1]. It is diagnosed through the European Society for Human Reproduction and Embryology/American Society for Reproductive Medicine (ESRHE/ASRM) criteria, requiring two of the following features: polycystic ovaries on ultrasound, oligoovulatory or anovulatory cycles and biochemical or clinical hyperandrogenism [2]. Women with PCOS experience a combination of reproductive (infertility, pregnancy complications) [3], metabolic (risk factors for and conditions of type 2 diabetes (T2DM) and cardiovascular disease (CVD)) [4, 5] and psychological (conditions including anxiety, depression, poor quality of life (QoL), disordered eating) comorbidities [6, 7].
Insulin resistance (IR) is defined as a key pathophysiological feature in PCOS, contributing to hyperandrogenism and worsening the clinical presentation of PCOS. While lean women present with IR in a form that is mechanistically different from IR caused by excess weight, overweight and obesity further exacerbate IR and consequent hyperinsulinaemia [8]. Women with PCOS also display a higher rate of weight gain over time [9] and a greater prevalence of overweight and obesity [10], which can further contribute to this worsening of IR and hence worsening of the presentation of PCOS [11]. The reason for this is unclear, but may be related to differences in intrinsic psychological and biological mechanisms [12,13,14,15], or extrinsic lifestyle factors such as diet and physical activity [16, 17]. Improving IR and excess adiposity are therefore key targets in PCOS management.
The International Evidence-Based Guideline for the Assessment and Management of PCOS [18], highlights lifestyle intervention as the primary early management strategy. Lifestyle interventions are traditionally defined as those designed to improve dietary intake or physical activity through appropriate behavioural support. In the 2018 PCOS guideline, lifestyle management is recommended for general health benefits [18]. Given that excess weight is associated with increased IR in PCOS [8], the guideline additionally promotes weight management, defined as: 1) weight gain prevention in all women with PCOS, and 2) achieving and maintaining modest weight loss in women with excess weight [18].
Lifestyle interventions in PCOS management can also be viewed as a broader construct beyond physical health. Since the emergence of the biopsychosocial model of healthcare in 1977, health disciplines have seen a gradual shift away from the classical biomedical model (where health is defined as the ‘absence of disease’) towards whole person or holistic care [19]. This is an approach that reflects many facets of the patient context, via integrating care that addresses biological, psychological, social, spiritual and ecological aspects [20]. It therefore requires a range of different treatment strategies to improve health. Provision of whole person or holistic care has been identified as a core objective of healthcare reforms internationally [21,22,23]. In line with these reforms the PCOS guideline recognises the importance of emotional wellbeing to overall health and QoL in women living with PCOS [18]. It also highlights evidence which suggests that the psychological impact associated with PCOS is under-appreciated in clinical care [4, 5], and that few women are satisfied with the mental health support they receive [6, 7]. Recommendations for appropriate screening, assessment and treatment strategies for anxiety, depression, psychosexual dysfunction, eating disorders and poor body image are provided [18]. These specific areas of emotional wellbeing are of particular concern, with research showing a higher prevalence and severity of depression and anxiety [24, 25], lower scores for satisfaction with sex life and feeling sexually attractive [26] and a higher prevalence of disordered eating and eating disorders [7] in women with PCOS. Features of PCOS, in particular hirsutism and increased weight, have also been shown to negatively affect body image [27, 28], with poor body image being strongly related to depression in women with PCOS [29, 30].
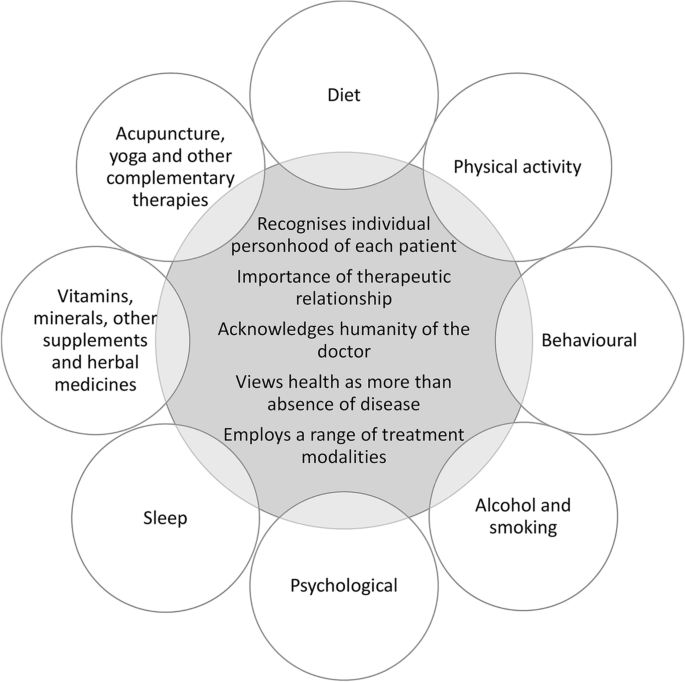
While the current PCOS guideline is comprehensive, considering all available evidence at the time of development and providing best-practice recommendations for necessary screening, risk assessment and management, it could not possibly cover all aspects of PCOS care. An International Delphi process was used to prioritise clinical questions, with consensus reached through extensive consultation with both consumers and multidisciplinary clinicians with expertise in PCOS care. Therapies, such as traditional, complementary and integrative medicine (TCIM), supplement use, sleep and meditation interventions are either briefly considered or not at all included in the 2018 PCOS guideline. Many of these therapies are novel and there is a paucity of evidence to support intervention efficacy on PCOS outcomes. However, as patient interest in these types of non-pharmacological interventions are growing [31,32,33,34,35], it is prudent to provide more guidance to healthcare providers in this area on their potential efficacy in PCOS. Whole person or holistic care recognises that the doctor-patient relationship should be one of open dialogue, where healthcare providers involve the patient in negotiating their care and recognises patient’s autonomy to guide treatment (Figure 1) [36].
Viewing lifestyle modifications through a whole person or holistic care lens. The key features of whole person or holistic care listed in the centre of the figure have been adapted from Thomas et al. [20]. ‘Recognises individual personhood’ relates to focusing on the unique needs of the person rather than the disease. ‘Importance of therapeutic relationship’ emphasises patient autonomy and responsibility. ‘Acknowledges humanity of the doctor’ considers the doctors’ ability to self-reflect on how they engage in the care of the patient. ‘Health as more than absence of disease’ incorporates the mental, emotional, physical, environmental and social needs of the patient. ‘Employs a range of treatment modalities’ promotes continuity of care across health disciplines, and while it may include traditional, complementary and integrative medicine (TCIM), TCIM is not holistic if used in isolation and without adequate integration into conventional healthcare
This review provides an extensive overview of evidence to date on lifestyle strategies used to optimise management of PCOS. Using a holistic definition of patient care, this review considers the traditional components of lifestyle change (diet, physical activity and behavioural change), psychological and sleep interventions, as well as TCIM approaches (supplements, herbal medicine, acupuncture and yoga). To improve translation of findings, evidence summaries are accompanied by an overview of relevant recommendations from the existing PCOS guideline. This highlights where emerging evidence supports current recommendations or provides new insights for research. As this is a narrative review, while evidence summaries include peer-reviewed journal articles identified from databases including Medline OVID, this is supplemented by expert opinion of the authors.
Traditional lifestyle and weight management
The PCOS guideline recommends the promotion of healthy lifestyle behaviours in all women with PCOS, to achieve and/or maintain a healthy weight and to optimise general health [18]. In women with excess weight, a weight loss of 5-10% is advised, aiming for an energy deficit of 30% or 500-750 kcal/day (1200-1500 kcal/day). While weight management is seen as a core component of lifestyle interventions, the guideline recognises that a healthy lifestyle provides benefits that occur independent of weight change.
A recent Cochrane review of 15 randomised controlled trials (RCT) and 498 participants, reported that lifestyle interventions compared with minimal intervention or usual care, significantly reduces weight (kg) and body mass index (BMI) and improves secondary reproductive outcomes such as free androgen index (FAI), testosterone (T), sex hormone-binding globulin (SHBG) and hirsutism (Ferriman-Gallwey score) [37]. In terms of metabolic outcomes, lifestyle intervention resulted in significant reductions in total cholesterol (TC), low density lipoprotein cholesterol (LDL-C) and fasting insulin (FINS). These findings are largely similar to that of other systematic reviews [38,40,41,41]. While no studies in the Cochrane review assessed clinical reproductive outcomes [37], individual trials that were not included in the review have reported that lifestyle interventions resulting in modest weight loss (2-5% total body weight) improve ovulation and menstrual regularity [42,44,45,45]. Losing >5% of weight is additionally associated with being able to conceive, having live births, reduction of ovarian volume and reduction in the number of follicles [46,48,49,50,51,52,52].
Although weight loss has shown clear benefits to PCOS outcomes, including not only on reproductive function, but also glucoregulatory status, androgen status and lipid profiles [42,44,45,46,47,48,49,50,51,52,52], there are varying degrees of responsiveness to weight loss in terms of improvement of PCOS symptoms. One study by Pasquali et al. [53] found that when women achieved similar levels of weight loss (>5% weight) only one-third displayed a full recovery from PCOS, with the remainder showing only partial or no recovery. Higher waist circumference (WC), waist-hip-ratio (WHR) and androstenedione at baseline were associated with a poorer chance of successful outcomes [53], suggesting that central adiposity and more severe hyperandrogenism may predict responsiveness to weight loss interventions in PCOS. Huber-Bucholz et al. [45] also reported women who achieve greater reductions in central fat and insulin sensitivity show greater symptom improvement with weight loss. This suggests that lifestyle interventions which simultaneously reduce IR and improve body composition (namely fat distribution), may help to optimise outcomes in PCOS management independent of changes in weight status.
Diet
The 2018 PCOS guideline recognises there is insufficient evidence to suggest that any specific dietary approaches provide greater benefits on health outcomes [18]. Dietary recommendations may take on a variety of balanced dietary strategies according to the individual’s lifestyle needs and preferences, as per general population recommendations [18]. This advice is based on a systematic review comparing different dietary compositions (e.g. low carbohydrate, low glycaemic index (GI) and glycaemic load (GL), high protein, monounsaturated fatty acid (MUFA) enriched and fat counting diets) to best manage PCOS, identifying minimal differences between diets on anthropometric outcomes, concluding weight loss improves the presentation of PCOS regardless of dietary composition [16, 54]. There is now an emerging body of evidence that suggests a range of dietary strategies may produce favourable effects on PCOS features that occur independent of weight loss. It is important that the emerging findings from these studies are thoroughly considered to support consumer and health professional interests. To summarise current evidence this review has grouped diets in terms of those that modify carbohydrates, protein and fat, as well as specific dietary patterns.
Carbohydrates
The use of altered carbohydrate composition remains the most researched dietary approach for PCOS management. Two systematic reviews published after guideline inception support altered carbohydrate intake to improve intermediate markers of PCOS [55, 56], finding that altering carbohydrate type, as opposed to content, is preferable to better manage PCOS [55]. RCTs [57,59,60,61,62,63,64,65,66,67,68,69,70,71,72,72] and pre-post intervention studies [73,75,76,77,78,79,80,80] demonstrate that following a low GI/GL diet for at least eight weeks significantly reduces WC [55, 73, 74] and BMI when compared to high GI/GL [56] or a regular diet [73,75,76,76], although levels of weight loss are generally comparable to other dietary compositions [59, 60, 72, 74]. These reductions are proposed to be a result of decreased hunger, which may reduce energy intake and make it easier to follow dietary recommendations in the long-term [78, 81,83,84,84]. Low GI/GL diets also improve insulin sensitivity and reproductive hormones (T, SHBG, FAI) compared to high carbohydrate [16, 55, 57, 79, 85] or control diets [56, 59, 73,75,76,76], contributing to improvements in reproductive function, specifically menstrual regularly [60, 79]. Lastly, low GI/GL diets can improve risk factors for T2DM and CVD, including glucose [86, 87], TC [55, 56, 59, 75, 77], LDL-C [55, 59, 75, 85], TAG [55, 59, 73] and HDL-C [75], when compared to a regular or high GI/GL diet. It must be noted that beneficial effects of low GI/GL diets may also be attributed to proportional increases in protein and/or fat loads.
Protein
In women with PCOS higher protein intakes may be superior at supressing androgen levels when compared to high carbohydrate diets. Postprandial research has shown that high protein meals can reduce insulin and dehydroepidiandrosteone stimulation compared to meals rich in glucose [88]. Research in the general population has also shown that reduced appetite and energy intakes from low GI/GL diets are related to increased protein intakes [89, 90]. RCTs and pre-post intervention studies found that high protein diets (defined here as protein constituting ≥25% energy [91]) consumed for at least four weeks reduce weight [12, 73, 74, 92,94,95,96,96], BMI [73, 74, 92, 95], WC [73, 74, 92, 97], WHR [73] and fat mass [74, 92, 97]. These reductions in anthropometric measures are accompanied by improved FINS [12, 74, 95, 98] and HOMA-IR [12, 73, 95, 98], blood lipids [12, 96], T [73, 92, 94] and hirsutism (Ferriman-Gallwey score) [73]. However, only three of these studies were able to show significant improvements in anthropometric measures [97], insulin sensitivity [98] and blood lipids [12] when compared to low/standard protein [12, 97] or control diets [98]. Only one study investigated effects on mental health outcomes and found that a high protein diet reduced depression and improved self-esteem [99].
Fats
Fatty acid composition is also an important consideration as metabolic disorders associated with PCOS can benefit from increased MUFA and polyunsaturated fatty acid (PUFA) intakes [63,65,65]. Postprandial research in PCOS reported prolonged reductions in T for high fat compared to low fat meals, which likely results from delayed nutrient absorption [86]. Two acute meal studies in lean and obese women with and without PCOS reported that proatherogenic inflammatory markers [100] and oxidative stress [101] were elevated, independent of but augmented by obesity, following saturated fat ingestion with this associated with worsened IR and androgens. Two experimental studies in PCOS investigated the effects of habitual walnut (PUFA rich diet) [102, 103] and almond (MUFA rich diet) [102] intake for at least six weeks and reported no differences in glucoregulatory status, lipids or androgens with the exception of HbA1c significantly decreasing in the walnut relative to the almond group. Kasim-Karakas et al. [103] reported increased fasting and postprandial glucose (oral glucose tolerance test (OGTT)) for increased walnut intake compared to habitual (control), which they postulated may be related to the control diet being rich in oleic acid. Together these findings suggest minimal benefit for improving dietary PUFA compared to MUFA content. Two RCTs in women with PCOS investigated the effects of diets rich in olive [104, 105], canola [105] and sunflower [105] oil. Yahay et al. [105] reported 25g/day canola oil caused reductions in TAG, TC/HDL-C, LDL-C/HDL-C, TAG/HDL-C and HOMA, but not androgens, compared to 25 g/day olive and sunflower oils [105]. This may be related to the more favourable fatty acid composition of canola oil, with comparable MUFA content to olive oil, higher alpha-linolenic acid, lower omega-6/omega-3 ratio and saturated fat than both olive and sunflower oils. Douglas et al. [104] reported weight and the acute insulin response (OGTT) were lower following a eucaloric low carbohydrate compared to a eucaloric MUFA-enriched olive oil diet, suggesting that reduced carbohydrate intake may have grater glucoregulatory benefits than increased MUFA intake [104]. Lastly, two RCTs compared hypocaloric low-fat diets to a low carbohydrate [106] or low GI [107] diets, with reductions in weight [106], WC [106], body fat [106, 107], FINS [106] and FAI [106] in both groups but no difference between groups.
Dietary and eating patterns
In addition to diets that focus on specific macronutrient manipulations, there are a range of dietary patterns which have been explored in PCOS management. A systematic review (including 19 studies and 1,193 participants) published after guideline development (2020) found that the Dietary Approaches to Stop Hypertension (DASH) diet (rich in fruit, vegetables, wholegrains, nuts, legumes and low-fat dairy and with a predominantly low-GI carbohydrate profile) was the optimal choice for reducing IR [85]. RCTs in PCOS also report beneficial effects on weight [63, 64], BMI [62, 63], IR [62] and hormonal profile, including SHBG [64], androstenedione [64] and FAI [62] for a DASH compared to a control diet after 8-12 weeks. A vegetarian diet also reduced inflammatory markers (CRP, resistin and adiponectin) compared to a meat inclusive diet [80]. A vegan diet improved weight loss at three, but not six months [68], and a pulse-based diet led to similar reductions in weight, insulin sensitivity and reproductive hormones compared to a healthy control diet [72]. All of these dietary patterns are high in fibre and plant proteins, producing favourable effects on microbial diversity and encouraging production of short-chain fatty acids that possess potential anti-inflammatory actions [108, 109]. With mechanistic animal studies suggesting a possible pathophysiological role of gut microbiota in IR and ovarian dysfunction, it is possible that metabolic and hormonal benefits associated with plant-based dietary patterns in PCOS are related to increased intakes of dietary prebiotics [110]. However, further mechanistic studies exploring the role of gut microbiota in PCOS and RCTs investigating effects of dietary prebiotics on PCOS outcomes are required.
Lastly, particular eating patterns, such as eating smaller more frequent meals across the day [111] and eating a larger breakfast and smaller dinner [66], have also been found to be beneficial for insulin sensitivity [66, 111] and androgen reductions [66]. This is an important finding, as women with PCOS are more likely to either skip breakfast or consume their breakfast and lunch later in the day [112].
Studies examining specific food items in relation to PCOS outcomes, including raw onions [65], concentrated pomegranate juice [69, 113,115,115] and flaxseed powder [70, 116] have yielded largely inconsistent results. A core limitation of these single food studies is that foods are never consumed alone within the diet, omitting the influence of the dietary matrix and the interactions that occur amongst dietary constituents within meals. These studies provide limited applicability in the context of formulating practical dietary recommendations [117]. Please see Table 1 for a summary of available evidence from reviews and experimental studies investigating the effects of different types of diets on PCOS outcomes.
Physical activity
The 2018 PCOS guideline recommends ≥150 minutes per week of moderate or ≥75 minutes per week of vigorous intensity exercise for weight gain prevention, and ≥250 minutes per week of moderate or ≥150 minutes per week of vigorous intensity exercise for weight loss and weight regain prevention [18]. Minimising sedentary time and the inclusion of strength training exercise for two days per week is also recommended [18].
To date the most comprehensive review in PCOS (including 27 papers from 18 trials up until June 2017) reported that exercise improved FINS, HOMA-IR, TC, LDL-C, TAG, body composition (body fat percentage and WC) and aerobic fitness (VO2max) [119] compared with usual care or control groups. In regards to exercise type, subgroup analysis reported aerobic exercise improved BMI, WC, body fat percentage, FINS, HOMA-IR, TC, TAG and VO2max. In contrast, while resistance training produced unfavourable effects on HDL-C (decrease) and BMI (increase), it improved other measures of anthropometry, including WC. Combined interventions (using both aerobic and resistance training) had no effect on any of the measured markers. Subgroup analysis also found that more outcomes improved when interventions were supervised, of a shorter duration (≤ 12 weeks) and were conducted in women who were above a healthy weight [119].
Three more recent systematic reviews have looked at the effects of specific types of exercise on PCOS outcomes [120,122,122]. These reviews found that vigorous aerobic exercise can improve measures of insulin responsiveness and resistance, including HOMA-IR [121] and the insulin sensitivity index [120]; body composition, including WC [121] and BMI [122]; and cardiorespiratory fitness (VO2max) [121]. High intensity interval training (HIIT) alone may be effective for improving IR and BMI [123], however this has not been consistently shown [124]. Interventions involving a combination of aerobic and resistance exercise [122] or resistance training only [120] did not result in improvements in BMI [122] or weight status [120]. Exercise involving resistance training did result in other beneficial improvements to body composition (reduced body fat, WC and increased lean mass) and strength. This is important, as the degree of central adiposity predicts responsiveness to weight loss interventions in PCOS [53], and women who achieve greater reductions in central fat show greater symptom improvement with weight loss [45]. Resistance training may also improve androgen levels, though findings are inconsistent and more research is needed to draw definite conclusions [120]. There was insufficient evidence from available data to assess the effects of exercise type on reproductive function [122]. Please see Table 2 for a summary of available evidence from meta-analyses investigating the effects of different types of exercise on PCOS outcomes.
When comparing the effects of exercise and diet combined with diet alone, a systematic review and meta-analysis (three studies) found no differences for any measured outcomes (glucose, insulin HOMA-IR, weight, BMI, WC, body fat, fat free mass, T, SHBG and FAI) [119]. In regards to exercise and diet combined compared to exercise alone, subgroup analysis (including 17 studies) from a large systematic review found that the addition of diet to exercise, particularly vigorous intensity aerobic exercise, resulted in greater reduction to BMI, WC, FAI and HOMA-IR than exercise only [121]. In regards to exercise (aerobic) alone versus diet alone, one intervention study found that exercise induced weight loss produced greater improvements in menstrual frequency and ovulation rates [125], with no differences in pregnancy rates [125]. However, this study was not randomised and treatments were self-selected, which may have biased the results and precludes firm conclusions [125].
Behavioural
The 2018 PCOS guideline promotes the use of behavioural interventions that foster self-efficacy [18]. These include the use of SMART (specific, measurement, achievable, realistic and timely) goals, self-monitoring, stimulus control, problem solving and relapse prevention [18].
Behavioural and cognitive interventions are required to improve sustainability of lifestyle changes, through considering not only the specific behaviour, but also their antecedents, consequences and cognition [126, 127]. Given that women with PCOS show higher rates of weight gain over time [9] and high attrition rates in clinical weight management research [37], there is a clear need to improve adherence to diet and physical activity interventions. However, the majority of research investigating lifestyle change in PCOS involve short-term dietary interventions with/without an exercise element, and there is a paucity of research on behavioural change strategies. As such, guideline development relied heavily on evidence taken from the general population. Only three RCTs in women with PCOS included a ‘behavioural intervention’ [128,130,130]. While these studies showed enhanced weight loss [128, 130] and improved androgen and lipid profiles [129] when compared with placebo, the interventions were not well defined, with negligible context provided regarding the theoretic framework or behavioural strategies utilised.
More recently, a cross-sectional study in 501 women with PCOS [131] and two RCTs [44, 132] explored the use of self-management strategies [131] and behavioural modification interventions [44, 132] in PCOS. In the cross-sectional study, implementation of physical activity self-management strategies improved the likelihood of meeting physical activity recommendations, but had no association with BMI. Dietary self-management strategies were associated with reductions in BMI, though were not related to weight or nutritional intake [131]. In the RCTs, only the behavioural modification programme and not the control (general healthy lifestyle recommendations) produced significant weight loss after four months. A significantly greater proportion of women in the intervention group also improved menstrual regularity [44] and psychological well-being (lower anxiety and depressive symptoms) [132] when compared to the control group. The women who achieved greater weight loss reported higher social desirability and lower embitterment scores on a personality trait assessment measure [132]. These findings are particularly novel, as they provide insight into the influence of personality traits and their contribution to success in following behavioural modifications [132].
Alcohol and smoking
In the clinical setting, smoking and alcohol consumption are often addressed alongside dietary and physical activity changes, employing the same behavioural and cognitive interventions to promote adherence. Hence, alcohol and cigarette use are considered here under traditional lifestyle strategies. The PCOS international guideline highlights the importance of assessing alcohol consumption and cigarette smoking when improving fertility and reproductive outcomes in women with PCOS [18]. Assessment of cigarette use is also recommended when evaluating CVD risk factors and thromboembolism risk associated with oral contraceptive pills [18]. These recommendations are based on existing practice guidelines used for the general population.
There is a paucity of observational research characterising alcohol consumption in women with PCOS. One Swedish study comparing women with PCOS (n=72) to healthy controls (n=30), demonstrated a lower alcohol intake in the PCOS group [133]. A larger study in Australia comparing women with (n=409) and without (n=7,057) PCOS, reported no significant difference in alcohol intake [134]. Similarly, a Spanish study (n=22 PCOS and n=59 controls) and a Chinese study (n=2,217 PCOS and n=279 controls), found no significant difference in alcohol intake between PCOS and non-PCOS groups [135, 136].
Current evidence on the impact of alcohol intake on anovulatory infertility (a common feature of PCOS) is controversial, with some studies showing adverse effects and others reporting no significant correlation [136, 137]. One prospective study including 18,555 married women from The Nurses’ Health Study II, who had no history of infertility, found no clinically significant impact of alcohol intake on anovulatory infertility, after adjusting for parity and other factors [138]. Similarly, a Danish study (n=6,120 women aged 21 to 45 years) found no fertility effect with alcohol consumption of less than 14 standard drinks per week [137]. In contrast, a study on 3,833 women who recently gave birth and 1,050 women with infertility, reported an increased risk of anovulatory infertility and endometriosis with increasing alcohol intake [139].
Current observational evidence does not reveal any significant difference in smoking between women with and without PCOS [135, 136, 140], with the exception of one study in pregnant women which showed a lower smoking rate in women with PCOS (n=354) compared to women without PCOS at 15 weeks gestation [3]. However, a significantly higher rate of smoking (including passive and active) is reported in women with PCOS and oligo-anovulation and/or reduced fertility compared to women with PCOS and normal menstruations or healthy controls [141, 142]. Smoking is also associated with PCOS risk independent of BMI and age [142]. A Mendelian randomisation study supports these findings, demonstrating a 38% higher risk of PCOS development in genetically predicted smokers (based on single-nucleotide polymorphisms associated with smoking initiation) compared with those who never smoked [143]. In PCOS, smoking is associated with increased levels of T, DHEAS, TC, LDL-C and FINS [141, 144, 145]. However, the underlying mechanisms are not fully understood and there are inconsistencies in findings from different studies. Furthermore, smoking is associated with lower conception and live birth rates and less favourable ART outcomes in women with PCOS [141, 146].
Psychological
The current guideline highlights the need for awareness, and appropriate assessment (such as stepwise screening) and management, of QoL, depression and anxiety, psychosexual dysfunction, negative body image and disordered eating [18]. The guideline emphasises the importance of clinicians and women working in partnership to address women’s individual priorities; understanding that the impact of PCOS on an individual’s QoL is key to delivering meaningful outcomes [147, 148]. To assist women to communicate with clinicians about what is important to them, the PCOS Question Prompt List [149] was developed and is consistent with the 2018 guideline. The 2018 guideline recommends screening for risk factors and symptoms of depression and anxiety at time of diagnosis. Women with positive screening results should be supported with further assessment and treatment by appropriately qualified clinicians. To screen for psychosexual dysfunction tools such as the Female Sexual Function Index [150] should be utilised. If negative body image, disordered eating or eating disorders are suspected, the PCOS guideline outlines a stepped approach for screening, and where appropriate promotes the use of psychological therapy offered by trained health professionals, which should be guided by regional clinical practice guidelines [18].
While the PCOS guideline provides justification and summarises evidence for mental health screening and diagnostic assessment, there is also a need for consideration of additional aspects, such as the efficacy of different types of psychological interventions and how psychological interventions influence engagement with lifestyle change. This is important, as poorer mental health outcomes at baseline are positively associated with higher rates of attrition in lifestyle interventions [13]. Cognitive behavioural interventions could be considered to improve engagement and adherence to healthy lifestyle in women with PCOS. Research has shown support for a range of different psychological interventions, such as counselling [151], cognitive behavioural therapy (CBT) [152,154,154] and mindfulness meditation [155, 156], helping to change the way clinicians’ approach and deliver optimal PCOS management.
CBT is one of the most widely-researched psychological interventions, and is well-recognised as the most effective psychological treatment for depression and anxiety [157]. One RCT showed that eight weekly group CBT sessions were effective in improving QoL ratings and reducing psychological fatigue in women with PCOS [152]. Another more recent RCT investigated the outcome of a 1 year three-component intervention focusing on CBT, diet and exercise [154] and reported improvements in self-esteem and depressive symptoms as compared to usual care [154]. Similarly, an RCT by Cooney et al. [153], comparing the effects of CBT and lifestyle modification versus lifestyle modification alone, reported the CBT/lifestyle modification group lost more than twice as much weight per week and had greater improvements in QoL compared to lifestyle only. Depression scores decreased in the overall group and there was no difference between the two groups [153]. Lastly, a pilot intervention study of adolescents with PCOS has shown promising results for the use of CBT in the reduction of weight and improvement in depressive symptoms [158].
Mindfulness meditation programs have gained increasing popularity over the past few decades, and are being included as part of clinical trials to reduce stress and improve psychological wellbeing across a range of medical conditions [159]. Mindfulness meditation can be used to reduce the production of adrenal androgens, activated via the adrenal glands as a direct result of psychological distress [156]. Despite the proposed benefits, there are very few studies investigating the use of mindfulness meditation as a treatment for psychological symptoms associated with PCOS. One RCT (n=86) compared the provision of an eight week mindfulness-based stress reduction (MBSR) program, and found that when compared to the control group (health education), the MBSR group produced greater reductions in perceived stress, depressive symptoms and fasting blood glucose [160]. Similarly, another RCT investigating the impact of mindfulness meditation for eight weeks in PCOS showed reduced stress, depression and anxiety symptoms, and increased life satisfaction and QoL in the intervention group compared to no treatment [156]. In adolescents with PCOS (n=37), a pilot RCT reported higher levels of nutrition and physical activity self-efficacy following a mindfulness and self-management program [161]. Mindfulness-based cognitive therapy (MBCT) combines both elements of MBSR and CBT, but as yet there are no trials investigating this intervention in PCOS.
In addition to CBT and mindfulness meditation, there is some evidence to support group counselling sessions as beneficial in conjunction with exercise programs to increase and support weight loss [151]. In one RCT (n=17) participants followed a high-intensity aerobic exercise program for eight weeks, followed by eight weeks of group counselling [151]. Qualitative analysis of data taken from the group counselling and physical exercise sessions revealed that development of supportive relationships was important for successful behavioural change. By fostering the exchange of narratives relating to their illness (e.g. effects of PCOS on aspects of everyday life), and generating feedback between group members, counselling sessions helped to reduce social isolation and improve adherence to the exercise intervention [151]. Please see Table 3 for a summary of experimental studies investigating effects of psychological interventions on PCOS outcomes.
Sleep
Women with PCOS have an increased risk of both clinical sleep disorders and non-clinical sleep disturbance, which is mediated by hormone derangement, in particular reduced oestrogen, progesterone and melatonin levels [164]. Oestrogen is required for the metabolism of neurotransmitters (norepinephrine and serotonin) involved in regulating sleep patterns, and plays an important role in maintaining a low body temperature at night [165]. Progesterone has sedative and anxiolytic actions that can support sleep quality, and acts as a respiratory stimulant that lessens airway resistance in obstructive sleep apnoea (OSA) [166]. Melatonin is a neuroendocrine hormone that is widely recognised as crucial in maintaining circadian rhythm regulation. However, melatonin is also involved in ovarian function, with actions including delaying ovarian senescence, promoting follicle formation and improving oocyte quality [167,169,170,171,172,173,173].
The current PCOS guideline recognises that OSA is 6.5-8.3 times more likely in women with PCOS [164, 174,176,177,177], and promotes routine screening to identify and treat associated symptoms, such as snoring, excessive sleepiness and the potential for fatigue to worsen mood disorders [18]. Screening should include a simple questionnaire, such as the Berlin tool [178], and where appropriate women should be referred onto a specialist for further assessment and treatment [18]. The guidelines also highlight that treatment of OSA in PCOS should not be used to improve metabolic features. Since guideline inception evidence has emerged reporting weight, PCOS and sleep are interrelated factors that can each contribute to the worsening presentation of one another, whereby sleep disorders and disturbance may worsen the presentation of PCOS related metabolic outcomes and vice versa [179].
Hypersomnia and insomnia are also common clinical sleep disorders in PCOS [164, 177, 180], with prevalence estimated at 11% versus 1% in women with versus those without PCOS [180]. Even in the absence of clinically diagnosed sleep disorders, women with PCOS have a higher prevalence of sleep disturbances, including poor sleep quality [181], issues with sleep initiation [182], severe fatigue [140], restless sleep [140] and difficulty sleeping overnight [140]. The prevalence of sleep disturbances may be up to 20% higher in women with PCOS compared to women without PCOS [183]. Emerging research also suggests that social restrictions arising from the COVID-19 pandemic have worsened sleep disturbances in women with PCOS [177]. Findings from key studies of non-clinical sleep disturbance can be found in Table 4.
In the general population short and disturbed sleep is consistently associated with excess weight [184], IR [185], T2DM [185] and CVD [186]. Similar relationships are observed in PCOS, where OSA and sleep disordered breathing exacerbates risk of IR and metabolic consequences of abnormal glucose tolerance [187, 188]. A cross-sectional study in adolescents with PCOS (n=103) reported those with sleep disordered breathing had significantly higher BMI Z-scores, and a higher prevalence of metabolic syndrome (METS) [188]. Similar metabolic consequences are seen in women with PCOS who suffer from non-clinical sleep disturbance [164]. Underlying mechanisms linking sleep disorders and disturbance with worsened metabolic outcomes include amplified sympathetic tone and oxidative stress [164], reduced adipose tissue lipolysis, and an increase in energy intake stemming from heightened hedonic and endocrine appetite signals [189].
Unfavourable effects on energy metabolism and appetite regulation, may explain why women with PCOS who display sleep disturbance have a reduced capacity to maintain dietary interventions [183]. Moreover, depression and anxiety share a bidirectional relationship with disrupted and reduced sleep [190], and as stated previously, interventions that improve mental health can help to increase engagement with dietary and physical activity recommendations [131]. Optimising sleep may therefore be an important consideration when promoting healthy lifestyle change in women with PCOS [183].
Traditional, complementary and integrative medicine
The 2018 PCOS guideline includes recommendations on inositol supplementation, though do not include evidence regarding the use of other supplements, herbal medicine or other TCIM approaches, including acupuncture and yoga [18].
Vitamins, vitamin-like supplements, minerals and other supplements
The 2018 guideline highlights that inositol (including myo-inositol (MI) and di-chiro inositol) is a nutritional supplement that may be involved in insulin signalling transduction [191]. MI in particular is a key endocrine regulator that displays impaired metabolism in PCOS [191]. MI supplementation has been explored in a meta-analysis of nine RCTs (n=496), which showed improved metabolic profiles and reduced hyperandrogenism [191]. These findings are supported by two earlier meta-analyses, reporting improved ovulation, menstrual cyclicity, and hormonal profiles following MI supplementation [192, 193]. The 2018 PCOS guideline recommends that inositol (in any form) should be considered as an experimental therapy in PCOS management. The guideline also recognises that women participating in any form of TCIM should be encouraged to advise their health professional. However, it does not consider emerging evidence for the use of other types of TCIM in PCOS treatment as this was outside of the scope of the 2018 guideline.
Vitamins
B-group vitamins (B1, B6 and B12), folic acid (B9) and vitamins D, E, and K are critical for several biological processes that can affect metabolic and reproductive features of PCOS. B-group vitamins work alongside folic acid (the synthetic form of folate) to regulate homocysteine (Hcy) via re-methylation of Hcy to methionine [194]. Hcy is an amino acid that confers an increased risk of CVD at high levels, and which is often deranged in women with PCOS [195], likely related to a higher prevalence of folate deficiency [196,197,198]. One RCT explored the use of B-group vitamins combined with folic acid in 60 women with PCOS, and reported a reduction in the Hcy increasing effect of metformin [198]. Folic acid alone has also been examined in two RCTs of women with PCOS (n=69 [199] and n=81 [200]), improving FINS, HOMA-IR, C-reactive protein, total antioxidant capacity (TAC) and glutathione with doses ≥ 5 mg/day when compared with placebo [199, 200]. Regarding vitamin D supplementation, three large-scale meta-analyses reported improvements in measures of IR (HOMA-IR [201, 202], FINS [201]), fasting glucose [201]), lipid profiles (LDL-C [201,202,203], TC [203] and TAG [203]) and androgens (T) [202], when compared with placebo. While vitamin E (or tocopherol) has various reported benefits on fertility outcomes in other populations [204], and has improved androgen profiles when co-supplemented with coenzyme Q10 (CoQ10) in women with PCOS [205], to date no RCTs have examined the use of vitamin E supplements alone in PCOS. Vitamin K also has limited available literature in PCOS, with only one RCT (n=84) demonstrating improvements in anthropometry, insulin and androgen profiles following supplementation (90 μg/day Menaquinone-7 for eight weeks), compared with placebo [206].
Vitamin-like supplements
Vitamin-like supplements including bioflavonoids, carnitine and alpha-lipoic acid (α-LA) have well-recognised antioxidant properties and play a role in fatty acid and glucose metabolism, providing possible metabolic benefits in PCOS [207]. Bioflavonoids consist of plant-derived polyphenolic compounds, some of which have been inversely associated with METS in women with PCOS [207]. In a pilot prospective study of 12 women with PCOS, 36 mg/day of the soy isoflavone genistein for six months improved lipid profiles but not anthropometry, IR, hormonal profiles or menstrual cyclicity [208]. Carnitine, particularly the active form L-carnitine, is reported to be lower in women with PCOS and linked with hyperandrogenism, hyperinsulinaemia and reduced oocyte quality [209, 210]. One RCT explored L-carnitine use in PCOS and found beneficial effects on mental health parameters and markers of oxidative stress [211], although the integrity of these have come under scrutiny and hence should be interpreted with caution [122212]. Regarding α-LA, a small pre-post study (n=6) administered 1200 mg/day for 16 weeks, and reported improved IR, LDL-C and TAG, though no effects on TAC or plasma oxidation metabolites [213]. Another RCT reported improved anthropometric (BMI), metabolic (FINS and HDL-C) and reproductive (menstrual cyclicity) features in 46 women with PCOS receiving α-LA supplementation (600 mg/day for 180 days) compared with controls [214]. However, as these women were co-supplemented with 1000 mg/day D-chiro-inostiol, findings are not isolated to the effects of α-LA alone [214].
Minerals
Minerals such as calcium, zinc, selenium, magnesium and chromium picolinate (CrP) have been explored in PCOS due to their reported insulin sensitising, antioxidant and anti-inflammatory properties [215,216,217]. A small number of studies have also reported women with PCOS are at higher risk of being deficient in calcium [218], zinc [215, 217] and selenium [195]. A recent systematic review (six RCTs) reported that vitamin D and calcium co-supplementation in women with PCOS improved lipid and androgen profiles, follicular health and menstrual cyclicity [219]. While these findings are promising, it is difficult to attribute benefits to calcium alone, given calcium is often co-supplemented with vitamin D due to their complementary mechanisms of action. One systematic review (five RCTs) in PCOS reported zinc (often co-supplemented with other nutrients such as calcium, vitamin D and magnesium), improved HOMA-IR, lipids, T, FSH and DHEAS [220] compared to placebo. Another systematic review (five RCTs) examining selenium supplementation reported reduced IR, oxidative stress and inflammation, while results for anthropometry, lipids, androgens and hirsutism were inconsistent [221]. Regarding magnesium (an intracellular cation involved in insulin metabolism), while supplementation in PCOS has been associated with reduced IR in observational research [222], these findings are not supported by data from RCTs, with considerable inconsistencies between studies [222]. Two meta-analyses examined CrP in women with PCOS [223, 224]. While one reported that CrP supplementation reduced BMI, FINS and free testosterone [223], the other reported decreased IR, but not BMI, and increased levels of T [224].
Other supplements
Other supplements purported to provide a range of antioxidant and anti-inflammatory benefits, including omega-3 fatty acids, N-acetyl-cysteine (NAC), CoQ10, probiotics, quercetin, resveratrol and melatonin have been explored in PCOS. A meta-analysis (nine RCTs) of women with PCOS (n=591) receiving omega-3 supplementation reported reductions in HOMA-IR, TC, TAG and LDL-C, though showed no effect on other metabolic parameters or T [225]. In a meta-analysis of eight RCTs (n=910) examining NAC supplementation (the acylated form of L-cysteine), researchers reported improved glucose regulation and a greater likelihood of conception and livebirths in women with PCOS compared with placebo [226]. In a single RCT (n=60) CoQ10 supplementation (100 mg/day for 12 weeks) improved fasting glucose and insulin, HOMA-IR, insulin sensitivity index and TC, compared with the placebo group [227]. Two meta-analyses reported probiotics improved FAI, SHBG, IR and blood lipids, with no differences in weight or hirsutism between intervention and placebo groups [228, 229]. These findings may be linked to lower microbial diversity and increased intestinal permeability in women with PCOS [230, 231]. In regards to quercetin and resveratrol, which are both food derived polyphenols with a strong antioxidant capacity, one systematic review (three experimental studies, n=246 women with PCOS) reported quercetin supplementation improved measures of IR and testosterone levels, but not anthropometry compared with placebo [232]. Similarly, one RCT in women with PCOS (n=61) reported resveratrol (800-1500 mg/day for four days) improved androgen and metabolic profiles and oocyte and embryo quality compared with placebo [233]. Finally, a systematic review (two RCTs and one cell-culture study) investigating the effects of melatonin supplementation in women with PCOS using assisted reproductive technologies reported melatonin significantly increased clinical pregnancy rates but not live birth rates [172]. A more recent RCT (n=56) reported improved levels of T, hirsutism, inflammatory and oxidative stress profiles in women receiving 10 g melatonin/day for 12 weeks, compared with placebo [234].
Herbal medicine
To date the most recent and comprehensive review (Cochrane review including five RCTs and n=414 women with PCOS) investigating the effects of herbal medicine on reproductive outcomes, reported no difference between the use of Chinese herbal medicine (CHM) and clomiphene for pregnancy rates, and limited evidence of increased pregnancy rate for CHM with clomiphene compared with clomiphene alone [235]. This review concluded that there was inadequate evidence to promote the use of CHM for the treatment of subfertility in women with PCOS [235]. Similarly, a smaller systematic review (five studies) investigating the effects of four herbal medicines (green tea, cinnamon, spearmint and black cohosh) on menstrual regularity in PCOS, found limited high-quality evidence from RCTs to support their clinical use and concluded that evidence for safety was lacking [236].
More recently, a number of small RCTs investigating metabolic and reproductive effects of a range of herbal medicines have been published. Curcumin, an active compound in turmeric (Curcuma longa), may exert hypoglycemic effects via a number of mechanisms, including attenuation of circulating levels of tumor necrosis factor-α [237]. One RCT (n=67) reported decreased levels of fasting glucose following supplementation compared with placebo [238], while another (n=51) which used a lower dose (1000 mg/day versus 1500 mg/day) and shorter duration (six weeks versus 12 weeks), reported no between group differences for fasting glucose, HOMA-IR or lipids [239]. Salvia officinalis or sage contains multiple active compounds that display antioxidant effects and therefore effects on glucose metabolism and insulin sensitivity [240]. One RCT (n=72) reported consuming sage extract for eight weeks improved IR and reduced BMI, with no effects on WHR or blood pressure [241] Foeniculum vulgare or fennel may provide protective effects on hormonal abnormalities in PCOS via its actions as a phytoestrogen [242]. One RCT (n=55) reported that six months of fennel tea and dry cupping was as effective as metformin for reducing BMI and menstrual cycle length [243]. Glycyrrhiza glabra or licorice contains active phytochemicals including isoflavane and glabridin, which have been shown to have antiandrogenic effects [244]. Two experimental studies in healthy women (n=9) [245] and women with PCOS (n=32) [246] reported that 3.5 g/day of licorice extract decreased T [245] and reduced side effects of spironolactone [246]. Mentha spicata (spearmint), Zingiber offinale Roscoe (ginger), Cinnamomum cassia (cinnamon) and Citrus sinensis (citrus) have been shown to exert anti-inflammatory and hypoglycemic effects [247,248,249,250]. One RCT in infertile women with PCOS (n=60) comparing the effects of a herbal mixture (citrus, ginger, cinnamon and spearmint) with clomiphene citrate (CC), herbal mixture alone, or CC alone reported that the herbal mixture, with or without CC, improved circulating antioxidant levels, IR and fasting blood glucose, but not menstrual regularity when compared to CC alone [251]. While observations from emerging research are promising, to support the safe translation of findings into the clinical setting there is a clear need for larger clinical trials investigating the efficacy and safety of herbal medicine use in PCOS.
Other traditional, complimentary and integrative medicine approaches
Acupuncture may provide beneficial impacts on sympathetic function [252] and ovarian blood flow [253] in women with PCOS. A recent meta-analysis of 22 RCTs (n=2315 women with PCOS) reported recovery of the menstrual period in the acupuncture group when compared with placebo, but no evidence for differences between groups in terms of live birth, pregnancy and ovulation [254]. While an earlier meta-analysis reported a significant reduction in BMI following acupuncture use, this was mainly due to one RCT (n=80) which compared acupuncture and the oral contraceptive pill to the oral contraceptive pill alone [255]. When this study was removed, the pooled analysis was no longer significant [255].
Yoga gymnastics have been recommended as an example of moderate physical activity in the 2018 evidence-based PCOS guideline [18]. However, as yoga is considered a mind-body therapy that incorporates aspects of meditation, it may provide additional benefits beyond those gained through other forms of exercise [256]. While one systematic review (16 observational and experimental studies, n=365 women with PCOS) reported yoga may provide a range of psychological, reproductive and metabolic benefits, no meta-analysis was performed and a limited summary of included studies made it difficult to confirm findings [257]. A more recent systematic review (11 experimental studies) included a meta-analysis of two RCTs and found that yoga significantly decreased clinical hyperandrogenism, menstrual irregularity and fasting glucose and insulin [258]. Lastly, findings from a recent RCT (n=67 women with PCOS) suggests that 90 minutes of yoga per day for six weeks can significantly reduce hirsutism, waist and hip circumference when compared to controls [259]. Please see Table 5 for a summary of available evidence from meta-analyses and experimental studies investigating the effects of TCIM on PCOS outcomes.
Summary of findings and research gaps
The 2018 International Evidence-Based Guideline for the Assessment and Management of PCOS highlights lifestyle (diet, physical activity and/or behavioural) management as the primary initial treatment strategy [18]. It is important to consider that the definition of lifestyle management may warrant expansion consistent with the whole person model of healthcare provision, which may include care addressing psychological and sleep interventions, as well as a range of TCIM approaches [20]. In line with patient interest [31,32,33,34,35], and to assist women and healthcare providers in understanding the evidence to aid safe implementation of adjunct therapies, rigorous assessment of the evidence for these alternative lifestyle strategies in PCOS management in warranted. Using a holistic definition of patient care, this review has summarised evidence to date on the traditional components of lifestyle change (diet, physical activity and behavioural change), psychological interventions and non-pharmacological strategies (sleep, supplements, herbal medicine and other TCIM approaches). Table 6 provides a overview of current guideline recommendations alongside the key findings from this review, summarising the identified research gaps that need to be addressed before evidence-based recommendations for clinical practice can be updated.
With regards to traditional lifestyle treatment, the majority of studies focussed on weight loss as a primary treatment goal. This indicates more research is warranted to understand the role of diet and exercise in lean women and/or in weight gain prevention. RCTs using lifestyle interventions under isocaloric conditions that investigate effects on IR, body composition and androgens independent of weight loss are needed. Given the high risk of failure with long-term weight management [9, 37, 40, 261] and high attrition in weight loss trials in PCOS [13], exploring interventions that focus on weight neural messaging around dietary quality and physical activity may also aid in optimising engagement, adherence and sustainability of lifestyle interventions. Future research should also identify subgroups who respond more favourably to weight loss [45, 53], to aid provision of a more targeted and personalised treatment approach.
With regards to diet strategies, there is a need for more research understanding the impact of low GI/GL diets on androgen status, as well as the biological mechanisms by which low GI/GL diets may impact reproductive and cardiometabolic outcomes associated with PCOS. With regards to physical activity, additional longer-term studies are required to guide exercise prescription in PCOS, although promising evidence supports the provision of vigorous aerobic exercise performed under supervised conditions (i.e. through referral to an exercise physiologist). While behavioural interventions are essential for long term sustainability of dietary and physical activity change, research in PCOS is scarce and interventions are not well defined. Future research should incorporate appropriate theoretical frameworks and clearly outline behavioural components utilised. This will aid intervention duplication and tailoring of active elements to ensure relevance in women with PCOS.
There is currently a lack of research investigating whether women with PCOS are at a higher risk of alcohol and smoking-related complications. This is particularly relevant given the well-established relationship between higher alcohol and cigarette use and rates of depression and anxiety in the general population [262,263,264,265]. There is also a need to better understand the relationship between alcohol intake and reproductive outcomes (particularly anovulatory infertility) [139], as safe alcohol limits in PCOS is currently unknown [139].
With regards to psychological interventions, the current evidence base for prevalence of mental health concerns in PCOS relies heavily on symptom prevalence. More adequately powered, gold standard prevalence studies using structured diagnostic interviews administered by appropriately qualified professionals are needed. While QoL has recently been highlighted as a core outcome in PCOS research [266], the application of QoL tools in clinical care is still unclear, with research yet to validate QoL tools longitudinally or identify clinically meaningful differences in QoL scores. The emerging evidence showing support for the use of CBT in PCOS [152,154,154] highlights an opportunity for tailoring of this psychological intervention to meet the specific mental health needs of women with PCOS, with a focus on how management of mental health symptoms affect lifestyle modifications. CBT that incorporates elements of mindfulness-based stress reduction also warrants further investigation.
Future research in PCOS and sleep disorders should include more high-quality research in subclinical disorders using objective sleep measures (polysomnography and actigraphy). Future work should also consider emerging evidence showing that disturbed sleep can detrimentally effect energy expenditure, which may increase adipose tissue deposition and exacerbate IR [164, 184, 186, 267,268,269,270,271], thereby worsening the presentation of PCOS. Further, a consideration of how sleep disturbance can reduce engagement with positive lifestyle changes, for example through the disruption of appetite regulation [272, 273] or via contributing to poor mental health outcomes [190, 274], is warranted. CBT interventions including elements of stimulus control and psychoeducation are effective non-pharmacological treatments for both clinical sleep disorders and sleep disturbances in the general population [275,276,277]. RCTs in women with PCOS that investigate effects of CBT on dietary intake, energy metabolism, appetite regulation, anthropometry, adherence to lifestyle changes and PCOS features are required.
With regards to TCIM, there is a vast array of literature suggesting some beneficial effects of vitamins (B-group vitamins, folate, vitamins D, E and K), vitamin-like nutrients (bioflavonoids, carnitine and α-LA), minerals (calcium, zinc, selenium, and CrP) and other formulations (such as melatonin, omega-3 fatty acids, probiotics, NAC and cinnamon) in PCOS [278]. However, the quality of evidence across studies ranges from meta-analyses of RCTs (vitamin D, omega-3 fatty acids and NAC) to single retrospective observational studies (vitamin K and carnitine). In addition, heterogeneity in results related to factors including variable PCOS presentation and study methodology make it difficult to draw definite conclusions. Future research should focus on specific populations within PCOS, for example age, BMI or phenotype (factors which substantially affect nutrient sufficiency), and outline more consistent approaches to supplement formulation, dosage, intervention duration and type of comparator used. Mechanistic studies are also needed to investigate herb- or nutrient-drug interactions (with common pharmacological treatments used in PCOS) and other possible interactions with the biological processes underpinning PCOS. In regards to acupuncture and yoga, more sufficiently powered RCTs are needed to determine clinical relevance and integration into PCOS management is not yet warranted.
While current research is not sufficiently robust to support integration of TCIM into routine clinical practice, healthcare providers should broaden their knowledge pertaining to how these therapies can be safely and appropriately utilised as adjuncts to conventional medical management [279,280,281]. TCIM is frequently used by women, with uptake of TCIM approaches increasing steadily over the past 10 years [31,32,33,34,35]. In women with PCOS, one cross-sectional study (n=493) found that 70% reported use of TCIM, namely nutritional and herbal supplements [282]. The most common reasons for use were to treat PCOS symptoms, improve general wellbeing and reduce depression. Of the women using TCIM, 77% had consulted with a complementary practitioner (acupuncturists, chiropractors, naturopaths and massage therapists) [282]. While the study did not report participants engagement with medical physicians, research in the general population has shown that patients are resistant to discuss TCIM use with their consulting physician [283,284,285,286,287,288]. Qualified healthcare providers should be involved in TCIM discussions to help ensure appropriate use, maximise possible benefits and minimize potential harm [289]. For example, to sustain patient engagement in women who express the desire to experiment with supplementation, healthcare providers could consider inositol supplementation, using a nuanced and case-specific approach that encapsulates the variety of pathologies in PCOS.
When considering all of the research summarised here, across traditional lifestyle, psychological, sleep and TCIM interventions, there is a clear need for more real-world PCOS research. This involves the translation of findings from clinical trials (where highly selected populations, intensive treatment protocols and expert multidisciplinary teams provide an ideal research setting), into the heterogenous situations that face clinicians [290,291,292]. Health professionals provide care to women from diverse social contexts, are often restrained by finite resources and are required to juggle many competing demands for their time [290,291,292]. While some barriers to implementation, including time, resource and access issues are considered in the current PCOS guideline, they were generated by the guideline development groups and research is needed to validate and clarify their proposed concerns. Real-world research is required to: a) fully understand whether lifestyle recommendations can be practically integrated into current healthcare settings; b) tailor interventions to meet the unique needs of women with PCOS; and c) generate evidence on clinical outcomes that are of great relevance to patients and clinicians, such as live birth, miscarriage and menstrual regularity, which can be collected through routine care.
It is also important to highlight that while lifestyle management is a first-line treatment for PCOS, the addition of pharmacological therapies to further improve clinical features of hyperandrogenism, menstrual irregularity and infertility are often indicated [293]. In these instances, prescribing physicians should consider how medical management and lifestyle change can be used in adjunct to optimise treatment. For example, the use of combined oral contraceptive pills may have detrimental effects on weight gain [294] and mental health [295], which can be mitigated by appropriate lifestyle intervention. Further, the combination of lifestyle modification and metformin has been shown to lower BMI, subcutaneous adipose tissue and improve menstruation compared with lifestyle modification alone, and hence may have an additive effect on improving cardio-metabolic outcomes in high risk groups [296].
Conclusion
Using the whole person or holistic definition of health, this review has highlighted emerging areas of research that could be considered for integration into future classifications of lifestyle management in PCOS. When developing lifestyle recommendations for PCOS management, interpreting and communicating evidence not only for diet, physical activity and behavioural interventions, but also psychological, sleep and TCIM approaches, will aid clinicians to deliver patient-centred care by affording women more choice and therefore autonomy over their treatment options. This sentiment aligns with the core objectives underpinning the 2018 PCOS guideline, which sought to understand the unmet needs of women with PCOS through continuing to engage consumers in co-design of guideline development, implementation, translation and dissemination.
Availability of data and materials
Not applicable.
Abbreviations
- α-LA:
-
Alpha-lipoic acid
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- CHM:
-
Chinese herbal medicine
- CrP:
-
Chromium picolinate
- CoQ10:
-
Coenzyme Q10
- CBT:
-
Cognitive behavioural therapy
- DASH:
-
Dietary Approaches to Stop Hypertension
- FINS:
-
Fasting insulin level
- FSH:
-
Follicle stimulating hormone
- FAI:
-
Free androgen index
- GI:
-
Glycaemic index
- GL:
-
Glycaemic load
- HDL-C:
-
High density lipoprotein cholesterol
- Hcy:
-
Homocysteine
- IR:
-
Insulin resistance
- LDL-C:
-
Low density lipoprotein cholesterol
- LH:
-
Luteinizing hormone
- VO2max :
-
Maximal rate of oxygen
- METS:
-
Metabolic syndrome
- MUFA:
-
Monounsaturated fatty acid
- MI:
-
Myo-inositol
- NAC:
-
N-acetyl-cysteine
- OGTT:
-
Oral glucose tolerance test
- PCOS:
-
Polycystic ovary syndrome
- PUFA:
-
Polyunsaturated fatty acid
- RCT:
-
Randomised controlled trial
- SHBG:
-
Sex hormone-binding globulin
- TAC:
-
Total antioxidant capacity
- TC:
-
Total cholesterol
- T:
-
Testosterone
- TCIM:
-
Traditional, complementary and integrative medicine
- TAG:
-
Triglycerides
- T2DM:
-
Type 2 diabetes
- WC:
-
Waist circumference
- WHR:
-
Waist-hip-ratio
References
Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841–55.
ESHRE TR, Group A-SPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
Bahri Khomami M, Moran LJ, Kenny L, Grieger JA, Myers J, Poston L, et al. Lifestyle and pregnancy complications in polycystic ovary syndrome: The SCOPE cohort study. Clin Endocrinol. 2019;90(6):814–21.
Kakoly N, Khomami M, Joham A, Cooray S, Misso M, Norman R, et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update. 2018;24(4):455–67.
Wekker V, Van Dammen L, Koning A, Heida K, Painter R, Limpens J, et al. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum Reprod Update. 2020;26(6):942–60.
Barry JA. Exploration of biological causes of psychological problems in polycystic ovary syndrome (PCOS). 2011. City Univerity London (thesis) doi: https://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.544446
Pirotta S, Barillaro M, Brennan L, Grassi A, Jeanes YM, Joham AE, et al. Disordered eating behaviours and eating disorders in women in Australia with and without polycystic ovary syndrome: a cross-sectional study. J Clin Med. 2019;8(10):1682.
Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic–hyperinsulaemic clamp. Hum Reprod. 2013;28(3):777–84.
Teede HJ, Joham AE, Paul E, Moran LJ, Loxton D, Jolley D, et al. Longitudinal weight gain in women identified with polycystic ovary syndrome: results of an observational study in young women. Obesity. 2013;21(8):1526–32.
Lim SS, Davies M, Norman RJ, Moran L. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(6):618–37.
Lim S, Norman RJ, Davies M, Moran L. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14(2):95–109.
Moran LJ, Noakes M, Clifton PM, Tomlinson L, Norman RJ. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(2):812–9.
Moran LJ, Noakes M, Clifton P, Buckley J, Brinkworth G, Thomson R, et al. Predictors of Lifestyle Intervention Attrition or Weight Loss Success in Women with Polycystic Ovary Syndrome Who Are Overweight or Obese. Nutrients. 2019;11(3):492.
Farid NR, Diamanti-Kandarakis E. Diagnosis and Management of Polycystic Ovary Syndrome: New York. United States: Springer; 2009.
Moran C, Garcia-Hernandez E, Barahona E, Gonzalez S, Bermudez JA. Relationship between insulin resistance and gonadotropin dissociation in obese and nonobese women with polycystic ovary syndrome. Fertil Steril. 2003;80(6):1466–72.
Moran LJ, Ko H, Misso M, Marsh K, Noakes M, Talbot M, et al. Dietary composition in the treatment of polycystic ovary syndrome: a systematic review to inform evidence-based guidelines. J Acad Nutr Diet. 2013;113(4):520–45.
Tay CT, Moran LJ, Harrison CL, Brown WJ, Joham AE. Physical activity and sedentary behaviour in women with and without polycystic ovary syndrome: An Australian population-based cross-sectional study. Clin Endocrinol. 2020;93(2):154–62.
Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602–18.
Checkland K, Harrison S, McDonald R, Grant S, Campbell S, Guthrie B. Biomedicine, holism and general medical practice: responses to the 2004 General Practitioner contract. Sociol Health Illness. 2008;30(5):788–803.
Thomas H, Mitchell G, Rich J, Best M. Definition of whole person care in general practice in the English language literature: a systematic review. BMJ Open. 2018;8(12):e023758.
Collins A, de Iongh A. Independent Commission on Whole Person Care for the UK Labour Party: British Medical Journal Publishing Group; 2014.
Primary Health Care Advisory Group, Department of Health. Better Outcomes for People with Chronic and Complex Health Conditions. Canberra: Commonwealth of Australia; 2015.
Ferrante JM, Balasubramanian BA, Hudson SV, Crabtree BF. Principles of the patient-centered medical home and preventive services delivery. Ann Fam Med. 2010;8(2):108–16.
Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod. 2017;32(5):1075–91.
Cesta CE, Månsson M, Palm C, Lichtenstein P, Iliadou AN, Landén M. Polycystic ovary syndrome and psychiatric disorders: Co-morbidity and heritability in a nationwide Swedish cohort. Psychoneuroendocrinology. 2016;73:196–203.
Pastoor H, Timman R, de Klerk C, W MB, Laan ET, Laven JS. Sexual function in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod BioMed Online. 2018;37(6):750–60.
Dawber RP. Guidance for the management of hirsutism. Curr Med Res Opin. 2005;21(8):1227–34.
Trent M, Austin SB, Rich M, Gordon CM. Overweight status of adolescent girls with polycystic ovary syndrome: body mass index as mediator of quality of life. Ambul Pediatr. 2005;5(2):107–11.
Pastore LM, Patrie JT, Morris WL, Dalal P, Bray MJ. Depression symptoms and body dissatisfaction association among polycystic ovary syndrome women. J Psychosom Res. 2011;71(4):270–6.
Himelein MJ, Thatcher SS. Depression and body image among women with polycystic ovary syndrome. J Health Psychol. 2006;11(4):613–25.
Smith CA, Bateson DJ, Weisberg E. A survey describing the use of complementary therapies and medicines by women attending a family planning clinic. BMC Complement Altern Med. 2013;13(1):1–8.
Sibbritt DW, Adams J, Young AF. A longitudinal analysis of mid-age women's use of complementary and alternative medicine (CAM) in Australia, 1996–1998. Women Health. 2005;40(4):41–56.
Gollschewski S, Anderson D, Skerman H, Lyons-Wall P. Associations between the use of complementary and alternative medications and demographic, health and lifestyle factors in mid-life Australian women. Climacteric. 2005;8(3):271–8.
Barnes LAJ, Barclay L, McCaffery K, Aslani P. Complementary medicine products used in pregnancy and lactation and an examination of the information sources accessed pertaining to maternal health literacy: a systematic review of qualitative studies. BMC Complement Altern Med. 2018;18(1):229.
Adams J, Lui CW, Sibbritt D, Broom A, Wardle J, Homer C, et al. Women's use of complementary and alternative medicine during pregnancy: a critical review of the literature. Birth. 2009;36(3):237–45.
van Velden D. The bio-psycho-social approach to health and disease. SADJ. 2003;58(5):191–6.
Lim SS, Hutchison SK, Van Ryswyk E, Norman RJ, Teede HJ, Moran LJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2019;3:CD007506.
Haqq L, McFarlane J, Dieberg G, Smart N. The Effect of Lifestyle Intervention on Body Composition, Glycemic Control, and Cardiorespiratory Fitness in Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis. Int J Sport Nutr Exerc Metab. 2015;25(6):533–40.
Abdolahian S, Tehrani FR, Amiri M, Ghodsi D, Yarandi RB, Jafari M, et al. Effect of lifestyle modifications on anthropometric, clinical, and biochemical parameters in adolescent girls with polycystic ovary syndrome: a systematic review and meta-analysis. BMC Endocr Disord. 2020;20(1):71.
Domecq JP, Prutsky G, Mullan RJ, Hazem A, Sundaresh V, Elamin MB, et al. Lifestyle modification programs in polycystic ovary syndrome: systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98(12):4655–63.
Haqq L, McFarlane J, Dieberg G, Smart N. Effect of lifestyle intervention on the reproductive endocrine profile in women with polycystic ovarian syndrome: a systematic review and meta-analysis. Endocrine Connect. 2014;3(1):36–46.
Ujvari D, Hulchiy M, Calaby A, Nybacka Å, Byström B, Hirschberg AL. Lifestyle intervention up-regulates gene and protein levels of molecules involved in insulin signaling in the endometrium of overweight/obese women with polycystic ovary syndrome. Hum Reprod. 2014;29(7):1526–35.
Soares NP, Santos AC, Costa EC, Azevedo GD, Damasceno DC, Fayh AP, et al. Diet-Induced Weight Loss Reduces DNA Damage and Cardiometabolic Risk Factors in Overweight/Obese Women with Polycystic Ovary Syndrome. Ann Nutr Metab. 2016;68(3):220–7.
Oberg E, Gidlöf S, Jakson I, Mitsell M, Tollet Egnell P, Hirschberg AL. Improved menstrual function in obese women with polycystic ovary syndrome after behavioural modification intervention-A randomized controlled trial. Clin Endocrinol. 2019;90(3):468–78.
Huber-Buchholz MM, Carey DG, Norman RJ. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. J Clin Endocrinol Metab. 1999;84(4):1470–4.
Ornstein RM, Copperman NM, Jacobson MS. Effect of weight loss on menstrual function in adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2011;24(3):161–5.
Marzouk TM, Sayed Ahmed WA. Effect of Dietary Weight Loss on Menstrual Regularity in Obese Young Adult Women with Polycystic Ovary Syndrome. J Pediatr Adolesc Gynecol. 2015;28(6):457–61.
Kuchenbecker WK, Groen H, van Asselt SJ, Bolster JH, Zwerver J, Slart RH, et al. In women with polycystic ovary syndrome and obesity, loss of intra-abdominal fat is associated with resumption of ovulation. Hum Reprod. 2011;26(9):2505–12.
Tolino A, Gambardella V, Caccavale C, D'Ettore A, Giannotti F, D'Antò V, et al. Evaluation of ovarian functionality after a dietary treatment in obese women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2005;119(1):87–93.
Lass N, Kleber M, Winkel K, Wunsch R, Reinehr T. Effect of lifestyle intervention on features of polycystic ovarian syndrome, metabolic syndrome, and intima-media thickness in obese adolescent girls. J Clin Endocrinol Metab. 2011;96(11):3533–40.
Crosignani PG, Colombo M, Vegetti W, Somigliana E, Gessati A, Ragni G. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod. 2003;18(9):1928–32.
Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13(6):1502–5.
Pasquali R, Gambineri A, Cavazza C, Ibarra Gasparini D, Ciampaglia W, Cognigni GE, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164(1):53–60.
Moran LJ, Tassone EC, Boyle J, Brennan L, Harrison CL, Hirschberg AL, et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: Lifestyle management. Obes Rev. 2020;21(10):e13046.
Kazemi M, Hadi A, Pierson RA, Lujan ME, Zello GA, Chilibeck PD. Effects of Dietary Glycemic Index and Glycemic Load on Cardiometabolic and Reproductive Profiles in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Adv Nutr. 2020;12(1):161–78.
Zhang X, Zheng Y, Guo Y, Lai Z. The Effect of Low Carbohydrate Diet on Polycystic Ovary Syndrome: A Meta-Analysis of Randomized Controlled Trials. Int J Endocrinol. 2019;2019:4386401.
Perelman D, Coghlan N, Lamendola C, Carter S, Abbasi F, McLaughlin T. Substituting poly- and mono-unsaturated fat for dietary carbohydrate reduces hyperinsulinemia in women with polycystic ovary syndrome. Gynecol Endocrinol. 2017;33(4):324–7.
Goss AM, Chandler-Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, et al. Effects of a eucaloric reduced-carbohydrate diet on body composition and fat distribution in women with PCOS. Metabolism. 2014;63(10):1257–64.
Gower BA, Chandler-Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, et al. Favourable metabolic effects of a eucaloric lower-carbohydrate diet in women with PCOS. Clin Endocrinol. 2013;79(4):550–7.
Atiomo W, Read A, Golding M, Silcocks P, Razali N, Sarkar S, et al. Local recruitment experience in a study comparing the effectiveness of a low glycaemic index diet with a low calorie healthy eating approach at achieving weight loss and reducing the risk of endometrial cancer in women with polycystic ovary syndrome (PCOS). Contemp Clin Trials. 2009;30(5):451–6.
Barr S, Reeves S, Sharp K, Jeanes YM. An isocaloric low glycemic index diet improves insulin sensitivity in women with polycystic ovary syndrome. J Acad Nutr Diet. 2013;113(11):1523–31.
Foroozanfard F, Rafiei H, Samimi M, Gilasi HR, Gorjizadeh R, Heidar Z, et al. The effects of dietary approaches to stop hypertension diet on weight loss, anti-Müllerian hormone and metabolic profiles in women with polycystic ovary syndrome: A randomized clinical trial. Clin Endocrinol. 2017;87(1):51–8.
Asemi Z, Esmaillzadeh A. DASH diet, insulin resistance, and serum hs-CRP in polycystic ovary syndrome: a randomized controlled clinical trial. Horm Metab Res. 2015;47(3):232–8.
Azadi-Yazdi M, Karimi-Zarchi M, Salehi-Abargouei A, Fallahzadeh H, Nadjarzadeh A. Effects of Dietary Approach to Stop Hypertension diet on androgens, antioxidant status and body composition in overweight and obese women with polycystic ovary syndrome: a randomised controlled trial. J Hum Nutr Diet. 2017;30(3):275–83.
Ebrahimi-Mamaghani M, Saghafi-Asl M, Pirouzpanah S, Asghari-Jafarabadi M. Effects of raw red onion consumption on metabolic features in overweight or obese women with polycystic ovary syndrome: A randomized controlled clinical trial. J Obstet Gynaecol Res. 2014;40(4):1067–76.
Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring). 2013;21(12):2504–12.
Karamali M, Kashanian M, Alaeinasab S, Asemi Z. The effect of dietary soy intake on weight loss, glycaemic control, lipid profiles and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomised clinical trial. J Hum Nutr Diet. 2018;31(4):533–43.
Turner-McGrievy GM, Davidson CR, Wingard EE, Billings DL. Low glycemic index vegan or low-calorie weight loss diets for women with polycystic ovary syndrome: a randomized controlled feasibility study. Nutr Res. 2014;34(6):552–8.
Abedini M, Ghasemi-Tehrani H, Tarrahi M, Amani R. The effect of concentrated pomegranate juice consumption on risk factors of cardiovascular diseases in women with polycystic ovary syndrome: A randomized controlled trial. Phytother Res. 2020;35:442–51.
Haidari F, Banaei-Jahromi N, Zakerkish M, Ahmadi K. The effects of flaxseed supplementation on metabolic status in women with polycystic ovary syndrome: a randomized open-labeled controlled clinical trial. Nutr J. 2020;19(1):8.
Johnson LK, Holven KB, Nordstrand N, Mellembakken JR, Tanbo T, Hjelmesæth J. Fructose content of low calorie diets: effect on cardiometabolic risk factors in obese women with polycystic ovarian syndrome: a randomized controlled trial. Endocrine Connect. 2015;4(3):144–54.
Kazemi M, McBreairty LE, Zello GA, Pierson RA, Gordon JJ, Serrao SB, et al. A pulse-based diet and the Therapeutic Lifestyle Changes diet in combination with health counseling and exercise improve health-related quality of life in women with polycystic ovary syndrome: secondary analysis of a randomized controlled trial. J Psychosom Obstet Gynecol. 2020;41(2):144–53.
Phy JL, Pohlmeier AM, Cooper JA, Watkins P, Spallholz J, Harris KS, et al. Low Starch/Low Dairy Diet Results in Successful Treatment of Obesity and Co-Morbidities Linked to Polycystic Ovary Syndrome (PCOS). J Obes Weight Loss Ther. 2015;5(2):259.
Pohlmeier AM, Phy JL, Watkins P, Boylan M, Spallholz J, Harris KS, et al. Effect of a low-starch/low-dairy diet on fat oxidation in overweight and obese women with polycystic ovary syndrome. Applied physiology, nutrition, and metabolism =. Physiol Appl Nutr Metab. 2014;39(11):1237–44.
Paoli A, Mancin L, Giacona MC, Bianco A, Caprio M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18(1):104.
Shishehgar F, Mirmiran P, Rahmati M, Tohidi M, Ramezani TF. Does a restricted energy low glycemic index diet have a different effect on overweight women with or without polycystic ovary syndrome? BMC Endocr Disord. 2019;19(1):93.
Szczuko M, Zapałowska-Chwyć M, Drozd A, Maciejewska D, Starczewski A, Wysokiński P, et al. Changes in the IGF-1 and TNF-α synthesis pathways before and after three-month reduction diet with low glicemic index in women with PCOS. Ginekol Pol. 2018;89(6):295–303.
Hoover SE, Gower BA, Cedillo YE, Chandler-Laney PC, Deemer SE, Goss AM. Changes in Ghrelin and Glucagon following a Low Glycemic Load Diet in Women with PCOS. J Clin Endocrinol Metab. 2021;106(5):e2151–e61.
Marsh KA, Steinbeck KS, Atkinson FS, Petocz P, Brand-Miller JC. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr. 2010;92(1):83–92.
Ganie MA, Sahar T, Rashid A, Wani IA, Nisar S, Sathyapalan T, et al. Comparative Evaluation of Biomarkers of Inflammation Among Indian Women With Polycystic Ovary Syndrome (PCOS) Consuming Vegetarian vs. Non-vegetarian Diet. Front Endocrinol. 2019;10:699.
McBreairty LE, Kazemi M, Chilibeck PD, Gordon JJ, Chizen DR, Zello GA. Effect of a pulse-based diet and aerobic exercise on bone measures and body composition in women with polycystic ovary syndrome: A randomized controlled trial. Bone Rep. 2020;12:100248.
Lujan ME, Brooks ED, Kepley AL, Chizen DR, Pierson RA, Peppin AK. Grid analysis improves reliability in follicle counts made by ultrasonography in women with polycystic ovary syndrome. Ultrasound Med Biol. 2010;36(5):712–8.
Kazemi M, Pierson RA, McBreairty LE, Chilibeck PD, Zello GA, Chizen DR. A randomized controlled trial of a lifestyle intervention with longitudinal follow-up on ovarian dysmorphology in women with polycystic ovary syndrome. Clin Endocrinol. 2020;92(6):525–35.
Kirchengast S, Huber J. Body composition characteristics and body fat distribution in lean women with polycystic ovary syndrome. Hum Reprod. 2001;16(6):1255–60.
Shang Y, Zhou H, Hu M, Feng H. Effect of Diet on Insulin Resistance in Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2020;105(10):3346–60.
Katcher HI, Kunselman AR, Dmitrovic R, Demers LM, Gnatuk CL, Kris-Etherton PM, et al. Comparison of hormonal and metabolic markers after a high-fat, Western meal versus a low-fat, high-fiber meal in women with polycystic ovary syndrome. Fertil Steril. 2009;91(4):1175–82.
McBreairty LE, Chilibeck PD, Gordon JJ, Chizen DR, Zello GA. Polycystic ovary syndrome is a risk factor for sarcopenic obesity: a case control study. BMC Endocr Disord. 2019;19(1):70.
Kasim-Karakas SE, Cunningham WM, Tsodikov A. Relation of nutrients and hormones in polycystic ovary syndrome. Am J Clin Nutr. 2007;85(3):688–94.
Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–73.
Larsen TM, Dalskov S-M, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363(22):2102–13.
Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96(6):1281–98.
Toscani MK, Mario FM, Radavelli-Bagatini S, Wiltgen D, Cristina Matos M, Spritzer PM. Effect of high-protein or normal-protein diet on weight loss, body composition, hormone, and metabolic profile in southern Brazilian women with polycystic ovary syndrome: a randomized study. Gynecol Endocrinol. 2011;27(11):925–30.
Stamets K, Taylor DS, Kunselman A, Demers LM, Pelkman CL, Legro RS. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil Steril. 2004;81(3):630–7.
Nadjarzadeh A, Ghadiri-Anari A, Ramezani-Jolfaie N, Mohammadi M, Salehi-Abargouei A, Namayande SM, et al. Effect of hypocaloric high-protein, low-carbohydrate diet supplemented with fennel on androgenic and anthropometric indices in overweight and obese women with polycystic ovary syndrome: A randomized placebo-controlled trial. Complement Ther Med. 2021;56:102633.
Moran LJ, Noakes M, Clifton PM, Wittert G, Tomlinson L, Galletly C, et al. Ghrelin and measures of satiety are altered in polycystic ovary syndrome but not differentially affected by diet composition. J Clin Endocrinol Metab. 2004;89(7):3337–44.
Moran LJ, Noakes M, Clifton PM, Norman RJ. The effect of modifying dietary protein and carbohydrate in weight loss on arterial compliance and postprandial lipidemia in overweight women with polycystic ovary syndrome. Fertil Steril. 2010;94(6):2451–4.
Sørensen LB, Søe M, Halkier KH, Stigsby B, Astrup A. Effects of increased dietary protein-to-carbohydrate ratios in women with polycystic ovary syndrome. Am J Clin Nutr. 2012;95(1):39–48.
Mehrabani HH, Salehpour S, Amiri Z, Farahani SJ, Meyer BJ, Tahbaz F. Beneficial effects of a high-protein, low-glycemic-load hypocaloric diet in overweight and obese women with polycystic ovary syndrome: a randomized controlled intervention study. J Am Coll Nutr. 2012;31(2):117–25.
Galletly C, Moran L, Noakes M, Clifton P, Tomlinson L, Norman R. Psychological benefits of a high-protein, low-carbohydrate diet in obese women with polycystic ovary syndrome—a pilot study. Appetite. 2007;49(3):590–3.
González F, Considine RV, Abdelhadi OA, Xue J, Acton AJ. Saturated fat ingestion stimulates proatherogenic inflammation in polycystic ovary syndrome. Am J Physiol Endocrinol Metab. 2021;321(5):E689–701.
González F, Considine RV, Abdelhadi OA, Acton AJ. Oxidative stress in response to saturated fat ingestion is linked to insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(11):5360–71.
Kalgaonkar S, Almario R, Gurusinghe D, Garamendi E, Buchan W, Kim K, et al. Differential effects of walnuts vs almonds on improving metabolic and endocrine parameters in PCOS. Eur J Clin Nutr. 2011;65(3):386–93.
Kasim-Karakas SE, Almario RU, Gregory L, Wong R, Todd H, Lasley BL. Metabolic and endocrine effects of a polyunsaturated fatty acid-rich diet in polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89(2):615–20.
Douglas CC, Gower BA, Darnell BE, Ovalle F, Oster RA, Azziz R. Role of diet in the treatment of polycystic ovary syndrome. Fertil Steril. 2006;85(3):679–88.
Yahay M, Heidari Z, Allameh Z, Amani R. The effects of canola and olive oils consumption compared to sunflower oil, on lipid profile and hepatic steatosis in women with polycystic ovarian syndrome: a randomized controlled trial. Lipids Health Dis. 2021;20(1):1–12.
Moran LJ, Noakes M, Clifton PM, Wittert GA, Williams G, Norman RJ. Short-term meal replacements followed by dietary macronutrient restriction enhance weight loss in polycystic ovary syndrome. Am J Clin Nutr. 2006;84(1):77–87.
Wong JM, Gallagher M, Gooding H, Feldman HA, Gordon CM, Ludwig DS, et al. A randomized pilot study of dietary treatments for polycystic ovary syndrome in adolescents. Pediatr Obes. 2016;11(3):210–20.
Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):1–17.
Wilson AS, Koller KR, Ramaboli MC, Nesengani LT, Ocvirk S, Chen C, et al. Diet and the human gut microbiome: an international review. Dig Dis Sci. 2020;65(3):723–40.
Giampaolino P, Foreste V, Di Filippo C, Gallo A, Mercorio A, Serafino P, et al. Microbiome and PCOS: state-of-art and future aspects. Int J Mol Sci. 2021;22(4):2048.
Papakonstantinou E, Kechribari I, Mitrou P, Trakakis E, Vassiliadi D, Georgousopoulou E, et al. Effect of meal frequency on glucose and insulin levels in women with polycystic ovary syndrome: a randomised trial. Eur J Clin Nutr. 2016;70(5):588–94.
Kulshreshtha B, Sharma N, Pant S, Sharma L, Pahuja B, Singh P. PCOS patients differ in meal timings rather than total caloric or macronutrient intake in comparison to weight matched controls. Eur J Obstet Gynecol Reprod Biol. 2022;270:11–6.
Hou C, Zhang W, Li J, Du L, Lv O, Zhao S, et al. Beneficial Effects of Pomegranate on Lipid Metabolism in Metabolic Disorders. Mol Nutr Food Res. 2019;63(16):e1800773.
Esmaeilinezhad Z, Babajafari S, Sohrabi Z, Eskandari MH, Amooee S, Barati-Boldaji R. Effect of synbiotic pomegranate juice on glycemic, sex hormone profile and anthropometric indices in PCOS: A randomized, triple blind, controlled trial. Nutr Metab Cardiovasc Dis. 2019;29(2):201–8.
Esmaeilinezhad Z, Barati-Boldaji R, Brett NR, de Zepetnek JOT, Bellissimo N, Babajafari S, et al. The effect of synbiotics pomegranate juice on cardiovascular risk factors in PCOS patients: a randomized, triple-blinded, controlled trial. J Endocrinol Investig. 2020;43(4):539–48.
Mani UV, Mani I, Biswas M, Kumar SN. An open-label study on the effect of flax seed powder (Linum usitatissimum) supplementation in the management of diabetes mellitus. J Diet Suppl. 2011;8(3):257–65.
Jacobs DR, Tapsell LC. Food synergy: the key to a healthy diet. Proc Nutr Soc. 2013;72(2):200–6.
Szczuko M, Zapałowska-Chwyć M, Maciejewska D, Drozd A, Starczewski A, Stachowska E. Significant improvement selected mediators of inflammation in phenotypes of women with PCOS after reduction and low GI diet. Mediat Inflamm. 2017;2017:5489523.
Kite C, Lahart IM, Afzal I, Broom DR, Randeva H, Kyrou I, et al. Exercise, or exercise and diet for the management of polycystic ovary syndrome: a systematic review and meta-analysis. Syst Rev. 2019;8(1):51.
Shele G, Genkil J, Speelman D. A Systematic Review of the Effects of Exercise on Hormones in Women with Polycystic Ovary Syndrome. J Funct Morphol Kinesiol. 2020;5(2):35.
Patten RK, Boyle RA, Moholdt T, Kiel I, Hopkins WG, Harrison CL, et al. Exercise Interventions in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front Physiol. 2020;11:606.
dos Santos IK, Ashe MC, Cobucci RN, Soares GM, de Oliveira Maranhão TM, Dantas PMS. The effect of exercise as an intervention for women with polycystic ovary syndrome: A systematic review and meta-analysis. Medicine. 2020;99(16):e19644.
dos Santos IK, Nunes F, Queiros VS, Cobucci RN, Dantas PB, Soares GM, et al. Effect of high-intensity interval training on metabolic parameters in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2021;16(1):e0245023.
Richards CT, Meah VL, James PE, Rees DA, Lord RN. HIIT'ing or MISS'ing the Optimal Management of Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of High-Versus Moderate-Intensity Exercise Prescription. Front Physiol. 2021;12:715881.
Palomba S, Giallauria F, Falbo A, Russo T, Oppedisano R, Tolino A, et al. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24-week pilot study. Hum Reprod. 2008;23(3):642–50.
Spahn JM, Reeves RS, Keim KS, Laquatra I, Kellogg M, Jortberg B, et al. State of the evidence regarding behavior change theories and strategies in nutrition counseling to facilitate health and food behavior change. J Am Diet Assoc. 2010;110(6):879–91.
Saelens BE, Gehrman CA, Sallis JF, Calfas KJ, Sarkin JA, Caparosa S. Use of self-management strategies in a 2-year cognitive-behavioral intervention to promote physical activity. Behav Ther. 2000;31(2):365–79.
Hoeger KM, Kochman L, Wixom N, Craig K, Miller RK, Guzick DS. A randomized, 48-week, placebo-controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: a pilot study. Fertil Steril. 2004;82(2):421–9.
Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93(11):4299–306.
Geier L, Bekx M, Connor E. Factors contributing to initial weight loss among adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2012;25(6):367–70.
Pirotta S, Joham AJ, Moran LJ, Skouteris H, Lim SS. Implementation of evidence-based PCOS lifestyle management guidelines: Perceived barriers and facilitators by consumers using the Theoretical Domains Framework and COM-B Model. Patient Educ Couns. 2021;104(8):2080–8.
Oberg E, Lundell C, Blomberg L, Gidlöf SB, Egnell PT, Hirschberg AL. Psychological well-being and personality in relation to weight loss following behavioral modification intervention in obese women with polycystic ovary syndrome: a randomized controlled trial. Eur J Endocrinol. 2020;183(1):1–11.
Larsson I, Hulthén L, Landén M, Pålsson E, Janson P, Stener-Victorin E. Dietary intake, resting energy expenditure, and eating behavior in women with and without polycystic ovary syndrome. Clin Nutr. 2016;35(1):213–8.
Moran LJ, Ranasinha S, Zoungas S, McNaughton SA, Brown WJ, Teede HJ. The contribution of diet, physical activity and sedentary behaviour to body mass index in women with and without polycystic ovary syndrome. Hum Reprod. 2013;28(8):2276–83.
Alvarez-Blasco F, Luque-Ramírez M, Escobar-Morreale HF. Diet composition and physical activity in overweight and obese premenopausal women with or without polycystic ovary syndrome. Gynecol Endocrinol. 2011;27(12):978–81.
de Angelis C, Nardone A, Garifalos F, Pivonello C, Sansone A, Conforti A, et al. Smoke, alcohol and drug addiction and female fertility. Reprod Biol Endocrinol. 2020;18(1):1–26.
Mikkelsen EM, Riis AH, Wise LA, Hatch EE, Rothman KJ, Cueto HT, et al. Alcohol consumption and fecundability: prospective Danish cohort study. BMJ. 2016;354:i4262.
Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Caffeinated and alcoholic beverage intake in relation to ovulatory disorder infertility. Epidemiology (Cambridge, Mass). 2009;20(3):374.
Grodstein F, Goldman MB, Cramer DW. Infertility in women and moderate alcohol use. Am J Public Health. 1994;84(9):1429–32.
Mo L, Mansfield DR, Joham A, Cain SW, Bennett C, Blumfield M, et al. Sleep disturbances in women with and without polycystic ovary syndrome in an Australian National cohort. Clin Endocrinol. 2019;90(4):570–8.
Legro RS, Chen G, Kunselman AR, Schlaff WD, Diamond MP, Coutifaris C, et al. Smoking in infertile women with polycystic ovary syndrome: baseline validation of self-report and effects on phenotype. Hum Reprod. 2014;29(12):2680–6.
Zhang B, Zhou W, Shi Y, Zhang J, Cui L, Chen Z-J. Lifestyle and environmental contributions to ovulatory dysfunction in women of polycystic ovary syndrome. BMC Endocr Disord. 2020;20(1):1–7.
Tao Y, Liu B, Chen Y, Hu Y, Zhu R, Ye D, et al. Genetically Predicted Cigarette Smoking in Relation to Risk of Polycystic Ovary Syndrome. Clin Epidemiol. 2021;13:527.
Cupisti S, Häberle L, Dittrich R, Oppelt PG, Reissmann C, Kronawitter D, et al. Smoking is associated with increased free testosterone and fasting insulin levels in women with polycystic ovary syndrome, resulting in aggravated insulin resistance. Fertil Steril. 2010;94(2):673–7.
Xirofotos D, Trakakis E, Peppa M, Chrelias C, Panagopoulos P, Christodoulaki C, et al. The amount and duration of smoking is associated with aggravation of hormone and biochemical profile in women with PCOS. Gynecol Endocrinol. 2016;32(2):143–6.
Waylen A, Metwally M, Jones G, Wilkinson A, Ledger W. Effects of cigarette smoking upon clinical outcomes of assisted reproduction: a meta-analysis. Hum Reprod Update. 2009;15(1):31–44.
Dokras A, Stener-Victorin E, Yildiz BO, Li R, Ottey S, Shah D, et al. Androgen Excess- Polycystic Ovary Syndrome Society: position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil Steril. 2018;109(5):888–99.
Alur-Gupta S, Lee I, Chemerinski A, Liu C, Lipson J, Allison K, et al. Racial differences in anxiety, depression, and quality of life in women with polycystic ovary syndrome. F S Rep. 2021;2(2):230–7.
Khan NN, Vincent A, Boyle JA, Burggraf M, Pillay M, Teede H, et al. Development of a question prompt list for women with polycystic ovary syndrome. Fertil Steril. 2018;110(3):514–22.
Rosen CB, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208.
Roessler KK, Glintborg D, Ravn P, Birkebaek C, Andersen M. Supportive relationships-psychological effects of group counselling in women with polycystic ovary syndrome (PCOS). Commun Med. 2012;9(2):125.
Abdollahi L, Mirghafourvand M, Babapour JK, Mohammadi M. Effectiveness of cognitive-behavioral therapy (CBT) in improving the quality of life and psychological fatigue in women with polycystic ovarian syndrome: a randomized controlled clinical trial. J Psychosom Obstet Gynecol. 2019;40(4):283–93.
Cooney LG, Milman LW, Hantsoo L, Kornfield S, Sammel MD, Allison KC, et al. Cognitive-behavioral therapy improves weight loss and quality of life in women with polycystic ovary syndrome: a pilot randomized clinical trial. Fertil Steril. 2018;110(1):161–71 e1.
Jiskoot G, Dietz de Loos A, Beerthuizen A, Timman R, Busschbach J, Laven J. Long-term effects of a three-component lifestyle intervention on emotional well-being in women with Polycystic Ovary Syndrome (PCOS): A secondary analysis of a randomized controlled trial. PLoS One. 2020;15(6):e0233876.
Raja-Khan N, Agito K, Shah J, Stetter CM, Gustafson TS, Socolow H, et al. Mindfulness-based stress reduction for overweight/obese women with and without polycystic ovary syndrome: design and methods of a pilot randomized controlled trial. Contemp Clin Trial. 2015;41:287–97.
Stefanaki C, Bacopoulou F, Livadas S, Kandaraki A, Karachalios A, Chrousos GP, et al. Impact of a mindfulness stress management program on stress, anxiety, depression and quality of life in women with polycystic ovary syndrome: a randomized controlled trial. Stress. 2015;18(1):57–66.
Cuijpers P, Cristea IA, Karyotaki E, Reijnders M, Huibers MJ. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta-analytic update of the evidence. World Psychiatry. 2016;15(3):245–58.
Rofey DL, Szigethy EM, Noll RB, Dahl RE, Lobst E, Arslanian SA. Cognitive–behavioral therapy for physical and emotional disturbances in adolescents with polycystic ovary syndrome: a pilot study. J Pediatr Psychol. 2009;34(2):156–63.
Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. Jama. 2008;300(11):1350–2.
Raja-Khan N, Agito K, Shah J, Stetter CM, Gustafson TS, Socolow H, et al. Mindfulness-based stress reduction in women with overweight or obesity: a randomized clinical trial. Obesity. 2017;25(8):1349–59.
Young CC, Monge M, Minami H, Rew L, Conroy H, Peretz C, et al. Outcomes of a Mindfulness-Based Healthy Lifestyle Intervention for Adolescents and Young Adults with Polycystic Ovary Syndrome. J Pediatr Adolesc Gynecol. 2021;35:305–13.
Jiskoot G, Timman R, Beerthuizen A, Dietz de Loos A, Busschbach J, Laven J. Weight reduction through a cognitive behavioral therapy lifestyle intervention in PCOS: the primary outcome of a randomized controlled trial. Obesity. 2020;28(11):2134–41.
Roessler KK, Birkebaek C, Ravn P, Andersen MS, Glintborg D. Effects of exercise and group counselling on body composition and VO 2max in overweight women with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2013;92(3):272–7.
Sam S, Ehrmann DA. Pathogenesis and consequences of disordered sleep in PCOS. Clin Med Insights. 2019;13:1179558119871269.
Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrinol Metab. 2001;86(2):517–20.
Ehrmann DA. Metabolic dysfunction in PCOS: relationship to obstructive sleep apnea. Steroids. 2012;77(4):290–4.
Shreeve N, Cagampang F, Sadek K, Tolhurst M, Houldey A, Hill C, et al. Poor sleep in PCOS; is melatonin the culprit. Hum Reprod. 2013;28(5):1348–53.
Tamura H, Nakamura Y, Korkmaz A, Manchester LC, Tan DX, Sugino N, et al. Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril. 2009;92(1):328–43.
Adriaens I, Jacquet P, Cortvrindt R, Janssen K, Smitz J. Melatonin has dose-dependent effects on folliculogenesis, oocyte maturation capacity and steroidogenesis. Toxicology. 2006;228(2-3):333–43.
Wei D, Zhang C, Xie J, Song X, Yin B, Liu Q, et al. Supplementation with low concentrations of melatonin improves nuclear maturation of human oocytes in vitro. J Assist Reprod Genet. 2013;30(7):933–8.
Sirotkin AV, Schaeffer HJ. Direct regulation of mammalian reproductive organs by serotonin and melatonin. J Endocrinol. 1997;154(1):1–5.
Hu KL, Ye X, Wang S, Zhang D. Melatonin Application in Assisted Reproductive Technology: A Systematic Review and Meta-Analysis of Randomized Trials. Front Endocrinol (Lausanne). 2020;11:160.
Raygan F, Ostadmohammadi V, Bahmani F, Reiter RJ, Asemi Z. Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38(1):191–6.
Tasali E, Van Cauter E, Ehrmann DA. Polycystic ovary syndrome and obstructive sleep apnea. Sleep Med Clin. 2008;3(1):37–46.
Helvaci N, Karabulut E, Demir AU, Yildiz BO. Polycystic ovary syndrome and the risk of obstructive sleep apnea: a meta-analysis and review of the literature. Endocrine Connect. 2017;6(7):437–45.
Sirmans SM, Parish RC, Blake S, Wang X. Epidemiology and comorbidities of polycystic ovary syndrome in an indigent population. J Investig Med. 2014;62(6):868–74.
Thannickal A, Brutocao C, Alsawas M, Morrow A, Zaiem F, Murad MH, et al. Eating, sleeping and sexual function disorders in women with polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Clin Endocrinol. 2020;92(4):338–49.
Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–91.
Kahal H, Kyrou I, Tahrani AA, Randeva HS. Obstructive sleep apnoea and polycystic ovary syndrome: a comprehensive review of clinical interactions and underlying pathophysiology. Clin Endocrinol. 2017;87(4):313–9.
Franik G, Krysta K, Madej P, Gimlewicz-Pięta B, Oślizło B, Trukawka J, et al. Sleep disturbances in women with polycystic ovary syndrome. Gynecol Endocrinol. 2016;32(12):1014–7.
Kutenaee MA, Amirjani S, Asemi Z, Taghavi S-A, Allan H, Kamalnadian S-N, et al. The impact of depression, self-esteem, and body image on sleep quality in patients with PCOS: a cross-sectional study. Sleep Breath. 2019;1:1–8.
Moran LJ, March WA, Whitrow MJ, Giles LC, Davies MJ, Moore VM. Sleep disturbances in a community-based sample of women with polycystic ovary syndrome. Hum Reprod. 2015;30(2):466–72.
Bennett CJ, Mansfield DR, Mo L, Joham A, Cain SW, Blumfield ML, et al. Sleep disturbances in women with and without polycystic ovary syndrome and their association with lifestyle factors (diet, physical activity and sitting time). Br J Nutr. 2021; Accepted in press.
Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–26.
Cappuccio FP, D'elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–20.
Cappuccio FP, Cooper D, D'elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–92.
Tasali E, Van Cauter E, Hoffman L, Ehrmann DA. Impact of obstructve sleep apnea on insulin resistance and glucose tolerance in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(10):3878–84.
Nandalike K, Strauss T, Agarwal C, Coupey SM, Sin S, Rajpathak S, et al. Screening for sleep-disordered breathing and excessive daytime sleepiness in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2011;159(4):591–6.
Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153(7):435–41.
Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059–68.
Unfer V, Facchinetti F, Orru B, Giordani B, Nestler J. Myo-inositol effects in women with PCOS: a meta-analysis of randomized controlled trials. Endocr Connect. 2017;6(8):647–58.
Pundir J, Psaroudakis D, Savnur P, Bhide P, Sabatini L, Teede H, et al. Inositol treatment of anovulation in women with polycystic ovary syndrome: a meta-analysis of randomised trials. BJOG. 2018;125(3):299–308.
Montanino Oliva M, Buonomo G, Calcagno M, Unfer V. Effects of myo-inositol plus alpha-lactalbumin in myo-inositol-resistant PCOS women. J Ovarian Res. 2018;11(1):38.
Asbaghi O, Ghanavati M, Ashtary-Larky D, Bagheri R, Rezaei Kelishadi M, Nazarian B, et al. Effects of Folic Acid Supplementation on Oxidative Stress Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants (Basel). 2021;10:6.
Gunalan E, Yaba A, Yilmaz B. The effect of nutrient supplementation in the management of polycystic ovary syndrome-associated metabolic dysfunctions: A critical review. J Turk Ger Gynecol Assoc. 2018;19(4):220–32.
Kazerooni T, Asadi N, Dehbashi S, Zolghadri J. Effect of folic acid in women with and without insulin resistance who have hyperhomocysteinemic polycystic ovary syndrome. Int J Gynaecol Obstet. 2008;101(2):156–60.
Guzelmeric K, Alkan N, Pirimoglu M, Unal O, Turan C. Chronic inflammation and elevated homocysteine levels are associated with increased body mass index in women with polycystic ovary syndrome. Gynecol Endocrinol. 2007;23(9):505–10.
Kilicdag EB, Bagis T, Tarim E, Aslan E, Erkanli S, Simsek E, et al. Administration of B-group vitamins reduces circulating homocysteine in polycystic ovarian syndrome patients treated with metformin: a randomized trial. Hum Reprod. 2005;20(6):1521–8.
Bahmani F, Karamali M, Shakeri H, Asemi Z. The effects of folate supplementation on inflammatory factors and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled clinical trial. Clin Endocrinol. 2014;81(4):582–7.
Asemi Z, Karamali M, Esmaillzadeh A. Metabolic response to folate supplementation in overweight women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Mol Nutr Food Res. 2014;58(7):1465–73.
Guo S, Tal R, Jiang H, Yuan T, Liu Y. Vitamin D Supplementation Ameliorates Metabolic Dysfunction in Patients with PCOS: A SystematicReview of RCTs and Insight into the Underlying Mechanism. Int J Endocrinol. 2020;2020:7850816.
Miao CY, Fang XJ, Chen Y, Zhang Q. Effect of vitamin D supplementation on polycystic ovary syndrome: A meta-analysis. Exp Ther Med. 2020;19(4):2641–9.
Gao H, Li Y, Yan W, Gao F. The Effect of Vitamin D Supplementation on Blood Lipids in Patients with Polycystic Ovary Syndrome: A Meta-Analysis of Randomized Controlled Trials. Int J Endocrinol. 2021;2021:8849688.
Cicek N, Eryilmaz OG, Sarikaya E, Gulerman C, Genc Y. Vitamin E effect on controlled ovarian stimulation of unexplained infertile women. J Assist Reprod Genet. 2012;29(4):325–8.
Izadi A, Ebrahimi S, Shirazi S, Taghizadeh S, Parizad M, Farzadi L, et al. Hormonal and Metabolic Effects of Coenzyme Q10 and/or Vitamin E in Patients With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2019;104(2):319–27.
Tarkesh F, Namavar Jahromi B, Hejazi N, Tabatabaee H. Beneficial health effects of Menaquinone-7 on body composition, glycemic indices, lipid profile, and endocrine markers in polycystic ovary syndrome patients. Food Sci Nutr. 2020;8(10):5612–21.
Oh JS, Ahn MJ, Han CJ, Kim H, Kwon O, Chung HW, et al. Relationship between flavonoids intake and metabolic syndrome in Korean women with polycystic ovary syndrome. J Nutr Health. 2014;47(3):176–85.
Romualdi D, Constantini B, Campagna G, Lanzone A, Guido M. Is there a role for soy isoflavones in the therapeutic approach to polycystic ovary syndrome? Results from a pilot study. Fertil Steril. 2008;90(5):1826–33.
Fenkci SM, Fenkci V, Oztekin O, Rota S, Karagenc N. Serum total L-carnitine levels in non-obese women with polycystic ovary syndrome. Hum Reprod. 2008;23(7):1602–6.
Dunning KR, Robker RL. Promoting lipid utilization with l-carnitine to improve oocyte quality. Anim Reprod Sci. 2012;134(1-2):69–75.
Jamilian H, Jamilian M, Samimi M, Afshar Ebrahimi F, Rahimi M, Bahmani F, et al. Oral carnitine supplementation influences mental health parameters and biomarkers of oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Gynecol Endocrinol. 2017;33(6):442–7.
Expression of concern: Comparison of myo-inositol and metformin on mental health parameters and biomarkers of oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial and The effects of fish oil omega-3 fatty acid supplementation on mental health parameters and metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. J Psychosom Obstet Gynaecol. 2020;41(4):I.
Masharani U, Gjerde C, Evans JL, Youngren JF, Goldfine ID. Effects of controlled-release alpha lipoic acid in lean, nondiabetic patients with polycystic ovary syndrome. J Diabetes Sci Technol. 2010;4(2):359–64.
Cianci A, Panella M, Fichera M, Falduzzi C, Bartolo M, Caruso S. d-chiro-Inositol and alpha lipoic acid treatment of metabolic and menses disorders in women with PCOS. Gynecol Endocrinol. 2015;31(6):483–6.
Guler I, Himmetoglu O, Turp A, Erdem A, Erdem M, Onan MA, et al. Zinc and homocysteine levels in polycystic ovarian syndrome patients with insulin resistance. Biol Trace Elem Res. 2014;158(3):297–304.
Gedik O, Zileli MS. Effects of hypocalcemia and theophylline on glucose tolerance and insulin release in human beings. Diabetes. 1977;26(9):813–9.
Jamilian M, Foroozanfard F, Bahmani F, Talaee R, Monavari M, Asemi Z. Effects of Zinc Supplementation on Endocrine Outcomes in Women with Polycystic Ovary Syndrome: a Randomized, Double-Blind, Placebo-Controlled Trial. Biol Trace Elem Res. 2016;170(2):271–8.
Szczuko M, Skowronek M, Zapalowska-Chwyc M, Starczewski A. Quantitative assessment of nutrition in patients with polycystic ovary syndrome (PCOS). Rocz Panstw Zakl Hig. 2016;67(4):419–26.
Shojaeian Z, Sadeghi R, Latifnejad RR. Calcium and vitamin D supplementation effects on metabolic factors, menstrual cycles and follicular responses in women with polycystic ocvary syndrome: A systematic review and meta-analysis. Caspian J Intern Med. 2019;10(4):359–69.
Nasiadek M, Stragierowicz J, Klimczak M, Kilanowicz A. The Role of Zinc in Selected Female Reproductive System Disorders. Nutrients. 2020;12:8.
Hajizadeh-Sharafabad F, Moludi J, Tutunchi H, Taheri E, Izadi A, Maleki V. Selenium and Polycystic Ovary Syndrome; Current Knowledge and Future Directions: A Systematic Review. Horm Metab Res. 2019;51(5):279–87.
Hamilton KP, Zelig R, Parker AR, Haggag A. Insulin Resistance and Serum Magnesium Concentrations among Women with Polycystic Ovary Syndrome. Curr Dev Nutr. 2019;3(11):nzz108.
Fazelian S, Rouhani MH, Bank SS, Amani R. Chromium supplementation and polycystic ovary syndrome: A systematic review and meta-analysis. J Trace Elem Med Biol. 2017;42:92–6.
Tang XL, Sun Z, Gong L. Chromium supplementation in women with polycystic ovary syndrome: Systematic review and meta-analysis. J Obstet Gynaecol Res. 2018;44(1):134–43.
Yang K, Zeng L, Bao T, Ge J. Effectiveness of Omega-3 fatty acid for polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2018;16(1):27.
Thakker D, Raval A, Patel I, Walia R. N-acetylcysteine for polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled clinical trials. Obstet Gynecol Int. 2015;2015:817849.
Samimi M, Zarezade Mehrizi M, Foroozanfard F, Akbari H, Jamilian M, Ahmadi S, et al. The effects of coenzyme Q10 supplementation on glucose metabolism and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol. 2017;86(4):560–6.
Liao D, Zhong C, Li C, Mo L, Liu Y. Meta-analysis of the effects of probiotic supplementation on glycemia, lipidic profiles, weight loss and C-reactive protein in women with polycystic ovarian syndrome. Minerva Med. 2018;109(6):479–87.
Shamasbi SG, Ghanbari-Homayi S, Mirghafourvand M. The effect of probiotics, prebiotics, and synbiotics on hormonal and inflammatory indices in women with polycystic ovary syndrome: a systematic review and meta-analysis. Eur J Nutr. 2020;59(2):433–50.
Torres PJ, Siakowska M, Banaszewska B, Pawelczyk L, Duleba AJ, Kelley ST, et al. Gut Microbial Diversity in Women With Polycystic Ovary Syndrome Correlates With Hyperandrogenism. J Clin Endocrinol Metab. 2018;103(4):1502–11.
Lindheim L, Bashir M, Munzker J, Trummer C, Zachhuber V, Leber B, et al. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS One. 2017;12(1):e0168390.
Pourteymour Fard Tabrizi F, Hajizadeh-Sharafabad F, Vaezi M, Jafari-Vayghan H, Alizadeh M, Maleki V. Quercetin and polycystic ovary syndrome, current evidence and future directions: a systematic review. J Ovarian Res. 2020;13(1):11.
Shojaei-Zarghani S, Rafraf M. Resveratrol and markers of polycystic ovary syndrome: a systematic review of animal and clinical studies. Reprod Sci. 2021;26:1–1.
Jamilian M, Foroozanfard F, Mirhosseini N, Kavossian E, Aghadavod E, Bahmani F, et al. Effects of Melatonin Supplementation on Hormonal, Inflammatory, Genetic, and Oxidative Stress Parameters in Women With Polycystic Ovary Syndrome. Front Endocrinol (Lausanne). 2019;10:273.
Zhou K, Zhang J, Xu L, Wu T, Lim CE. Chinese herbal medicine for subfertile women with polycystic ovarian syndrome. Cochrane Database Syst Rev. 2016;10(10):Cd007535.
Arentz S, Smith CA, Abbott J, Bensoussan A. Nutritional supplements and herbal medicines for women with polycystic ovary syndrome; a systematic review and meta-analysis. BMC Complement Altern Med. 2017;17(1):500.
Zhang D-W, Fu M, Gao S-H, Liu J-L. Curcumin and diabetes: a systematic review. Evid Based Complement Alternat Med. 2013;2013:636053.
Heshmati J, Moini A, Sepidarkish M, Morvaridzadeh M, Salehi M, Palmowski A, et al. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomedicine. 2021;80:153395.
Sohaei S, Amani R, Tarrahi MJ, Ghasemi-Tehrani H. The effects of curcumin supplementation on glycemic status, lipid profile and hs-CRP levels in overweight/obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled clinical trial. Complement Ther Med. 2019;47:102201.
Ghowsi M, Yousofvand N, Moradi S. Effects of Salvia officinalis L. (common sage) leaves tea on insulin resistance, lipid profile, and oxidative stress in rats with polycystic ovary: An experimental study. Avicenna J Phytomed. 2020;10(3):263–72.
Amini L, Mojab F, Jahanfar S, Sepidarkish M, Raoofi Z, Maleki-Hajiagha A. Efficacy of Salvia officinalis extract on the prevention of insulin resistance in euglycemic patients with polycystic ovary syndrome: A double-blinded placebo-controlled clinical trial. Complement Ther Med. 2020;48:102245.
Badgujar SB, Patel VV, Bandivdekar AH. Foeniculum vulgare Mill: a review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Res Int. 2014;2014:842674.
Mokaberinejad R, Rampisheh Z, Aliasl J, Akhtari E. The comparison of fennel infusion plus dry cupping versus metformin in management of oligomenorrhoea in patients with polycystic ovary syndrome: a randomised clinical trial. J Obstet Gynaecol. 2019;39(5):652–8.
Minnetti M, De Alcubierre D, Bonaventura I, Pofi R, Hasenmajer V, Tarsitano MG, et al. Effects of licorice on sex hormones and the reproductive system. Nutrition. 2022;103-104:111727.
Armanini D, Mattarello MJ, Fiore C, Bonanni G, Scaroni C, Sartorato P, et al. Licorice reduces serum testosterone in healthy women. Steroids. 2004;69(11-12):763–6.
Armanini D, Castello R, Scaroni C, Bonanni G, Faccini G, Pellati D, et al. Treatment of polycystic ovary syndrome with spironolactone plus licorice. Eur J Obstet Gynecol Reprod Biol. 2007;131(1):61–7.
Sadeghi Ataabadi M, Alaee S, Bagheri MJ, Bahmanpoor S. Role of Essential Oil of Mentha Spicata (Spearmint) in Addressing Reverse Hormonal and Folliculogenesis Disturbances in a Polycystic Ovarian Syndrome in a Rat Model. Adv Pharm Bull. 2017;7(4):651–4.
Medagama AB. The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Nutr J. 2015;14:108.
Chen Z-T, Chu H-L, Chyau C-C, Chu C-C, Duh P-D. Protective effects of sweet orange (Citrus sinensis) peel and their bioactive compounds on oxidative stress. Food Chem. 2012;135(4):2119–27.
Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46(2):409–20.
Ainehchi N, Khaki A, Farshbaf-Khalili A, Hammadeh M, Ouladsahebmadarek E. The Effectiveness of Herbal Mixture Supplements with and without Clomiphene Citrate in Comparison to Clomiphene Citrate on Serum Antioxidants and Glycemic Biomarkers in Women with Polycystic Ovary Syndrome Willing to be Pregnant: A Randomized Clinical Trial. Biomolecules. 2019;9(6):215.
Stener-Victorin E, Jedel E, Manneras L. Acupuncture in polycystic ovary syndrome: current experimental and clinical evidence. J Neuroendocrinol. 2008;20(3):290–8.
Stener-Victorin E, Fujisawa S, Kurosawa M. Ovarian blood flow responses to electroacupuncture stimulation depend on estrous cycle and on site and frequency of stimulation in anesthetized rats. J Appl Physiol (1985). 2006;101(1):84–91.
Wu J, Chen D, Liu N. Effectiveness of acupuncture in polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99(22):e20441.
Qu F, Wu Y, Hu X-Y, Barry JA, Zhou J, Wang F-F, et al. The effects of acupuncture on polycystic ovary syndrome: A systematic review and meta-analysis. Eur J Integr Med. 2016;8(1):12–8.
Monro R. Yoga therapy. J Bodyw Mov Ther. 1997;1(4):215–8.
Thakur D, Saurabh Singh DS, Tripathi DM, Lufang D. Effect of yoga on polycystic ovarian syndrome: A systematic review. J Bodyw Mov Ther. 2021;27:281–6.
Verma A, Upadhyay V, Saxena V. Effect of Yoga Therapy on Health Outcomes in Women With Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Am J Lifestyle Med. 2021;1:15598276211029221.
Mohseni M, Eghbali M, Bahrami H, Dastaran F, Amini L. Yoga Effects on Anthropometric Indices and Polycystic Ovary Syndrome Symptoms in Women Undergoing Infertility Treatment: A Randomized Controlled Clinical Trial. Evid Based Complement Alternat Med. 2021;2021:5564824.
Heydarpour F, Hemati N, Hadi A, Moradi S, Mohammadi E, Farzaei MH. Effects of cinnamon on controlling metabolic parameters of polycystic ovary syndrome: A systematic review and meta-analysis. J Ethnopharmacol. 2020;254:112741.
Kataoka J, Tassone EC, Misso M, Joham AE, Stener-Victorin E, Teede H, et al. Weight management interventions in women with and without PCOS: a systematic review. Nutrients. 2017;9(9):996.
Fluharty M, Taylor AE, Grabski M, Munafò MR. The association of cigarette smoking with depression and anxiety: a systematic review. Nicotine Tob Res. 2016;19(1):3–13.
Jane-Llopis E, Matytsina I. Mental health and alcohol, drugs and tobacco: a review of the comorbidity between mental disorders and the use of alcohol, tobacco and illicit drugs. Drug Aalcohol Rev. 2006;25(6):515–36.
Mendelsohn C. Smoking and depression: a review. Aust Fam Physician. 2012;41(5):304–7.
Sullivan LE, Fiellin DA, O’Connor PG. The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med. 2005;118(4):330–41.
Al Wattar BH, Teede H, Garad R, Franks S, Balen A, Bhide P, et al. Harmonising research outcomes for polycystic ovary syndrome: an international multi-stakeholder core outcome set. Hum Reprod. 2020;35(2):404–12.
Theorell-Haglöw J, Berne C, Janson C, Sahlin C, Lindberg E. Associations between short sleep duration and central obesity in women. Sleep. 2010;33(5):593.
Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14(4):402.
Dong Z, Xu X, Wang C, Cartledge S, Maddison R, Islam SMS. Association of overweight and obesity with obstructive sleep apnoea: a systematic review and meta-analysis. Obes Med. 2020;17:100185.
Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5(5):253–61.
Wu Y, Zhai L, Zhang D. Sleep duration and obesity among adults: a meta-analysis of prospective studies. Sleep Med. 2014;15(12):1456–62.
Chaput J-P, St-Onge M-P. Increased food intake by insufficient sleep in humans: are we jumping the gun on the hormonal explanation? Front Endocrinol. 2014;5:116.
Chaput J-P, Tremblay A. Insufficient sleep as a contributor to weight gain: an update. Curr Obes Rep. 2012;1(4):245–56.
Weaver MD, Vetter C, Rajaratnam SM, O’Brien CS, Qadri S, Benca RM, et al. Sleep disorders, depression and anxiety are associated with adverse safety outcomes in healthcare workers: A prospective cohort study. J Sleep Res. 2018;27(6):e12722.
Ham OK, Lee BG, Choi E, Choi SJ. Efficacy of cognitive behavioral treatment for insomnia: a randomized controlled trial. West J Nurs Res. 2020;42(12):1104–12.
Murawski B, Wade L, Plotnikoff RC, Lubans DR, Duncan MJ. A systematic review and meta-analysis of cognitive and behavioral interventions to improve sleep health in adults without sleep disorders. Sleep Med Rev. 2018;40:160–9.
Sharma MP, Andrade C. Behavioral interventions for insomnia: Theory and practice. Indian J Psychiatry. 2012;54(4):359.
Alesi S, Ee C, Moran LJ, Rao V, Mousa A. Nutritional Supplements and Complementary Therapies in Polycystic Ovary Syndrome. Advanc Nutr. 2021;13:1243–66.
Raja-Khan N, Stener-Victorin E, Wu X, Legro RS. The physiological basis of complementary and alternative medicines for polycystic ovary syndrome. Am J Physiol Endocrinol Metab. 2011;301(1):E1–E10.
Jazani AM, Azgomi HND, Azgomi AND, Azgomi RND. A comprehensive review of clinical studies with herbal medicine on polycystic ovary syndrome (PCOS). DARU J Pharm Sci. 2019;27(2):863–77.
Bargiota A, Diamanti-Kandarakis E. The effects of old, new and emerging medicines on metabolic aberrations in PCOS. Ther Advanc Endocrinol Metab. 2012;3(1):27–47.
Arentz S, Smith CA, Abbott JA, Bensoussan A. A survey of the use of complementary medicine by a self-selected community group of Australian women with polycystic ovary syndrome. BMC Complement Altern Med. 2014;14(1):1–6.
Thomson P, Jones J, Evans JM, Leslie SL. Factors influencing the use of complementary and alternative medicine and whether patients inform their primary care physician. Complement Ther Med. 2012;20(1-2):45–53.
Milden SP, Stokols D. Physicians' attitudes and practices regarding complementary and alternative medicine. Behav Med. 2004;30(2):73–84.
Ge J, Fishman J, Vapiwala N, Li SQ, Desai K, Xie SX, et al. Patient-physician communication about complementary and alternative medicine in a radiation oncology setting. Int J Radiat Oncol Biol Phys. 2013;85(1):e1–6.
Berretta M, Rinaldi L, Taibi R, Tralongo P, Fulvi A, Montesarchio V, et al. Physician attitudes and perceptions of complementary and alternative medicine (CAM): a Multicentre Italian study. Front Oncol. 2020;10:594.
Barraco D, Valencia G, Riba AL, Nareddy S, Draus CB, Schwartz SM. Complementary and alternative medicine (CAM) use patterns and disclosure to physicians in acute coronary syndromes patients. Complement Ther Med. 2005;13(1):34–40.
Adler SR, Wrubel J, Hughes E, Beinfield H. Patients' interactions with physicians and complementary and alternative medicine practitioners: older women with breast cancer and self-managed health care. Integr Cancer Ther. 2009;8(1):63–70.
Schiff E, Frenkel M, Shilo M, Levy M, Schachter L, Freifeld Y, et al. Bridging the physician and CAM practitioner communication gap: suggested framework for communication between physicians and CAM practitioners based on a cross professional survey from Israel. Patient Educ Couns. 2011;85(2):188–93.
Venkat Narayan K, Gregg E, Engelgau M, Moore B, Thompson T, Williamson D, et al. Translation research for chronic disease: the case of diabetes. Diabetes Care. 2000;23(12):1794–8.
Hiss RG. The concept of diabetes translation: addressing barriers to widespread adoption of new science into clinical care. Diabetes Care. 2001;24(7):1293–6.
Sung NS, Crowley WF Jr, Genel M, Salber P, Sandy L, Sherwood LM, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289(10):1278–87.
Neven AC, Laven J, Teede HJ, Boyle JA. A summary on polycystic ovary syndrome: diagnostic criteria, prevalence, clinical manifestations, and management according to the latest international guidelines. Semin Reprod Med. 2018;36(1):5–12.
Tay CT, Joham AE, Hiam DS, Gadalla MA, Pundir J, Thangaratinam S, et al. Pharmacological and surgical treatment of nonreproductive outcomes in polycystic ovary syndrome: An overview of systematic reviews. Clin Endocrinol. 2018;89(5):535–53.
Cinar N, Harmanci A, Demir B, Yildiz BO. Effect of an oral contraceptive on emotional distress, anxiety and depression of women with polycystic ovary syndrome: a prospective study. Hum Reprod. 2012;27(6):1840–5.
Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21(5):560–74.
Acknowledgements
Not applicable.
Funding
CE is supported by an endowment from the Jacka Foundation of Natural Therapies. SL and AM are supported by National Health and Medical Research Council of Australia fellowships. LM is supported by a National Heart Foundation Future Leader Fellowship.
Author information
Authors and Affiliations
Contributions
SC, SL, CA, SP, RT, MG, RB, NN, CB, CE, VR, AM, SA and LM reviewed the literature and wrote the first draft of the manuscript. SC, SL, CA, SP, RT, MG, RB, NN, CB, CE, VR, AM, SA and LM revised and edited the manuscript. SC and LM conceptualised and determined the scope of the manuscript and had primary responsibility for the final content. LM supervised the review process. All authors meet ICMJE criteria for authorship and approved the final version for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cowan, S., Lim, S., Alycia, C. et al. Lifestyle management in polycystic ovary syndrome – beyond diet and physical activity. BMC Endocr Disord 23, 14 (2023). https://doi.org/10.1186/s12902-022-01208-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-022-01208-y