Abstract
Background
Prolonged laparoscopic nephroureterectomy (LNU) for upper tract urothelial cancer (UTUC) can increase the frequency of intravesical recurrence after surgery. Therefore, it is important for urological surgeons to have knowledge on preoperative risk factors for prolonged LNU. However, few studies have investigated the risk factors for prolonged LNU. We hypothesized that the quantity of perirenal fat affects the pneumoretroperitoneum time (PRT) of retroperitoneal LNU (rLNU). This study aimed to investigate the preoperative risk factors for prolonged PRT during rLNU.
Methods
We reviewed the data of 115 patients who underwent rLNU for UTUC between 2013 and 2021. The perirenal fat thickness (PFT) observed on preoperative computed tomography (CT) images was used to evaluate the perinephric fat quantity. Preoperative risk factors for PRT during rLNU were analyzed using logistic regression models. The cutoff value for PRT was determined based on the median time.The cutoff values for fat-related factors influencing PRT were defined according to receiver operating characteristic curve analysis.
Results
The median PRT for rLNU was 182 min (interquartile range, 155–230 min). The cutoff values of posterior, lateral, and anterior PFTs were 15 mm, 24 mm, and 6 mm, respectively. Multivariate analysis revealed that a posterior PFT ≥ 15 mm (odds ratio [OR], 2.72; 95% confidence interval, 1.04–7.08; p = 0.0410) was an independent risk factor for prolonged PRT.
Conclusions
Thick posterior PFT is a preoperative risk factor for prolonged PRT during rLNU. For patients with UTUC and thick posterior PFT, surgeons should develop optimal surgical strategies, including the selecting an expert surgeon as a primary surgeon and the selecting transperitoneal approach to surgery or open surgery.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Upper tract urothelial cancer (UTUC) is a relatively uncommon condition that accounts for 5 to 10% of all urothelial malignancies [1]. Although nephroureterectomy (NU) with bladder cuff excision is the gold standard treatment for non-metastatic UTUC, laparoscopic nephroureterectomy (LNU) is performed in patients with UTUC worldwide [2]. However, one of the major concerns associated with NU is intravesical recurrence (IVR). Interestingly, some studies have reported that prolonged pneumoperitoneum time of transperitoneal LNU and pnemoretroperitoneum time (PRT) of retroperitoneal LNU (rLNU) are risk factors for IVR after surgery; furthermore, these studies have speculated that long CO2 gas pressure times resulted in intraluminal seeding of cancer cells to the bladder [3, 4]. Additionally, prolonged surgical times could result in surgical team fatigue, increase the likelihood of technical errors, and increase perioperative complications, such as pulmonary embolism and rhabdomyolysis [5, 6]. Accordingly, it is important for urological surgeons to be aware of the preoperative risk factors for prolonged laparoscopy times during LNU when determining surgical strategies because these times can affect oncological outcomes and perioperative complications. However, few studies have investigated preoperative risk factors for prolonged laparoscopy times during LNU.
Perirenal fat is the one that encapsulates the kidney and fills out the space between the kidney and the adjacent retroperitoneal tissue, renal parenchyma, and adrenal glands [7]. It is surrounded by a complete renal fascia with complete system of blood supply, lymphatic fluid drainage, and innervation [8]. It is adjacent to the kidneys, active in metabolism and adipokine secretion, and shares the same developmental origin as the typical visceral fat [9]. Visceral obesity has a detrimental impact on surgery. Perirenal fat thickness (PFT), an indirect indicator of visceral obesity, has been identified as an independent predictor of postoperative complications in surgeries for gastric and colorectal cancers [10, 11]. Severe complications such as anastomotic leakage are believed to result from challenging dissections caused by excessive visceral fat and a restricted operative field [10, 11]. Additionally, thick perirenal fat has been associated with prolonged operating times and increased intraoperative blood loss during transperitoneal laparoscopic adrenalectomy [12]. In addition, it was reported that the amount of perirenal fat, especially that of anterior perirenal fat, was correlated with the operative time during laparoscopic donor nephrectomy using transperitoneal approach [13]. Moreover, there was a case report of rLNU that had to be converted to hand assisted laparoscopic surgery because the operative field could not be secured due to the massive amount of perirenal fat [14].
Based on previous reports and our long-term experience with rLNU, we hypothesized that the amount of perirenal fat affects PRT during rLNU for UTUC. Therefore, this study aimed to investigate whether the amount of perirenal fat is a risk factor for prolonged PRT during rLNU.
Methods
Patient selection
We retrospectively identified 115 patients who underwent rLNU for non-metastatic UTUC at our institution between 2013 and 2021.
Surgical procedure
During retroperitoneoscopic procedure of rLNU, dissection of the kidney and upper ureter was performed using four ports (one laparoscopic trocar and three instrument trocars) in the lateral position. Sequentially, a small iliac incision (Gibson incision) in the lateral position or lower abdominal midline incision in the supine position was created to retrieve the kidney and ureter and perform bladder cuff resection with a sufficient surgical margin using the extravesical approach. At our institution, we perform lymphadenectomy only in cases suspected of visible lymph node metastasis on computed tomography (CT) and lymphadenectomy is performed using laparotomy. Therefore, lymphadenectomy was not performed in this study. Additionally, our hospital is an educational institution; therefore, it employs many non-expert surgeons. However, only non-expert surgeons with adequate skills in laparoscopic surgery are allowed to serve as primary surgeons. The criteria for a non-expert surgeon to qualify as the primary surgeon include: having acted as a scopist in at least 50 laparoscopic surgery cases, possessing adequate experience in performing laparoscopic surgeries at affiliated hospitals, having over 4 years of experience in urological surgery, being certified as a urologist by the Japanese Urological Association, and receiving an endorsement from three supervisors confirming the surgeon’s capability to perform the procedure. During surgery, the supervisor is the first assistant surgeon, providing guidance and assisting in difficult or urgent situations such as rapid hemostatic maneuvers. We defined the PRT during rLNU as the time from pressured CO2 gas infusion to the completion of the retroperitoneoscopic procedure.
Evaluation of variables
We collected clinical and surgical information from the medical records of the patients. This information included age, sex, body mass index (BMI), laterality and location of the main tumor, clinical T stage, hydronephrosis grade, visceral fat area (VFA), subcutaneous fat area (SFA), posterior PFT, lateral PFT, anterior PFT, presence of preoperative diagnostic ureteroscopic biopsy, quantity of preoperatively assessed ipsilateral renal arteries and veins in the hilum, and PRT during rLNU. At our institution, preoperative diagnostic ureteroscopic biopsy is not routinely performed because it has been reported that diagnostic ureteroscopic biopsy might increase the risk of IVR [15]. In addition, adjuvant intravesical chemotherapy is not administered in our institution.
Imaging evaluation
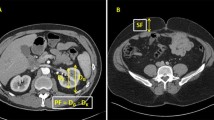
CT findings within 3 months before surgery were evaluated by a staff radiologist (T.K.) with 21 years of experience performing urological imaging using CT. Hydronephrosis was classified as grades 0 to 4 according to the classification of Cho et al. [16]. Cases without calix or pelvic dilation were classified as grade 0, cases with pelvic dilation only as grade 1, cases accompanying mild calix dilation as grade 2, cases with severe calix dilation as grade 3, and those with calix dilation accompanied by renal parenchyma atrophy as grade 4 [16]. The PFT was measured according to the methods of Anderson et al. and Davidiuk et al. (Fig. 1) [13, 17]. Posterior, lateral, and anterior PFTs at the level of the renal veins on preoperative CT images were measured (Fig. 1). Perirenal fat stranding was classified following the method outlined by Kim et al. (Fig. 2) [18]. Cases without fat stranding were categorized as “none”; those with a few thin visible strands were categorized as “mild”; cases with numerous thick visible bands were categorized as “severe”; and cases falling between mild and severe were categorized as “moderate.” The Mayo adhesive probability (MAP) score was computed based on the sum of the posterior PFT score (1 cm = 0 points, 1.1–1.9 cm = 1 point, > 2.0 cm = 2 points) and the type of perirenal fat stranding (no stranding = 0 points, mild/moderate = 2 points, severe = 3 points), following the methodology described by Davidiuk et al. [17]. The VFA and SFA at the level of the umbilicus on preoperative images were measured using a dedicated workstation with Synapse Vincent software (Fujifilm Co. Ltd., Tokyo, Japan) (Fig. 3). Additionally, the ipsilateral renal arteries and veins on CT images were measured preoperatively.
Endpoint of the present study
The primary endpoint of this study was the preoperative risk factors for prolonged PRT during rLNU.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 29 (IBM, Armonk, NY, USA). Statistical significance was set at p < 0.05. Univariate and multivariate analyses were performed using a logistic regression model to determine independent preoperative factors that predict prolonged operative times during rLNU. The cutoff value for PRT during rLNU was established based on the median of 115 patients. Additionally, cutoff values for BMI, VFA, SFA, posterior PFT, lateral PFT, and anterior PFT were determined using sensitivity and specificity levels derived from the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. These values were calculated using the Youden Index formula based on the presence or absence of prolonged PRT. Age (75 years or older vs. younger than 75 years), sex, BMI, laterality, main tumor location (ureter vs. renal pelvis), tumor size (≥ 3 cm vs. < 3 cm), clinical T stage (≥ 3 vs. < 3), hydronephrosis (grade ≥ 3 vs. grade < 3), VFA, SFA, posterior PFT, lateral PFT, anterior PFT, presence or absence of perirenal fat stranding, MAP score (≥ 3 vs. ≤ 2), presence or absence of diagnostic ureteroscopic biopsy, and the quantity of ipsilateral renal arteries and veins (2 vs. ≥ 3) were assessed to identify independent preoperative factors that predict prolonged PRT during rLNU.
Results
Patient population
Table 1 shows the characteristics of patients who underwent rLNU. The median (interquartile [IQR], 25th–75th) values of the BMI, VFA, SFA, posterior PFT, lateral PFT, and anterior PFT were 22.7 kg/m2 (IQR, 20.4–25.0), 111 cm2 (61–150), 126 cm2 (102–165), 10 mm (5–15), 16 mm (8–22), and 5 mm (3–10), respectively. During this study, 13 surgeons including 3 expert surgeons performed rLNU. The median PRT and total operative time of rLNU were 182 mm (IQR, 155–230) and 341 min (IQR, 295–374), respectively.
Evaluation of preoperative factors associated with PRT during rLNU
We defined the cutoff value of PRT during rLNU as 182 min. Figure 4 demonstrates the ROC curves of the BMI, VFA, SFA, posterior PFT, lateral PFT, and anterior PFT. Table 2 presents the results of the ROC curve analysis. The AUC for posterior PFT was 0.646 (p = 0.004, 95% confidence interval [CI] 0.545–0.746). The cutoff values for BMI, VFA, posterior PFT, lateral PFT, and anterior PFT were defined as 21.9 kg/m2, 92 cm2, 15 mm, 24 mm, and 6 mm, respectively. The median was used as the cutoff value for SFA because the AUC for SFA was < 0.5. Univariate logistic regression analysis revealed that a posterior PFT ≥ 15 mm (p = 0.0067) and MAP score ≥ 3 (p = 0.0262) were risk factors for prolonged PRT during rLNU. Multivariate logistic regression analysis revealed that a posterior PFT ≥ 15 mm (p = 0.0410; odds ratio [OR], 2.72; 95% CI, 1.04–7.08) was an independent risk factor for prolonged PRT during rLNU (Table 3).
Discussion
In the present study, multivariate analyses using a logistic regression model revealed that thick posterior PFT was the only independent risk factor for prolonged PRT during rLNU (Tables 2 and 3). In contrast, indicators of obesity, such as BMI, VFA, and SFA, were not significant risk factors for prolonged PRT during rLNU. This is the first study to reveal that the PFT is associated with the PRT during rLNU. Anderson et al. reported that the amount of perirenal fat, especially thats of anterior perirenal fat, rather than the amount of intraperitoneal fat, was correlated with the operative time during laparoscopic donor nephrectomy using the peritoneal approach [13]. Furthermore, they speculated that perirenal fat obscured anatomic landmarks, thus making it difficult to identify the location of the renal vessels and their branches, whereas intraperitoneal fat had little influence on surgery [13]. The retroperitoneal approach to laparoscopic renal surgery has the advantage of avoiding thick subcutaneous fat and visceral fat in patients with obesity [19, 20]. However, the narrow working space associated with the retroperitoneal approach during laparoscopic renal surgery requires a skilled and experienced surgeon. Additionally, thick perirenal fat poses the difficulty of dissecting the renal hilum, including the renal vessels, which is a process that requires careful manipulation during rLNU. Therefore, patients with thick posterior perirenal fat require careful renal hilum manipulation in a narrow surgical field and appropriate traction of the posterior perirenal fat covering the renal hilum to secure the surgical field. Based on published research and our own experience, we speculate that a greater posterior PFT prolongs the PRT during rLNU.
Posterior PFT measurements are easily performed using plain CT images because a dedicated workstation is not required; therefore, they can be routinely performed in clinical practice. Using these measurements, prolonged PRT during rLNU can be predicted. Based on the results of this study, rLNU should be performed by an experienced surgeon and not by a trainee or non-expert surgeon, when patients have thick posterior PFT on preoperative CT images and are at higher risk for prolonged PRT. Moreover, when patients have very thick posterior PFT on preoperative CT images and are at higher risk for prolonged PRT, selecting transperitoneal approach to surgery or open surgery should be considered. It has been reported that a single early intravesical chemotherapy cycle using mitomycin C or pirarubicin after NU decreases the risk of IVR [21, 22]. Adjuvant intravesical chemotherapy might be considered for cases with prolonged PRT during rLNU.
While the influence of a thick PFT on surgical and oncological outcomes remains uncertain, several studies have reported negative effects of thick PFT on these outcomes in certain types of cancer [10,11,12, 23]. Generally, oncological surgeries in patients with a thick PFT may lead to increased postoperative complications and poorer oncological results. Therefore, it is advisable to complete procedures swiftly and safely in such cases. Expert surgeons should ideally handle the entire procedure from start to finish for patients with thick PFT, especially in those with low surgical tolerance, such as older adults or those with multiple complications. Conversely, cases with thin PFT and high tolerance may be suitable for non-expert surgeons.
This study had several limitations. UTUC is relatively uncommon, and this study was conducted at a single institution; therefore, the cohort was small. The characteristics of the facility, including the availability of surgical equipment such as energy devices and retractors, as well as facility-specific surgical techniques, could impact the insufflation time. These facility-related factors might affect the applicability of the present study findings. To address these limitations, future prospective studies involving multiple institutions and larger cohorts are needed. Because this study was a retrospective analysis, and because our institution is an educational institution, selection bias for surgeons may have occurred. In this study, 10 non-expert surgeons with adequate technical skills but not yet experts performed rLNU. However, three expert surgeons supervised all rLNU procedures. Therefore, we believe that the selection of surgeons did not affect the ranking of the operating times significantly. However, previous reports indicate that factors prolonging operative time in laparoscopic surgery may vary between expert and non-expert surgeons [24]. Thus, future studies in non-educational settings are warranted. Additionally, this study exclusively involved Japanese patients whose body size and other patient characteristics may differ from those in other countries. Therefore, further investigations in countries beyond Japan are necessary. Currently, lymphadenectomy is recommended for pathological T ≥ 2 UTUC. However, lymphadenectomy was not performed in this study because there are several discrepancies between the clinical T stage and pathological T stage, and there are technical issues with rLNU. However, lymphadenectomy only in cases suspected of visible lymph node metastasis on imaging might result in worse oncological outcome. We need to perform lymphadenectomy for clinical T ≥ 2 UTUC and improve the accuracy of diagnostic imaging for clinical T ≥ 2 UTUC staging and lymph node dissection, as well as technical skills to perform laparoscopic lymphadenectomy. In the future, analysis including cases with laparoscopic lymphadenectomy is required.
Conclusions
Thick posterior PFT is a preoperative risk factor for prolonged PRT during rLNU. For patients with UTUC and thick posterior PFT, surgeons should develop optimal surgical strategies, including the selecting an expert surgeon as a primary surgeon and the selecting transperitoneal approach to surgery or open surgery.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- IQR:
-
Interquartile range
- IVR:
-
Intravesical recurrence
- LNU:
-
Laparoscopic nephroureterectomy
- MAP:
-
Mayo adhesive probability
- NU:
-
Nephroureterectomy
- OR:
-
Odds ratio
- PFT:
-
Perirenal fat thickness
- PRT:
-
Pneumoretroperitoneum time
- rLNU:
-
Retroperitoneal laparoscopic nephroureterectomy
- ROC:
-
Receiver operating characteristic
- SFA:
-
Subcutaneous fat area
- UTUC:
-
Upper tract urothelial cancer
- VFA:
-
Visceral fat area
References
Rouprêt M, Seisen T, Birtle AJ, Capoun O, Compérat EM, Dominguez-Escrig JL, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2023 update. Eur Urol. 2023;84:49–64.
O’Sullivan NJ, Naughton A, Temperley HC, Casey RG. Robotic-assisted versus laparoscopic nephroureterectomy; a systematic review and meta-analysis. BJUI Compass. 2023;4:246–55.
Shigeta K, Kikuchi E, Hagiwara M, Ando T, Mizuno R, Miyajima A, et al. Prolonged pneumoperitoneum time is an independent risk factor for intravesical recurrence after laparoscopic radical nephroureterectomy in upper tract urothelial carcinoma. Surg Oncol. 2017;26:73–9.
Yanagi M, Hamasaki T, Akatsuka J, Endo Y, Takeda H, Kondo Y. Risk factor analysis of intravesical recurrence after retroperitoneoscopic nephroureterectomy for upper tract urothelial carcinoma. BMC Urol. 2021;21:167.
Cheng H, Chen BP, Soleas IM, Ferko NC, Cameron CG, Hinoul P. Prolonged operative duration increases risk of surgical site infections: A systematic review. Surg Infect (Larchmt). 2017;18:722–35.
Yanagi M, Hamasaki T, Morita K, Takeda H, Akatsuka J, Endo Y, et al. Rhabdomyolysis after retroperitoneal laparoscopic radical nephrectomy in the lateral decubitus position. J Nippon Med Sch. 2022;89:466–8.
Marx WJ, Patel SK. Renal fascia: its radiographic importance. Urology. 1979;13:1–7.
Hu H, Zhang Z, Liu Z, Chu F, Ran J, Liang W. Thickened perirenal fat predicts poor renal outcome in patients with immunoglobulin a nephropathy: a population-based retrospective cohort study. Kidney Dis (Basel). 2023;10:51–60.
Chau YY, Bandiera R, Serrels A, Martínez-Estrada OM, Qing W, Lee M, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16:367–75.
Sönmez MR, Aydin İC, Biçer G, Havan N, Sunar AO, Ademoğlu S, et al. Perirenal fat thickness as a risk factor for postoperative complications in elective colorectal cancer surgery. Med (Baltim). 2023;102:e34072.
Eto K, Ida S, Ohashi T, Kumagai K, Nunobe S, Ohashi M, et al. Perirenal fat thickness as a predictor of postoperative complications after laparoscopic distal gastrectomy for gastric cancer. BJS Open. 2020;4:865–72.
Hayashida M, Sakaguchi K, Yasuoka S, Tanaka M, Oshina T, Oka S, et al. Perirenal fat thickness is a powerful predictor for surgical outcomes of transperitoneal laparoscopic adrenalectomy. Int J Urol. 2024;31:56–63.
Anderson KM, Lindler TU, Lamberton GR, Baron PW, Ojogho OK, Baldwin DD. Laparoscopic donor nephrectomy: Effect of perirenal fat upon donor operative time. J Endourol. 2008;22:2269–74.
Yanagi M, Hamasaki T, Katsu A, Kono H, Kimata R, Nishimura T, et al. Usefulness of hand-assisted retroperitoneal laparoscopic radical nephrectomy for extreme obese patients -a case report. J Med Invest. 2024;71:187–90.
Sharma V, Miest TS, Juvet TS, Toussi A, Packiam V, Chamie K, et al. The impact of upper tract urothelial carcinoma diagnostic modality on intravesical recurrence after radical nephroureterectomy: A single institution series and updated meta-analysis. J Urol. 2021;206:558–67.
Cho KS, Hong SJ, Cho NH, Choi YD. Grade of hydronephrosis and tumor diameter as preoperative prognostic factors in ureteral transitional cell carcinoma. Urology. 2007;70:662–6.
Davidiuk AJ, Parker AS, Thomas CS, Leibovich BC, Castle EP, Heckman MG, et al. Mayo adhesive probability score: An accurate image-based scoring system to predict adherent perinephric fat in partial nephrectomy. Eur Urol. 2014;66:1165–71.
Kim S, Choi SK, Lee SM, Choi T, Lee DG, Min GE, et al. Predictive value of preoperative unenhanced computed tomography during ureteroscopic lithotripsy: A single institute’s experience. Korean J Urol. 2013;54:772–7.
Abreu SC, Kaouk JH, Steinberg AP, Gill IS. Retroperitoneoscopic radical nephrectomy in a super-obese patient (body mass index 77 kg/m2). Urology. 2004;63:175–6.
Yanagi M, Kimura G, Sekine T, Takeda H, Akatsuka J, Endo Y, et al. Factors associated with prolonged retroperitoneal laparoscopic radical nephrectomy performed by non-expert surgeons. J Nippon Med Sch. 2021;88:109–12.
Ito A, Shintaku I, Satoh M, Ioritani N, Aizawa M, Tochigi T, et al. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: the THP Monotherapy Study Group Trial. J Clin Oncol. 2013;31:1422–7.
O’Brien T, Ray E, Singh R, Coker B, Beard R. British association of urological surgeons section of oncology prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial). Eur Urol. 2011;60:703–10.
Eto K, Yoshida N, Iwatsuki M, Iwagami S, Nakamura K, Morita K, et al. Clinical impact of perirenal thickness on short- and long-term outcomes of gastric cancer after curative surgery. Ann Gastroenterol Surg. 2022;6:496–504.
Yuge K, Miyajima A, Jinzaki M, Kaneko G, Hagiwara M, Hasegawa M, et al. How does visceral obesity affect surgical performance in laparoscopic radical nephrectomy? Jpn J Clin Oncol. 2015;45:373–7.
Acknowledgements
The authors thank Editage for editing the manuscript.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Conception and design: MY; Collection of data: MY, YE, HT, and TH; Data analysis: MY, TK, and JA; Manuscript writing: MY, TK, TH, TN, and YK; final approval of the manuscript: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki. This study was approved by the Ethics Committee of the Nippon Medical School Hospital (approval number: 30–03-1100). Based on the retrospective nature of the study, the need for written informed consent was waived by the Ethics Committee of the Ethics Committee of the Nippon Medical School Hospital. However, all participants had the opportunity to opt-out on a homepage of the Ethics Committee of the Nippon Medical School Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yanagi, M., Kiriyama, T., Akatsuka, J. et al. Preoperative analysis of factors associated with prolonged pneumoretroperitoneum time during retroperitoneal laparoscopic nephroureterectomy for upper tract urothelial carcinoma. BMC Urol 24, 155 (2024). https://doi.org/10.1186/s12894-024-01538-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-024-01538-0