Abstract
Background and objective
This study comprehensively evaluates the distribution patterns and antimicrobial resistance profiles of urinary pathogens in Preoperative midstream urine cultures collected from patients with urinary calculi in China over the last two decades.
Methods
A cross-sectional analysis of 41 studies was conducted. A systematic search across various databases, including Wanfang Data, CNKI, SinoMed, Embase, PubMed, and Web of Science, was carried out, covering the time period from 2002 to 2022. Using R 4.2.1 software, a meta-analysis was performed to assess heterogeneity using Cochran’s Q test and the I2 statistic.
Results
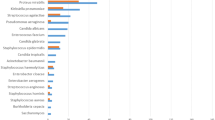
In the analysis of preoperative midstream urine cultures from Chinese patients with urinary calculi, gram-negative bacteria dominated at 69%, with Escherichia coli (43%), Klebsiella pneumoniae (8%), Proteus mirabilis (6%), Pseudomonas aeruginosa (5%), Acinetobacter baumannii (3%), and Enterobacter cloacae (4%) being prominent. Gram-positive organisms included Enterococcus faecalis (9%), Enterococcus faecium (5%), and Staphylococcus aureus (4%). Over time, proportions of Proteus mirabilis, Enterococcus faecalis, and Staphylococcus aureus decreased, while Klebsiella pneumoniae and Pseudomonas aeruginosa increased. Notably, Escherichia coli proportion reduced from 37 to 33% within the last two decades. Antimicrobial resistance analysis indicated declining resistance in E. coli (e.g., co-trimoxazole from 73 to 55%, gentamicin from 64 to 40%), but rising resistance in piperacillin and cefotaxime (34–60%). Enterococcus faecalis exhibited increasing resistance to ampicillin (5–69%), gentamicin (59–94%), and tetracycline (77–89%) over time, while resistance to levofloxacin and ciprofloxacin notably decreased (72–16% and 49–8%, respectively).
Conclusion
Over the past two decades, the proportion of gram-negative bacteria was declined, while the proportion of gram-positive bacteria increased. Escherichia coli remained the most common pathogen in the urine culture of patients with urinary calculi in China and the resistance of Escherichia coli to commonly used antibiotics increased. Clinicians should select appropriate antibiotics according to the results of urine culture and drug sensitivity test to reduce the occurrence of antibiotic resistance.
Similar content being viewed by others
Introduction
Urinary lithiasis represents a prevailing condition within the domain of urology, frequently culminating in urinary obstructive complications, subsequently predisposing individuals to urinary tract infections [1]. The intricate interplay among microbial consortia within urinary milieu contributes substantively to a complex succession of events, encompassing the facilitation of urinary alkalinization, deposition of phosphate, and aggregation of crystals [2]. These intricate processes are profoundly intertwined with the underlying pathogenesis of urinary stone formation, thereby fostering the emergence of consequential clinical ramifications, such as urosepsis, systemic inflammation, and potentially, septic shock [3].
In spite of the evolving diagnostic landscape, the midstream urine culture persists as the foundational cornerstone in the clinical evaluation of urinary tract infections. Notably, while both pelvis urine culture and stone culture may yield augmented rates of positivity, their utility is confined predominantly to intraoperative settings, typified by prolonged intervals required for the determination of drug susceptibility outcomes [4, 5]. Compellingly, empirical evidence underscores the heightened vulnerability of patients harboring positive preoperative midstream urine cultures to postoperative infectious sequelae, marked by heightened occurrences of fever, systemic inflammatory response syndrome (SIRS), and sepsis, in stark contrast to their counterparts with negative urine cultures [6].
In view of these considerations, the imperative of comprehensively elucidating the intricate composition of the urinary microbiota, coupled with unraveling the intricate tapestry of antibiotic resistance patterns displayed by uropathogens in individuals afflicted by urinary calculi, becomes unequivocal. The ramifications of this understanding extend broadly, encompassing judicious antibiotic deployment, adept perioperative infection management, and the attenuation of the likelihood of calculus recurrence [7].
Despite the notable paucity of comprehensive nationwide cross-sectional investigations delineating the panorama of pathogen distribution and the landscape of drug resistance in midstream urine cultures of urinary calculus patients, a wealth of pertinent literature emanates from both domestic and international spheres. Against this backdrop, the current study undertakes a systematic analytical voyage, probing the microbial panorama and discerning the susceptibility profiles to antibiotics of pathogens resident within preoperative midstream urine cultures among patients afflicted by urinary calculi over the course of the preceding two decades in China. Through this endeavor, our aspiration is to furnish the clinical milieu with a pivotal reference, conducive to the judicious and standardized application of antibiotics, thereby yielding a salutary impact upon optimal clinical practices.
Methods
Literature searching protocol
The research was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the meta-analysis protocol was registered on the PROSPERO database on June 3, 2023 (CRD42023428298). A systematic and meticulous approach was employed for conducting a comprehensive literature search, with the objective of retrieving pertinent studies elucidating the distribution patterns and drug resistance profiles of pathogens present in preoperative midstream urine cultures from individuals afflicted with urinary calculi. This methodological strategy entailed a thorough exploration of esteemed academic databases, including Wanfang Data, Chinese Journal Full-text Database (CNKI), SinoMed, Embase, PubMed, and Web of Science. The search encompassed studies published within the time frame spanning January 2002 to December 2022, effectively covering a duration of two decades. Our formulated search queries were thoughtfully tailored to incorporate pivotal terminologies such as “urinary calculus,” “urolithiasis,” “urinary stone,” “bacterial distribution,” “microbial spectrum,” “antibiotic sensitivity,” “drug resistance,” “bacterial culture,” and “midstream urine culture.” It is noteworthy that unpublished studies were intentionally omitted to safeguard the scholarly integrity inherent in our findings. This methodologically robust approach was directed at identifying studies that offer insights into the intricate interplay between pathogens and urinary calculi, thereby enriching our comprehension of antibiotic resistance trends and microbial dynamics within this specific contextual domain.
Inclusion and exclusion criteria
To ensure the quality and reliability of our study, we established strict inclusion and exclusion criteria. In this meta-analysis, we included research that closely examined changes in urine culture and drug resistance patterns among individuals with urinary stones from 2002 to 2022. These studies had to focus exclusively on confirmed cases of urinary calculi, use a specific research design, collect preoperative midstream urine samples, follow consistent bacteriological culturing methods, and provide comprehensive and clear information on bacterial diversity and drug resistance outcomes, and the type of all studies was cross-sectional study. On the other hand, we excluded studies with incomplete or repetitive data, as well as abstracts or reviews that didn’t align with our research focus. Additionally, research involving participants from outside China, using different culture techniques, or relying on animal experiments were also excluded.
Data extraction
The data extraction process was independently conducted by two researchers by screening the titles and abstracts according to the inclusion and exclusion criteria, and irrelevant literatures were excluded. The main extracted information included: name of the first author, publication year, study period during which the data were collected, study location, total number of bacterial culture specimens, number of bacteria of each genus, and drug susceptibility results.
Quality assessment
Ensuring the integrity of our research, we meticulously evaluated the quality of the studies integrated into our analysis. Given their inherent cross-sectional design, we turned to the esteemed quality assessment criteria endorsed by The Agency for Healthcare Research and Quality (AHRQ) for observational research. Through the formulation of a tailored set of four distinct criteria, each study underwent rigorous scrutiny: (1) Precision in defining research subjects and establishing transparent inclusion/exclusion parameters; (2) Comprehensive elucidation of drug susceptibility testing methods, leaving no room for ambiguity; (3) Transparent documentation of evidence related to drug resistance patterns; (4) Achievement of sequencing outcomes surpassing the robust threshold of 70%. Assigning points to each criterion, with an aggregate score of three or more indicating a high level of methodological quality, we independently assessed the studies using a standardized data collection tool. Any discrepancies or potential biases were meticulously addressed through collaborative discussions, often involving a third researcher. This collective effort enhanced the overall robustness of our quality assessment, thus reinforcing the credibility of our findings.
Statistical analysis
In the context of this Meta-analysis, the statistical analysis was conducted using the R 4.2.1 software. The assessment of heterogeneity among the included studies was executed through the implementation of Cochran’s Q test and subsequent computation of the I2 statistic. In cases where the I2 statistic exceeded the established threshold of 50%, indicating substantial heterogeneity, a random-effects model was employed to calculate combined rates. Given the extensive time span covered by the scrutinized literature and the potential variability in terms of publication and research durations, a strategic approach involving subgroup analyses was undertaken. These analytical subdivisions were guided by the identification of median time periods derived from the literature itself. This nuanced methodology facilitated the provision of a more comprehensive perspective that transcended mere chronological considerations.
Results
Search results and study characteristics
Initially, 3,321 relevant articles were identified. After excluding 733 duplicates, the titles and abstracts of the remaining 2,588 articles were comprehensively reviewed. Among them, 71 articles were thematically aligned with the research focus. Upon detailed examination of the full texts, 6 articles pertained to postoperative urine culture, 6 articles addressed stone culture and renal pelvic urine culture, while 18 articles had incomplete outcomes for drug sensitivity and bacterial spectrum. Subsequently, 41 cross-sectional studies [6, 8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] meeting strict inclusion criteria were included in the meta-analysis—35 in Chinese and 6 in English. Figure 1 illustrates the literature retrieval process.
The studies included in this analysis uniformly adopted a cross-sectional study design and demonstrated quality scores exceeding 3 points. These studies were published between the years 2002 and 2022. Among them, two studies were conducted during the period of 2002 to 2007, with one each in the regions of Guangdong and Jiangxi. Shifting to the timeframe of 2008 to 2012, a total of six studies emerged, encompassing two from Zhejiang and one each from Guizhou, Anhui, Tianjin, and Yunnan. Subsequently, between 2013 and 2017, a cohort of 21 studies were published. Among these, three originated from each of Guangdong, Xizang, and Beijing, while two each emerged from Zhejiang. Additionally, there was one study from Shandong, Henan, Shaanxi, Shenzhen, Guizhou, Chongqing, Jiangxi, Hunan, Hebei, and Jiangsu. Finally, within the period of 2018 to 2022, 12 studies were published. This subset comprised three from Hunan, two each from Jiangxi and Hebei, and one each from Guizhou, Shenzhen, Henan, Fujian, and Shaanxi. In total, the studies collectively examined 17,555 pathogenic strains. Further insights into the characteristics of each individual study are available in Table 1.
Characteristics of pathogen distribution
The meta-analysis findings indicated that Chinese individuals with urinary calculi undergoing preoperative midstream urine cultures predominantly showed gram-negative bacteria, making up 69% (95%CI: 0.66–0.72) of cases, while gram-positive bacteria constituted 23% (95%CI: 0.21–0.26). Subgroup analysis displayed a declining trend in gram-negative bacteria over the past two decades (see Fig. 2).
Further genus-level analysis indicated that Escherichia coli was the predominant gram-negative pathogen (43%), followed by Klebsiella pneumoniae (8%), Proteus mirabilis (6%), Pseudomonas aeruginosa (5%), Acinetobacter baumannii (3%), and Enterobacter cloacae (4%). Among gram-positive bacteria, the key pathogens were Enterococcus faecalis (9%), Enterococcus faecium (5%), and Staphylococcus aureus (4%) (Table 2). From 2002 to 2022, there was a decline in Proteus mirabilis (7–5%), Enterococcus faecalis (13–9%), and Staphylococcus aureus (7–3%). Conversely, Klebsiella pneumoniae (6–10%) and Pseudomonas aeruginosa (3–8%) increased. Notably, Escherichia coli detection initially rose and then fell, dropping from 37% (95%CI: 0.09–0.71) to 33% (95%CI: 0.28–0.39) over two decades. Also, Enterococcus faecium rates varied between 4% (95%CI: 0.02–0.08) and 6% (95%CI: 0.04–0.07), while Acinetobacter baumannii (2-3%) and Enterobacter cloacae (3-4%) remained relatively stable (Table 2 - Annual variation of genus proportions).
Pathogen drug resistance characteristics
In terms of common gram-negative bacteria, both Escherichia coli and Klebsiella pneumoniae displayed considerable resistance rates exceeding 80% against ampicillin. Moreover, resistance rates surpassing 50% were observed for co-trimoxazole, cefazolin, and piperacillin. However, these pathogens remained susceptible to amikacin, cefoperazone/sulbactam, and piperacillin/tazobactam, with resistance rates consistently remaining below 20%. Notably, minimal resistance was observed for imipenem, as Escherichia coli and Klebsiella pneumoniae exhibited rates of 2% (95%CI: 0.00-0.05) and 1% (95%CI: 0.00-0.03), respectively. Overall, Escherichia coli demonstrated a higher antibiotic resistance rate compared to Klebsiella pneumoniae (Table 3).
Subsequent subgroup analyses revealed a gradual reduction in Escherichia coli’s resistance rates to various antibiotics in the study, encompassing ampicillin, gentamicin, levofloxacin, co-trimoxazole, and ceftazidime. Particularly noteworthy was the evident decline in the co-trimoxazole resistance rate, which decreased from an initial 73% (95%CI: 0.45–0.94) to a more recent 55% (95%CI: 0.46–0.63), and levofloxacin resistance decreased from 60% (95%CI: 0.49–0.70) to 55% (95%CI: 0.43–0.64) over the last two decades, however, both of them consistently remained more than 50%. Additionally, resistance to gentamicin dropped substantially from 64% (95%CI: 0.55–0.74) to 40% (95%CI: 0.35–0.45). A similar trend was observed for ceftriaxone, where resistance decreased from 64% (95%CI: 0.45–0.81) to 43% (95%CI: 0.32–0.54). In contrast, both piperacillin and cefotaxime exhibited a sustained upward trajectory. Piperacillin’s resistance rate increased from 63% (95%CI: 0.51–0.74) to 79% (95%CI: 0.70–0.86), and cefotaxime notably rose from 34% (95%CI: 0.04–0.65) to 60% (95%CI: 0.41–0.79) (Fig. 3).
Variation in antibiotic resistance of gram-positive bacteria
In the context of common gram-positive, Enterococcus faecalis displayed a consistent upward trajectory in resistance rates to ampicillin, gentamicin, and tetracycline. Moreover, both Enterococcus faecalis and Enterococcus faecium exhibited notable resistance rates surpassing 70% against gentamicin and erythromycin; nevertheless, their resistance levels towards linezolid and vancomycin were notably minimal, approaching 0%. Enterococcus faecalis showed relative sensitivity to penicillin (29%, 95%CI: 0.11–0.52), levofloxacin (38%, 95%CI: 0.23–0.55), and ciprofloxacin (22%, 95%CI: 0.33–0.70).
In contrast, Enterococcus faecium primarily displayed lower resistance to tetracycline (48%, 95%CI: 0.33–0.70), while other antibiotic resistance rates exceeded 60% (refer to Table 4). Notably, the resistance rate to ampicillin had substantially increased from 5% (95%CI: 0.01–0.14) to 69% (95%CI: 0.14-1.00), alongside a significant surge in the gentamicin resistance rate from 59% (95%CI: 0.45–0.71) to 94% (95%CI: 0.14-1.00), reaching its zenith over a two-decade span. Conversely, the resistance rate to levofloxacin had declined from 72% (95%CI: 0.59–0.83) to 16% (95%CI: 0.08–0.25), and the ciprofloxacin resistance rate had dropped from 49% (95%CI: 0.32–0.33) to 8% (95%CI: 0.00-0.33), showcasing significant recent reductions (Fig. 4).
Discussion
Examining complex interactions between individuals with urinary calculi, commonly called kidney stones, and their heightened susceptibility to urinary tract infections (UTIs), highlights a vital concern in the medical field. This exploration gains significance in assessing infection risks post lithotripsy, a key medical procedure posing a notable challenge. This prompts diverse diagnostic methods, including urine sample analysis before surgery, culture examination from various urinary segments, and stone scrutiny [48].
Earlier studies emphasized detecting infections via renal pelvis and stone cultures over midstream urine samples. However, concerns arise due to delayed antibiotic administration linked to time-consuming techniques. Thus, midstream urine culture analysis remains the primary UTI diagnostic route [49]. Through this meta-analysis, we explored evolving pathogenic bacteria and antibiotic resistance in Chinese urinary calculus patients (2002–2022). Findings underscore Gram-negative bacteria prevalence in midstream urine cultures over two decades. Notably, Escherichia coli and Klebsiella pneumoniae dominated Gram-negative bacteria, while Enterococcus faecalis and Enterococcus faecium were primary Gram-positive species. Klebsiella pneumoniae and Pseudomonas aeruginosa proportions grew, with a minor Enterococcus faecalis decreased. And the proportion of gram-negative bacteria declined, while gram-positive bacteria increased. These changes were basically consistent with the trends of bacterial spectrum monitored on China Antimicrobial Surveillance Network (CHINET) from 2018 to 2022 [50], which may be attributed to the continuous improvement of the antimicrobial management system in China [51].
The distribution and antibiotic resistance profiles of uropathogens among urinary calculus patients exhibited discrepancies due to uneven antibiotic practices and regional disparities. Our previous investigation in Guangdong province unveiled prominent trends. In this study, the most common uropathogens included Escherichia coli (48.7%), Klebsiella pneumoniae (10.4%), Enterococcus faecalis (8.7%), and Proteus mirabilis (5.2%). Importantly, among female patients, proportions of Escherichia coli (60.8%) and Proteus mirabilis (7.5%) were higher than in males, signifying gender-based microbial distinctions [32]. Another inquiry underscored that Escherichia coli and Enterococcus faecalis prevailed as the major bacteria, with a higher prevalence of Escherichia coli in females (53.2%) compared to males (26.6%). Furthermore, Klebsiella pneumoniae was more prevalent among elderly patients (9.6%) than younger ones (4.7%), while Enterococcus faecium exhibited a parallel trend. Importantly, Escherichia coli and Klebsiella pneumoniae exhibited susceptibility to piperacillin/tazobactam, imipenem, and amikacin (over 70%), while demonstrating resistance to penicillin, tetracycline, and vancomycin. Notably, the resistance rates of pathogens to antibiotics in male patients were significantly higher than that in female patients [52]. In addition, pathogens in older patients also showed more resistant to antibiotics than younger patients [34].
In this study we identified Escherichia coli as the predominant pathogen in midstream urine cultures of urinary calculus patients (43%), followed by Enterococcus faecalis (9%), Klebsiella pneumoniae (8%), Proteus mirabilis (6%), Pseudomonas aeruginosa (5%), Enterococcus faecium (5%), Enterobacter cloacae (4%), Staphylococcus aureus (4%), and Acinetobacter baumannii (3%). Similarly, a 2020 European multicenter study revealed Escherichia coli (41.3%) as the leading pathogen, followed by gram-positive bacteria (25.1%), KES (Klebsiella spp., Enterobacter spp., Serratia spp.) (14.2%), Proteus spp. (11.7%), and Pseudomonas aeruginosa (4.1%). Resistance rates to various antibiotics were notably high for quinolones, cephalosporins, and TMP/SMX, while Escherichia coli exhibited resistance rates below 10% to carbapenems, piperacillin/tazobactam, and amikacin, congruent with our findings [53]. Conversely, a U.S. single-center study reported contrasting findings, with Staphylococcus (22.2%) as the dominant pathogen in preoperative urine cultures, trailed by Proteus species (15.3%), Escherichia coli (13.1%), and Enterococcus (8.8%) [3].
The recent emergence of multidrug-resistant bacteria has raised significant public health concerns [35]. A comprehensive study conducted in 2019 estimated that bacterial antimicrobial resistance (AMR) contributed to around 495 million deaths, with approximately 127 million directly attributed to bacterial AMR. Notably, drug-resistant Escherichia coli was identified as the primary lethal pathogen, followed by Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa [54].
Varying economic and healthcare circumstances among nations lead to distinct patterns of bacterial distribution and drug resistance [55]. Interestingly, some lower- and middle-income countries exhibit higher rates of antibiotic resistance compared to wealthier nations, despite the former’s lower per-capita antibiotic usage. This discrepancy is largely due to the inappropriate and excessive use of antibiotics [56]. Thus, enhancing the surveillance of pathogen distribution and drug resistance is crucial to prevent the dissemination of resistant strains and curb the rise of multidrug-resistant bacteria.
This pioneering study aims to elucidate the taxonomy and pharmacological resistance of pathogenic bacteria in Chinese urinary calculi patients on a national scale. The research uncovers intricate distribution patterns and reactivity to pharmaceutical agents through preoperative midstream urinary cultures, offering valuable clinical guidance on antibiotic usage. Despite its merits, certain limitations affect the scope and applicability of the findings. The assembled literature’s quality varies due to predominant Chinese sources. Efforts to stratify studies still yield dissimilarities, partly due to literature volume. Limited inclusion of studies from 2002 to 2007 (only two) hampers understanding shifts in bacterial distribution during this period. Varying proportions of bacterial strains and resistance tendencies across institutions introduce complexity due to differing patient cohort sizes. Geographical and temporal factors also influence results, potentially causing geographic and temporal biases. Despite comprehensive scope, a focus on specific locales may create geographic bias, while absent nationwide studies introduce temporal bias. In summary, this study advances our knowledge of harmful bacteria and resistance in Chinese urinary stones patients, but limitations must be addressed for future research reliability.
Conclusion
Over the past 20 years in China, the proportion of gram-negative bacteria was on the decline, while the proportion of gram-positive bacteria increased. Escherichia coli remained the most common pathogen in the urine culture of patients with urinary calculi in China, and the resistance of Escherichia coli to commonly used antibiotics increased, such as resistance to piperacillin and cefotaxime exhibited a gradual upward trend. Clinicians should select appropriate antibiotics according to the results of urine culture and drug sensitivity test to reduce the occurrence of antibiotic resistance. Furthermore, it is significant to strengthen the monitoring of the distribution and drug resistance of pathogens in patients with urinary calculi to prevent resistant strains increasing.
Abbreviations
- E. coli:
-
Escherichia coli
- SIRS:
-
Systemic inflammatory response syndrome
- CNKI:
-
Chinese Journal Full-text Database
- AHRQ:
-
The Agency for Healthcare Research and Quality
- CI:
-
Confidence interval
- UTIs:
-
Urinary tract infections
- CHINET:
-
China Antimicrobial Surveillance Network
- AMR:
-
Antimicrobial resistance
References
Cho S, Park MG, Lee KC, Cho SY, Lee JW. Microbiological features and clinical factors Associated with empirical antibiotic resistance in Febrile patients with Upper urinary tract Calculi. J Korean Med Sci. 2021;36(1):e3.
Espinosa-Ortiz EJ, Eisner BH, Lange D, Gerlach R. Current insights into the mechanisms and management of infection stones. Nat Rev Urol. 2019;16(1):35–53.
Paonessa JE, Gnessin E, Bhojani N, Williams JC, Jr, Lingeman JE. Preoperative bladder urine culture as a predictor of Intraoperative Stone Culture results: clinical implications and relationship to Stone Composition. J Urol. 2016;196(3):769–74.
Chen D, Jiang C, Liang X, Zhong F, Huang J, Lin Y, Zhao Z, Duan X, Zeng G, Wu W. Early and rapid prediction of postoperative infections following percutaneous nephrolithotomy in patients with complex kidney stones. BJU Int. 2019;123(6):1041–7.
Karsiyakali N, Yucetas U, Karatas A, Karabay E, Okucu E, Erkan E. Renal pelvis urine gram stain as a traditional, but new marker in predicting postoperative fever and stone culture positivity in percutaneous nephrolithotomy: an observational, prospective, non-randomized cohort study. World J Urol. 2021;39(6):2135–46.
Yang Z, Lin D, Hong Y, Hu M, Cai W, Pan H, Li Q, Lin J, Ye L. The effect of preoperative urine culture and bacterial species on infection after percutaneous nephrolithotomy for patients with upper urinary tract stones. Sci Rep. 2022;12(1):4833.
Kandil H, Cramp E, Vaghela T. Trends in Antibiotic Resistance in Urologic Practice. Eur Urol Focus. 2016;2(4):363–73.
Huang JK, Li X, Wu KJ, Luo JT. Bacterial pattern and drug resistance pattern of complex renal calculi. China Med, 2006(02):110–2.
Huang JY, Hu R. Distribution and antibiotic resistance of pathogens causing urinary infection among patients with urinary calculus. Chin J Nosocomiol. 2008;18(10):1463–6.
He CH, Wang HT, Tang FC, Lei HQ. Analysis of bacteria spectrum and drug resistance in patients with renal calculi complicated with urinary tract infection. J Mod Urol. 2017;22(10):738–42.
Ju GW. Distribution and drug resistance of pathogenic bacteria in ureteral calculi complicated with urinary tract infection. China High Med Educ, 2014(09):136–7.
Xu SX, Luo XR, Shi H, Li K, Chu ZG, Wang YL, Sun ZL. Distribution and drug resistance of pathogens causing urinary tract infections in patients with urolithiasis. Chin J Nosocomiol. 2015;25(14):3173–5.
Hong X, Xu QK, Duan Y, Shen WH, Yu TQ, Sun XJ. The distribution and drug resistance of pathogens in midstream urine culture of 1368 patients with ureteral calculi. Zhejiang J Trauma Surg. 2014;19(06):904–6.
Huang JB. The distribution and drug resistance of pathogens in preoperative urine culture of patients with urinary calculi. Yunnan Med Pharm. 2014;35(04):482–4.
Zhu M. Analysis of urinary pathogenic microorganisms and drug resistance in patients with ureteral calculi complicated with urinary tract infection. Clin Res. 2017;25(6):177–8.
Liu XL. Analysis of bacterial spectrums in urine cultures of patients with upper urinary stones before operations and the uses of antibiotics. Tianjin Yi Ke Da Xue Bao; 2014.
Li J, Chen Y, Zhou QB. Distribution and drug resistance of pathogens causing urinary tract infections in patients with kidney stones. Chin J Nosocomiol. 2015;25(17):3881–3.
Silang JC, Luo F, Li CH, Wang F, Zhang BP, Zhaxi PC. Mid-stream urine culture and drug sensitivity analysis of upper urinary tract calculi: a single center. Tibet Med. 2018;39(03):17–9.
Zhu CL. A single center retrospective analysis of related risk factors of urinary stone disease concurrent urinary tract infection and the etiological characteristics in Tibet. Tibet University; 2018.
Xiao N, Wang LJ, Duan N, Zhu TY, Li RQ, Zhao XQ. The analysis of the species and antibiotics sensitivity of the urological pathogens isolated from nephrolithiasis patients. Chin J Lab Diagn. 2018;22(06):999–1002.
Li H, Luo JX, Mao XM. Distribution and drug resistance of pathogens in urine samples of patients with urinary tract calculi complicated with infections. Chin J Public Health Eng. 2017;16(02):245–6.
Yi SF, Wang JL, De J. Bacterial spectrum change and drug susceptibility characteristics of urinary calculi complicating infection in a hospital. Lab Med Clin. 2019;16(03):397–9.
Li Y, Zhao MZ. Drug resistance analysis of pathogenic bacteria in ureteral calculi complicated with urinary tract infection. Chin J Health Lab. 2019;29(23):2851–3.
Li BG, Li X, Lou JB, Li XG, Du Y, Liang TC. Distribution and drug resistance of pathogens isolated from urine specimens of urinary tract calculi patients complicated with infection. Chin J Nosocomiol. 2018;28(21):3282–5.
Li J, Wang J. Distribution and drug resistance of 60 strains of pathogenic bacteria in patients with urinary calculi complicated with urinary tract infection. AntiInfect Pharm. 2018;15(08):1337–9.
Zhao WH, Wang ZL, Wang M, Sun FD. Analysis of the pathogens, drug sensitivity and risks of postoperative urinary tract infection in patients with ureteral and renal pelvic calculi. Guizhou Med J. 2022;46(01):117–8.
Yang J, Li GH, Li QW, Jiang H, Li J, Wang L. Analysis the distribution and drug resistance of pathogens causing urinary tract infection in patients with urinary calculi. Jilin Med J. 2019;40(07):1482–3.
Zhang ZB, Li MZ, Qiu L. Distribution and drug resistance of pathogens causing urinary tract infection in patients with urinary calculi from 2018 to 2019 in a hospital. AntiInfect Pharm. 2021;18(07):1018–21.
Liu LZ, Fu TB. Analysis of pathogenic bacteria and drug sensitivity in urine culture of patients with urinary calculi. Baojian Wenhui, 2020(14):159–60, 226.
Zhou H, Wu SP, Xie M, Jiang YH, Hu DH, Liu YH. Analysis on distribution and drug resistance of pathogenic bacteria in 350 patients of urinary calculi with urinary tract infection. AntiInfect Pharm. 2021;18(05):644–7.
Ao J, Sun XX, Wang QY. Distribution and drug resistance of pathogenic bacteria in urine culture of patients with upper urinary tract calculi complicated with urinary tract infection. Jiangxi Med J. 2021;56(12):2194–6.
Chen D, Zhang Y, Huang J, Liang X, Zeng T, Lan C, Duan X, Zhao Z, Zeng G, Tiselius HG, et al. The analysis of microbial spectrum and antibiotic resistance of uropathogens isolated from patients with urinary stones. Int J Clin Pract. 2018;72(6):e13205.
Cui H. Distribution and drug resistance of pathogens causing urinary tract infection in patients with urinary calculi. Am J Transl Res. 2021;13(9):10554–61.
Gu J, Song P, Chen X, Yang Z, Zhang X, Bai Y. Comparative study of the bacterial distribution and antimicrobial susceptibility of uropathogens in older and younger patients with urinary stones. BMC Geriatr. 2022;22(1).
Wang S, Zhang Y, Zhang X, Li J. An evaluation of multidrug-resistant (MDR) bacteria in patients with urinary stone disease: data from a high-volume stone management center. World J Urol. 2020;38(2):425–32.
Bai Y, Liu Q, Gu J, Zhang X, Hu S. Analysis of urinary Pathogen cultures and drug sensitivity in patients with urinary stones for five consecutive years in Xiangya Hospital, China. Infect Drug Resist. 2020;13:1357–63.
Zhang HF, Ou YB, Xia Y, Yang DY, Xing CM. Distribution and antibiotic sensitivity of urinary bacteria in patients with lower urinary tract stones complicated with infection in Xuanwu District of Nanjing City. Med J Chin People Health. 2019;31(9):114–5.
Cao RL, Zhang Y, Chen LZ, He ST, Liu PH, Zhang FY. Detection of bacteriology in the obstructed segment of urinary calculi and its clinical significance. Int J Nephrol Urol, 2020(01):130–2.
Liu JJ. Risk factors of urinary stone disease concurrent infections and the investigation of etiology. J Shantou Univ Med Coll. 2016.
Quan KL. Analysis the types and drug sensitivity of pathogenic bacteria in patients with urinary calculi. J Med Theory Pract. 2020;33(19):3267–9.
Shang XT, Wu YD. Etiology and risk factors of urolithiasis complicated with urinary tract infection. Chin J Front Med Sci. 2017;9(9):87–91.
Wang Y, Liang ZZ, Hao ZY, Ye YP, Zhang L. Investigation of the bacterial distribution of urine in the patients with upper urinary stones. J Clin Urol. 2015;30(12):1092–5.
Wang D, Xiong T. Analysis of urine culture results in patients with urinary calculi complicated with urinary tract infection. Health Guide, 2018(26):306.
Xu YY, Chen YF, Jiang Y. Analysis the risk factors and etiology of upper ureteral calculi complicated with infection. Chongqing Med. 2019;48(7):1210–2.
Ye JB. Analysis of pathogen distribution and drug sensitivity characteristics of urinary calculi complicated with infection. Chin Med Univ. 2021.
Yang C, Liu J, Shen ZH, Cui HZ, Zhang HM, Hao JX, Pei X. Bacterial spectrum changes and drug sensitivity characteristics of patients with urinary stone infection in Tangshan Workers Hospital from 2018 to 2021. Chin J Mod Med. 2022;32(15):79–84.
Cui HJ, Yang C, Liu J. Pathogen distribution and drug resistance and risk factors of urinary tract infection in patients with urinary calculi. Chin J Mod Med. 2022;32(14):83–8.
Benson AD, Juliano TM, Miller NL. Infectious outcomes of nephrostomy drainage before percutaneous nephrolithotomy compared to concurrent access. J Urol. 2014;192(3):770–4.
Mariappan P, Smith G, Bariol SV, Moussa SA, Tolley DA. Stone and pelvic urine culture and sensitivity are better than bladder urine as predictors of urosepsis following percutaneous nephrolithotomy: a prospective clinical study. J Urol. 2005;173(5):1610–4.
Gu J, Chen X, Yang Z, Bai Y, Zhang X. Gender differences in the microbial spectrum and antibiotic sensitivity of uropathogens isolated from patients with urinary stones. J Clin Lab Anal. 2022;36(1).
Yang W, Ding L, Han R, Yin D, Wu S, Yang Y, Zhu D, Guo Y, Hu F. Current status and trends of antimicrobial resistance among clinical isolates in China: a retrospective study of CHINET from 2018 to 2022. One Health Adv. 2023;1:8.
Xiao Y. Antimicrobial stewardship in China: Systems, actions and future strategies. Clin Infect Dis. 2018;67(suppl2):135–S141.
De Lorenzis E, Alba AB, Cepeda M, Galan JA, Geavlete P, Giannakopoulos S, Saltirov I, Sarica K, Skolarikos A, Stavridis S, et al. Bacterial spectrum and antibiotic resistance of urinary tract infections in patients treated for upper urinary tract calculi: a multicenter analysis. Eur J Clin Microbiol Infect Dis. 2020;39(10):1971–81.
Global burden of bacterial antimicrobial resistance. In 2019: a systematic analysis. Lancet (London England). 2022;399(10325):629–55.
Luz CF, Van Niekerk JM, Keizer J, Beerlage-de Jong N, Braakman-Jansen LMA, Stein A, Sinha B, van Gemert-Pijnen J, Glasner C. Mapping twenty years of antimicrobial resistance research trends. Artif Intell Med. 2022;123:102216.
Tornimbene B, Eremin S, Abednego R, Abualas EO, Boutiba I, Egwuenu A, Fuller W, Gahimbare L, Githii S, Kasambara W, et al. Global Antimicrobial Resistance and Use Surveillance System on the African continent: early implementation 2017–2019. Afr J Lab Med. 2022;11(1):1594.
Funding
This work was funded by a grant from the National Natural Science Foundation of China (No.82070719, No.82270807), Guangzhou key discipline of urology, and the Key Clinical Technique of Guangzhou, China (2024CL-ZD04).
Author information
Authors and Affiliations
Contributions
MX and WWQ were responsible for study conception and design. YBT and TRZ contributed to literature search and data extraction. ZSK, XP and HZC performed the quality evaluation. MX and ZSK performed statistical analysis and wrote the manuscript. IMS and WWQ revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable for that section.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

























Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mei, X., Zhang, S., Xu, P. et al. Distribution and antimicrobial resistance patterns of urinary pathogens in preoperative midstream urine cultures from Chinese patients with urinary calculi: a meta-analysis. BMC Urol 24, 46 (2024). https://doi.org/10.1186/s12894-024-01415-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-024-01415-w