Abstract
Background
Previous studies have indicated inconsistent relationships of diabetes with thyroid cancer risk, yet little is known in China. In this study, we aimed to investigate the associations between diabetes, diabetes duration and the risk of thyroid cancer in Chinese population.
Methods
A 1:1 matched case-control study was performed between 2015 and 2017 in Zhejiang Province including 2,937 thyroid cancer cases and 2,937 healthy controls. Odds ratios (ORs) with 95 % confidence intervals (CIs) for thyroid cancer were estimated in logistic regression models. Specific effects stratified by age, as well as sex, body mass index (BMI) and family history of diabetes were also examined.
Results
Overall, neither diabetes (OR = 0.75, 95 % CI: 0.21–2.73) nor diabetes duration (OR = 0.14, 95 % CI: 0.02–1.22 for diabetes duration ≦ 5 years; OR = 2.10, 95 % CI: 0.32–13.94 for diabetes duration > 5 years) was significantly associated with thyroid cancer. In stratified analyses, significant lower risk of thyroid cancer was observed among subjects with diabetes and shorter diabetes duration ( ≦ 5 years), but limited to those who were aged more than 40 years, female, overweight/obese and had positive family history of diabetes.
Conclusions
Diabetes and shorter diabetes duration were significantly associated with decreased risk of thyroid cancer in individuals characterized by older age, female sex, higher BMI and positive family history of diabetes.
Similar content being viewed by others
Background
Diabetes is one of the fastest growing health challenges of the 21st century. Estimated by the International Diabetes Federation, there were currently 463 million adults with diabetes in 2019 and the number was expected to increase to 700 million by 2045 globally [1]. Cancer is also a major public health issue. The World Health Organization reported that there were an estimated 18.1 million new cancer cases and 9.6 million cancer deaths in 2018 worldwide [2]. As the global burden of diabetes and cancer grow, an increasing number of studies have been exploring the interrelationships between them, among which the majority focuses on the occurrence and prognosis of cancer among people with diabetes. Interestingly, elevated incidence and mortality in overall or specific types of cancer in diabetes patients have been reported in some studies [3,4,5,6], but not in others [7,8,9]. Among diabetes patients, the inconsistent incident risk also extends to thyroid cancer, which is the most common endocrine cancer [10]. In recent decades, with a significant increase in incidence of thyroid cancer worldwide [11], public health concerns have been raised on the association between diabetes and thyroid cancer risk. Specifically, some studies lend support to the hypothesis that diabetes increases the risk of thyroid cancer [12,13,14], while other studies report either null or protective effect [15,16,17,18]. Along with the inconclusive findings on diabetes-cancer effects across the literature, the possible mechanisms linking diabetes and cancer are partially understood. It is widely assumed that the association of diabetes with cancer is due to the hyperglycemia, hyperinsulinemia and inflammation [19,20,21]. Some studies have reported that the shared risk factors (e.g. age, sex, body mass index, etc.) for diabetes and cancer are also involved. Besides, the diabetes duration [22] and treatments [23, 24] may play a contributory role in the link between diabetes and cancer, which still needs further study to verify.
Methods
Study subjects
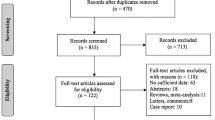
With a 1:1 matched (by age and sex) hospital-based case-control study, we aimed to explore the associated factors of thyroid cancer in Zhejiang Province. The study design and subjects selection have been described in detail elsewhere [25, 26], and are thus only briefly recounted here. Totally, 2,937 case subjects were selected from thyroid cancer patients diagnosed in local hospitals and were identified by physician review of medical records and pathology reports. Besides, based on ICD-10 (malignant neoplasm of thyroid gland [C73]), the cases were further identified. In the same area and same year, 2,937 control subjects were selected from thyroid- healthy examinees who underwent the annual routine physical examination in local hospitals.
Questionnaire
According to a designed questionnaire, the interviewer collected the socio-demographic characteristics, individual history and family history of chronic diseases, lifestyle behaviors, environmental hazardous substances exposure, dietary habits and other information face-to-face at enrollment. The process of questionnaire design and specific contents of questionnaire have been described in detail elsewhere [25].
Definition of the exposure variables
In this study, self-reported diabetes and diabetes duration were the exposure of interests. The diabetes status (yes, no) was based on a questionnaire item asking about whether the subjects had ever been diagnosed with diabetes by a doctor. In this study, we did not collect the information on the diabetes drugs taken by the subjects. Diabetes duration was calculated from the year of diabetes diagnosis to the year of thyroid cancer diagnosis in the case subjects and from the year of diabetes diagnosis to the year of thyroid ultrasound screening in the control subjects, respectively. In this study, the diabetes duration was grouped as no diabetes, ≦5 years, and > 5 years.
Definition of the covariates
Consistent with the existing literature and the definition of confounding factors, the following variables was collected at enrollment and chosen as the potential confounders, which included age, sex, education level, average monthly household income, smoking, alcohol drinking, body mass index (BMI), annual X-ray examination, family history of diabetes, family history of thyroid cancer. Notably, smoking was assessed by the questionnaire item: “How often do you smoke now?” (never, ever smoked but have quitted, currently smoked but not daily, currently and daily). Subjects answered ever smoked but have quitted were considered as ever smokers and those answered currently smoked daily / non-daily were considered as current smokers. Alcohol drinking was assessed by the questionnaire item: “During the past 12 months, how often did you drink any alcohol?” (never, only occasionally, only at certain seasons, every month but less than weekly, usually at least once a week). Subjects answered alcohol drinking only occasionally and at certain seasons were classified as occasional and those drank alcohol every month weekly or less than weekly were classified as current regular. Besides, BMI was calculated based on the Chinese adult BMI classification [27].
Statistical analysis
The associations between socio-demographic characteristics and thyroid cancer were compared with Chi-square tests (Table 1).
To examine the associations between diabetes, diabetes duration and thyroid cancer risk in total subjects, we used multivariate conditional logistic regression models with adjusting for covariates. Furthermore, to compare the associations between diabetes, diabetes duration and thyroid cancer risk among subjects with different characteristics of age, sex, BMI and family history of diabetes, we also performed relevant stratified analyses. For this part of analyses, multivariate logistic regression models were conducted with adjusting for the covariates. The specific results were showed in Fig. 1.
Associations between diabetes, diabetes duration and thyroid cancer in total subjects and stratified by age, as well as sex, body mass index (BMI) and family history of diabetes (FHD). OR odds ratio, CI confidence interval. ORs were adjusted for all variables listed in Table 1
The associations of diabetes, diabetes duration with thyroid cancer risk were reported by odds ratios (ORs) with 95 % confidence intervals (CIs). All statistical tests were based on the 2-sided 5 % level of significance using SAS statistical package (version 9.2, SAS Institute, Inc., Cary, NC, USA).
Results
General information of study subjects
A total of 2,937 pairs of subjects participated in the study. Matched by age and sex, the mean age in case and control subjects was 49.27 ± 1.19 years and 49.15 ± 1.20 years, respectively. Among them, there were 2,261 pairs (77.0 %) of females and 676 pairs (23.0 %) of males. In this study, 293 subjects reported that a doctor had told them they had diabetes. Frequencies and proportions of socio-demographic characteristics among case and control subjects are shown in Table 1. Through comparison, the cases and control had significant differences in the characteristics of average monthly household income (P = 0.01), alcohol category (P < 0.001), BMI (P < 0.001), annual X-ray examination (P < 0.001), family history of diabetes (P < 0.001) and family history of thyroid cancer (P = 0.001).
The association of diabetes with thyroid cancer risk
Overall, we found no significant association between diabetes and thyroid cancer risk (OR = 0.75, 95 % CI: 0.21–2.73). In stratified analyses, compared to subjects without diabetes, significant lower risk of thyroid cancer was seen in subjects with diabetes, but limited to those who were aged more than 40 years (OR = 0.53, 95 % CI: 0.30–0.92), female (OR = 0.48, 95 % CI: 0.25–0.91), overweight/obese (OR = 0.34, 95 % CI: 0.17–0.68) and had positive family history of diabetes (OR = 0.35, 95 % CI: 0.15–0.86) (Fig. 1).
The association of diabetes duration with thyroid cancer risk
In this study, we compared the risk of thyroid cancer for subjects with diabetes duration ≦ 5 years and > 5 years, with reference to subjects without diabetes. Overall, we found no significant risk of thyroid cancer in subjects with diabetes duration ≦ 5 years (OR = 0.14, 95 % CI: 0.02–1.22) and > 5 years (OR = 2.10, 95 % CI: 0.32–13.94). In stratified analyses, significant lower risk of thyroid cancer was seen in subjects with diabetes duration ≦ 5 years, but restricted to those who were aged more than 40 years (OR = 0.42, 95 % CI: 0.18–0.98), overweight/obese (OR = 0.35, 95 % CI: 0.14–0.88) and had positive family history of diabetes (OR = 0.27, 95 % CI: 0.08–0.92) (Fig. 1).
Discussion
Overall, the findings of the present study suggest no associations between self-reported diabetes, diabetes duration and risk of thyroid cancer, after adjustment for potential confounders. Since consistent with some previous studies showing that diabetes, diabetes duration was not associated with risk of thyroid cancer [15,16,17,18], our results may aggravate the heated debate on the potential effects of diabetes on thyroid cancer. Besides, to make our study more comparable with existing literature on this topic, we further conduct the stratified analyses according to the baseline characteristics of age, as well as sex, BMI and family history of diabetes. Based on the findings, a significant lower risk of thyroid cancer was observed among subjects with diabetes and shorter diabetes duration ( ≦ 5 years) within some strata.
There is increasing evidence that the thyroid cancer risk among patients with diabetes is various by sex [28,29,30] and our study is no exception. In the current study, our results indicate that the risk of thyroid cancer is lower only in females with diabetes compared to females without diabetes. This observed decreased risk of thyroid cancer among women with diabetes is consistent with findings from studies conducted in Korea, as well as in Japan and Italy [17, 31, 32], although some estimates were of borderline significance. The older age and higher BMI were proposed as common risk factors for diabetes and thyroid cancer, while their role in the diabetes-cancer linkage has not been well studied. In this study, we find lower risk of thyroid cancer among patients with diabetes who are aged more than 40 years or overweight/obese. Inconsistently, a recent meta-analysis including 5 US-based prospective cohort studies suggested no statistically-significant results within strata of baseline age (< 60 and ≥ 60 years) and BMI (< 25 and ≥ 25 kg/m2) [15]. Furthermore, a population-based cohort study in Italy also investigated the thyroid cancer risk among patients with diabetes by strata of age. After stratification, the study only reported a significant positive association between diabetes and thyroid cancer risk for older patients (65–74 years) [33]. Family history of diabetes is an established risk factor for diabetes [34], which reflects the gene-environment interactions within families. A previous study has reported that a positive family history of diabetes increased the effects of diabetes on overall and specific types of cancer risks, including stomach, rectum, corpus uteri and pancreas [31]. However, due to the limited number of cases, the corresponding effect on thyroid cancer risk was not investigated. According to the analyses, our results reveal that the risk of thyroid cancer is lower in patients with diabetes who had a positive family history of diabetes.
In this study, the underlying mechanisms for the decreased risk of thyroid cancer among patients with diabetes in different strata are not clear. Regarding the sex-specific effect of diabetes on thyroid cancer, one possible explanation is the inverse association between blood glucose level and thyroid cancer risk in women, which has been reported by Metabolic syndrome and Cancer project [35]. Regarding the lower risk of thyroid cancer among overweight/obese patients with diabetes, there is little convincing evidence existing for this observation. In addition, regarding the observed decreased risk of thyroid cancer in this study, another potential factor is the diabetes drugs. Metformin is the most commonly used drug and recent studies have indicated that metformin use in diabetes patients may reduce the risk of thyroid cancer [36, 37]. Regretfully, we did not collect the information about intake of diabetes drugs and thus, their specific effects on thyroid cancer cannot be evaluated in this study.
Recent evidence has suggested that patients with diabetes have a higher risk of cancer in the decade before and shortly after diabetes diagnosis [22]. In this study, we observe lower risk of thyroid cancer in the first five years after diabetes diagnosis, which is in line with results from studies conducted in Korea [17] and Taiwan [16]. As for the decreased risk of thyroid cancer in patients with diabetes duration ≦ 5 years, we have no obvious explanation. However, in the study of Taiwan [16], the author proposed that metformin was widely used in patients with early diabetes and the protective effect of metformin against thyroid cancer may account for such a finding.
Our study has several strengths. This study is one of the few studies investigating the specific associations between diabetes, diabetes duration and thyroid cancer among individuals with different characteristics in mainland China. It is a hospital-based 1:1 case-control study with relatively large sample of 2,937 pairs of subjects. All incident thyroid cancer cases are diagnosed in hospitals and further identified by physician review of medical records and pathology reports, which will minimize the probability of misclassification.
However, some limitations are also observed. Firstly, the number of subjects with diabetes is relatively small in the study, which could decrease the statistical power to investigate the associations with thyroid cancer, especially in the stratified analyses. Secondly, due to the fact that the related information of diabetes and other variables is collected via self-reporting but not confirmed in the medical records, recall bias may be inevitable in this study. However, evidence was emerging that there was a substantial agreement in determinations of diabetes status by self-reports and those based on actual diagnoses [38]. Meanwhile, indeed, the method of self-reporting may decrease the reliability of findings with limiting our ability to conduct the study based on clinic classification of diabetes. Thirdly, although some potential confounders have been considered in this study, the fact we did not collect information on the diabetes treatment, to some extent, may also affect reliability of the study.
Conclusions
Although there is no association between overall diabetes and thyroid cancer, our study suggest that diabetes and short diabetes duration are significantly associated with decreased risk of thyroid cancer among individuals characterized by older age, female sex, higher BMI and positive family history of diabetes. These findings have certain clinical and public health implications for early identification of individuals who are at higher risk for thyroid cancer and providing support for the development of thyroid cancer preventive strategies among patients with diabetes.
Availability of data and materials
The data will be available upon reasonable request from the corresponding author.
Abbreviations
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- BMI:
-
Body mass index
- CDC:
-
Center for Disease Control and Prevention
References
International Diabetes Federation. IDF Diabetes atlas, 9th. 2019. Available from https://diabetesatlas.org.
World Health Organization. The Cancer Atlas, 3rd. 2019. Available from http://publications.iarc.fr.
Pan XF, He M, Yu C, Lv J, Guo Y, Bian Z, et al. Type 2 Diabetes and Risk of Incident Cancer in China: A Prospective Study Among 0.5 Million Chinese Adults. Am J Epidemiol. 2018;187(7):1380–91. doi:https://doi.org/10.1093/aje/kwx376.
Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care. 2015;38(2):264–70. doi:https://doi.org/10.2337/dc14-1996.
Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35(2):299–304. doi:https://doi.org/10.2337/dc11-1313.
Zhang D, Zhao Y, Wang T, Xi Y, Li N, Huang H. Diabetes mellitus and long-term mortality of ovarian cancer patients. A systematic review and meta-analysis of 12 cohort studies. Diabetes Metab Res Rev. 2017;33(4):e2868. doi:https://doi.org/10.1002/dmrr.2868.
Rastad H, Parsaeian M, Shirzad N, Mansournia MA, Yazdani K. Diabetes mellitus and cancer incidence: the Atherosclerosis Risk in Communities (ARIC) cohort study. J Diabetes Metab Disord. 2019;18(1):65–72. doi:https://doi.org/10.1007/s40200-019-00391-5.
Chen Y, Wu F, Saito E, Lin Y, Song M, Luu HN, et al. Association between type 2 diabetes and risk of cancer mortality: a pooled analysis of over 771,000 individuals in the Asia Cohort Consortium. Diabetologia. 2017;60(6):1022–32. doi:https://doi.org/10.1007/s00125-017-4229-z.
Pladys A, Couchoud C, LeGuillou A, Siebert M, Vigneau C, Bayat S. Type 1 and type 2 diabetes and cancer mortality in the 2002–2009 cohort of 39,811 French dialyzed patients. PLoS One. 2015;10(5):e0125089. doi:https://doi.org/10.1371/journal.pone.0125089.
Burman KD, Wartofsky L. Thyroid Nodules. N Engl J Med. 2015;373(24):2347–56. doi:https://doi.org/10.1056/NEJMcp1415786.
Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2020;16(1):17–29. doi:https://doi.org/10.1038/s41574-019-0263-x.
Yeo Y, Ma SH, Hwang Y, Horn-Ross PL, Hsing A, Lee KE, et al. Diabetes mellitus and risk of thyroid cancer: a meta-analysis. PLoS One. 2014;9(6):e98135. doi:https://doi.org/10.1371/journal.pone.0098135.
Aschebrook-Kilfoy B, Sabra MM, Brenner A, Moore SC, Ron E, Schatzkin A, et al. Diabetes and thyroid cancer risk in the National Institutes of Health-AARP Diet and Health Study. Thyroid. 2011;21(9):957–63. doi:https://doi.org/10.1089/thy.2010.0396.
Liu X, Hemminki K, Försti A, Sundquist K, Sundquist J, Ji J. Cancer risk in patients with type 2 diabetes mellitus and their relatives. Int J Cancer. 2015;137(4):903–10. doi:https://doi.org/10.1002/ijc.29440.
Kitahara CM, Platz EA, Beane Freeman LE, Black A, Hsing AW, Linet MS, et al. Physical activity, diabetes, and thyroid cancer risk: a pooled analysis of five prospective studies. Cancer Causes Control. 2012;23(3):463–71. doi:https://doi.org/10.1007/s10552-012-9896-y.
Tseng CH. Thyroid cancer risk is not increased in diabetic patients. PLoS One. 2012;7(12):e53096. doi:https://doi.org/10.1371/journal.pone.0053096.
Seo YG, Choi HC, An AR, Park DJ, Park YJ, Lee KE, et al. The Association between Type 2 Diabetes Mellitus and Thyroid Cancer. J Diabetes Res. 2017;2017:5850879. https://doi.org/10.1155/2017/5850879.
Luo J, Phillips L, Liu S, Wactawski-Wende J, Margolis KL. Diabetes. Diabetes Treatment, and Risk of Thyroid Cancer. J Clin Endocrinol Metab. 2016;101(3):1243–8. doi:https://doi.org/10.1210/jc.2015-3901.
Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293(2):194–202. doi:https://doi.org/10.1001/jama.293.2.194.
Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–85. doi:https://doi.org/10.2337/dc10-0666.
Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373(9682):2215–21. doi:https://doi.org/10.1016/S0140-6736(09)60619-X.
Lega IC, Wilton AS, Austin PC, Fischer HD, Johnson JA, Lipscombe LL. The temporal relationship between diabetes and cancer: A population-based study. Cancer. 2016;122(17):2731–8. doi:https://doi.org/10.1002/cncr.30095.
Shlomai G, Neel B, LeRoith D, Gallagher EJ. Type 2 Diabetes Mellitus and Cancer: The Role of Pharmacotherapy. J Clin Oncol. 2016;34(35):4261–9. doi:https://doi.org/10.1200/JCO.2016.67.4044.
Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013;8(8):e71583. doi:https://doi.org/10.1371/journal.pone.0071583.
Wang M, Gong WW, He QF, Hu RY, Yu M. Menstrual, reproductive and hormonal factors and thyroid cancer: a hospital-based case-control study in China. BMC Womens Health. 2021;21(1):13. doi:https://doi.org/10.1186/s12905-020-01160-w.
Wang M, Gong WW, Lu F, He QF, Hu RY, Zhong JM, et al. Associations of intensity, duration, cumulative dose, and age at start of smoking, with thyroid cancer in Chinese males: A hospital-based case-control study in Zhejiang Province. Tob Induc Dis. 2020;18:97. doi:https://doi.org/10.18332/tid/130350.
Zhou B, Coorperative Meta-Analysis Group Of China Obesity Task Force. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23(1):5–10. (In Chinese).
Ohkuma T, Peters SAE, Woodward M. Sex differences in the association between diabetes and cancer: a systematic review and meta-analysis of 121 cohorts including 20 million individuals and one million events. Diabetologia. 2018;61(10):2140–54. doi:https://doi.org/10.1007/s00125-018-4664-5.
Qi J, He P, Yao H. Cancer risk among patients with type 2 diabetes: A real-world study in Shanghai, China. J Diabetes. 2019;11(11):878–83. doi:https://doi.org/10.1111/1753-0407.12926.
Saarela K, Tuomilehto J, Sund R, Keskimäki I, Hartikainen S, Pukkala E. Cancer incidence among Finnish people with type 2 diabetes during 1989–2014. Eur J Epidemiol. 2019;34(3):259–65. doi:https://doi.org/10.1007/s10654-018-0438-0.
Kuriki K, Hirose K, Tajima K. Diabetes and cancer risk for all and specific sites among Japanese men and women. Eur J Cancer Prev. 2007;16(1):83–9.
La Vecchia C, Negri E, Franceschi S, D’Avanzo B, Boyle P. A case-control study of diabetes mellitus and cancer risk. Br J Cancer. 1994;70(5):950–3.
Gini A, Bidoli E, Zanier L, Clagnan E, Zanette G, Gobbato M, et al. Cancer among patients with type 2 diabetes mellitus: A population-based cohort study in northeastern Italy. Cancer Epidemiol. 2016;41:80–7. doi:https://doi.org/10.1016/j.canep.2016.01.011.
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi:https://doi.org/10.1038/nrendo.2017.151.
Almquist M, Johansen D, Björge T, Ulmer H, Lindkvist B, Stocks T, et al. Metabolic factors and risk of thyroid cancer in the Metabolic syndrome and Cancer project (Me-Can). Cancer Causes Control. 2011;22(5):743–51. doi:https://doi.org/10.1007/s10552-011-9747-2.
Cho YY, Kang MJ, Kim SK, Jung JH, Hahm JR, Kim TH, et al. Protective Effect of Metformin Against Thyroid Cancer Development: A Population-Based Study in Korea. Thyroid. 2018;28(7):864–70. doi:https://doi.org/10.1089/thy.2017.0550.
Tseng CH. Metformin reduces thyroid cancer risk in Taiwanese patients with type 2 diabetes. PLoS One. 2014;9(10):e109852. doi:https://doi.org/10.1371/journal.pone.0109852.
Bowlin SJ, Morrill BD, Nafziger AN, Jenkins PL, Lewis C, Pearson TA. Validity of cardiovascular disease risk factors assessed by telephone survey: the Behavioral Risk Factor Survey. J Clin Epidemiol. 1993;46(6):561–71.
Acknowledgements
We thank the all colleagues at hospitals, health services centers, and CDCs participated in the study for their important contributions.
Funding
This work was supported by grant from the National Key Research and Development Program of China “precision medical research” (2016YFC0900502) from the Ministry of Science and Technology; grant from Zhejiang Medical and Health Technology Project (2021KY614). The funding body plays no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
MW designed the study and wrote the manuscript. MW collected, analyzed the data with WWG, QFH and FL. Professor RYH and MY gave much advice and directions in both study design and preparing of the manuscript. All the authors have read and approved the final submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was carried out in accord with the “Declaration of Helsinki” and the verbal consents were obtained from participants and approved by the Ethics Committee of Zhejiang Provincial Center for Disease Control and Prevention (CDC). The ethics committee approved the procedure for verbal consent because Zhejiang CDC has the authority of the Zhejiang provincial government to collect and publish the cancer related information, which is part of disease surveillance scope in Zhejiang CDC. All the participants (cases and controls) were notified that they have the right to refuse or terminate the study at any point of the interview. Because we obtained verbal consent, documentation of consent was not required. However, the information provided by each participant was kept confidential in Zhejiang CDC.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, M., Gong, WW., Lu, F. et al. The association between diabetes and thyroid cancer risk: a hospital-based case-control study in China. BMC Endocr Disord 21, 21 (2021). https://doi.org/10.1186/s12902-021-00684-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-021-00684-y