Abstract
Background
The incidence rate of malignant tumors after solid organ transplantation is higher than the normal population. The aim of our study is to identify the risk of renal cell carcinoma (RCC) after liver, kidney, heart and lung transplantation, respectively, and suggest that transplant patients can be screened early for tumors to avoid risk.
Methods
PubMed, Embase and the Cochrane Library from their inception until August 16,2023. Retrospective and cohort studies which focus on the statistical data of standardized incidence ratios (SIRs) of RCC after solid organ transplantation (SOT) more than one year have been included and extracted. The study was registered with PROSPERO, CRD4202022343633.
Results
Sixteen original studies have been included for meta-analysis. Liver transplantation could increase the risk of RCC (SIR = 0.73, 95%CI: 0.53 to 0.93) with no heterogeneity(P = 0.594, I2 = 0.0%). And kidney transplantation could increase the risk of RCC(8.54, 6.68 to 10.40; 0.000,90.0%). Besides, heart and lung transplantation also could increase the risk of RCC(SIR = 0.73, 95%CI: 0.53 to 0.93; SIR = 1.61, 95%CI:0.50 to 2.71). Moreover, significance could also be found in most subgroups, especially the European group and retrospective study group. What’s more, after removing studies which have a greater impact on the overall outcome in RCC rate after kidney transplantation, heterogeneity did not solve and significant different was also observed in the European group (7.15, 5.49 to 8.81; 0.000, 78.6%).
Conclusion
Liver, kidney, heart and lung transplantation patients have an increased risk of processing RCC compared to the general population and most subgroups, especially in geographic location of European subgroup, which suggested that patients should be screened frequently after transplantation.
Similar content being viewed by others
Introduction
Although solid organ transplantation (SOT) has been the most useful alternative therapeutic strategy for solid-organ diseases nowadays [1, 2], which offers life-saving treatment for end-stage diseases considered terminal or those associated with a premonitory impairment in patients’ quality of life. Although immunosuppression therapy has also seen advantages with the expansion of immunosuppressive protocols to dampen the host immune response and improve short and long-term graft survival, which is accompanied by the risk of developing a postoperative malignant tumor [1, 3], and the incidence of malignant tumors after SOT is 2–5 times that of the normal population [4, 5]. Therefore, we need to conduct a combined analysis on the incidence of malignant tumors after important SOT.
Immunosuppressive therapy after transplantation reduces transplant recipients’ ability to control virus infections, thus exposing the recipients to increase risk of infection-associated cancers [6]. Moreover, the use of immunosuppressive agents to prevent allograft rejection increases the long-term risk of malignant tumor. Among the risks of solid tumors after transplantation, renal cell carcinoma (RCC) is the most common type. The cause may be driven primarily by kidney failure-related factors, with immunosuppression contributing to a lesser degree [7, 8]. So we determine to research the effect of important SOT on the incidence rate of RCC.
In this systematic review and meta-analysis, we determine to identify the risk of RCC after liver, kidney, heart and lung transplantation, respectively. And suggests transplant patients can be screened early for tumors to avoid risk. None of previous systematic review [9, 10] has provided a comprehensive overview with meta-analysis and meta-regression which to evaluate the transplant population most likely to progress to RCC.
Materials and methods
This systematic review and meta-analysis followed systematic reviews and meta-analyses (PRISMA) guideline [11] and registered with International prospective register of systematic reviews(PROSPERO) website (NO. CRD4202022343633) [12].
Search strategy and eligibility criteria
We meticulously searched electronic databases of PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Embase (https://ersp.lib.whu.edu.cn/s/com/ovid/dc1/ovidsp/G.https/ovid-b/ovidweb.cgi) and the Cochrane Library (https://www.cochranelibrary.com/) from their inception until May 31, 2022; and update literature search was performed in August 16,2023. Search keywords were organ transplantation, incidence of renal cell carcinoma and their MeSH terms (see details in Table S1). Studies which focus on the statistical data of standardized incidence ratios (SIRs) of RCC after SOT more than one year have been included and extracted. Research type of either retrospective study or cohort study with no language restriction. Transplantation types include kidney, liver, heart and lung transplantation have been included, and no restrictions on nationality, region, time of transplants or follow-up time. The search process was performing by two independent researches (WY and LYN), and the controversy and disagreement have been resolved by the third experienced review(GHF or ZYS). Besides, the reference lists of relevant meta-analysis was also identified for potential eligible publications.
Data collection and risk of bias
For each eligible study, the above pairs of researches extracted data independently using a standardized table. Baseline characteristic of first author, publication year, region, research type, research period, research design, number of transplant recipients, age, gender, follow-up period and incidence cases of RCC. For outcomes in this systematic review and meta-analysis, only SIR data with combined effect size model have been extracted. Moreover, subgroups analysis were grouping by transplant type (liver transplantation, kidney transplantation, heart transplantation, lung transplantation), geographic location (Europe, Asia, North America) and research type (retrospective study, cohort study). And the quality of eligible studies was assessed using the Newcastle-Ottawa Scale (NOS) score [13], and the score greater than 4 is acceptable. We also used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) scales [14] to evaluate the quality of the outcomes from standard meta-analysis. All the above procedures were evaluated by two research separately, and disagreement have been discussion.
Data synthesis and analysis
For combined effect size pairwise meta-analysis, in order to avoid homogeneity between included studies, random-effect models were used. And the heterogeneity among studies were assessed by P-value and I2 statistic, and P-values less than 0.05 or I2 more than 50% indicated heterogeneity in these outcome [15]. And, SIR data with their 95% credible intervals (95%CI) upper and lower limits have been extracted. Moreover, we take the natural logarithm of the above data and then merge them to avoid discrepancies in the data. Besides, the P value from meta-regression was used to determine whether the factor was the source of heterogeneity, P value less than 0.05 proved existences [16, 17]. Moreover, the Begg’s and Egger’s test were used to assess the publication bias for available comparisons, P value less than 0.05 means yes. We also used Galbraith plot to determine heterogeneity among included studies. All the aforementioned analyses were performed using StataSE version 15.1.
Results
Description of included studies
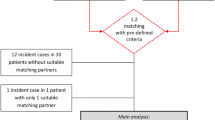
The electronic database search yielded 2622 unique publications, after screening by titles, 61 publications were left for checking. Then moving researches with no incidence of RCC, non-solid transplantation and case analysis, 25 publications were left for full-text checking. Later, studies of sub-type of RCC, no-extractable data available and researches between different sexes have been excluded. Ultimately, 16 original studies [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] have been included for meta-analysis, eleven of them reported incidence RCC data of liver transplant, kidney transplant (n = 11), heart transplant (n = 6) and lung transplant (n = 3)(Fig. 1). And seven of them were retrospective study, 9 of them were cohort study; the sample size was from 280 to 102,654; the baseline characteristic of incidence of RCC risk after transplantation were summarized in Table 1. The quality of all included studies was itemized in Table S2, they all scored 7–8 scales which were acceptable.
Incidence of renal cell carcinoma risk after transplantation
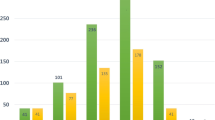
Table 2 and Fig. 2 summarized total data and subgroup analysis from original studies of the incidence of RCC in the liver, kidney, heart, and lung transplant patients. We can notice that in the first section, liver transplant could significant increase incident rate of RCC(SIR = 0.73, 95%CI: 0.53 to 0.93) with no heterogeneity (P = 0.594, I2 = 0.0%), and the publication bias could be tested from the Egger’s test (P = 0.036), and the GRADE assessment was low grade. Besides, significance could also be found in geographic locations of Europe (0.91, 0.50 to 1.32) and Asia (0.93, 0.27 to 1.59) subgroups, and research type of both retrospective study (1.89, 1.46 to 2.31) and cohort study (2.07, 0.88 to 3.26), with low heterogeneity, moderate grade and no publication bias.
For SIR of RCC after kidney transplantation, significant different could be found in overall (8.54, 6.68 to 10.40), geographic location of Europe (6.43, 4.55 to 8.31) and Asia (18.50, 4.33 to 32.68) subgroups, retrospective study (11.85, 7.82 to 15.89) and cohort study (6.83, 5.23 to 8.43). Strangely, there was substantial heterogeneity within overall and all subgroups, and no source of heterogeneity was determined by meta-analysis. Therefore, sensitivity analysis was done for further analysis. From the outcome of sensitivity analysis, we found that the researches of Yeh CC(2020) and Engels EA(2011) may have a greater impact on the overall outcome (Fig. 3A). Subsequently, significance could be found in overall after the adjustment also with substantial heterogeneity (7.15, 5.49 to 8.81; 0.000, 78.6%; Fig. 3B), and geographic location of Europe (6.43, 4.55 to 8.31; 0.000, 82.2%). Galbraith plots were used for heterogeneity testing and we found that none of the adjusted data fell outside the confidence interval, suggesting less heterogeneity among included studies (Fig. 3C).
For incident risk of RCC after heart transplantation, significance could be found in overall(3.37, 2.02 to 4.71), geographic location of Europe (4.17, 1.49 to 6.85) and Asia (2.89, 2.26 to 3.53) subgroups and retrospective study (3.93, 1.58 to 6.28), with low heterogeneity and low-moderate grade. Besides, meta-regression detected different geographic regions may influence the existence of heterogeneity (P = 0.047). For the risk of RCC after lung transplantation, significant different with no heterogeneity could be found (1.61, 0.50 to 2.71, Table 2 and Fig. 2).
In summary, the incident of RCC risk could increase after solid organ transplantation, especially in the European subgroup.
Discussion
This research followed standard PRISMA statement and registered with PROSPERO website. First, 16 original studies [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] were yield through the layers processing, 11of them provided SIR and their 95% data of liver transplantation, 11 of them provide data of kidney transplantation, 6 of them in heart transplantation and 3 of them with data of lung transplantation(Table 1; Fig. 1). Second, from pairwise meta-analysis, incident of RCC risk could increase from liver, kidney, heart and lung transplantation, and significance could find in most subgroups (Table 2; Fig. 2). Besides, meta-regression showed that grouping by geographic location maybe the source of heterogeneity. Third, sensitivity analysis determine that Yeh CC and Engels EA may had great influence on overall outcome of incident risk of RCC. After removing them, we could notice that SOT increase risk of RCC mainly in Europe group (Fig. 3).
From our research, although the incidence rate of RCC will increase after SOT, we also found that the incidence rate RCC after kidney transplantation is higher (8.54, 6.68 to 10.40) than that of incidence rate such as liver, heart and lung transplantation. Moreover, we do not know what causes the clinical heterogeneity among adjustment forest. Kidney transplant recipients have at least a 2–5 fold higher risk of developing or dying from malignant tumor than the general population. The increased risk of primary and recurrence cancer in transplant recipients is complex and attributed to oncogenic viruses, immunosuppression and altered T cell immunity [34]. The cause of heterogeneity may include different immunosuppressive agents, dose regimens, duration, etc. Immunosuppressant medication promotes impaired immunosurveillance, activation of oncogenic viruses, and have carcinogenic effects [34, 35]. Different studies have described an increased risk of RCC development in patients with chronic kidney disease (CKD). As a results of the chronic uremic state in patients and other former risk factors associated with both CKD and malignancy [36, 37]. Nowadays, risk factors for cancer-based on recipient characteristics known a pre-exist at the time of transplantation, such as sex differences that have not been well explored. And many medical disciplines have shown increasing awareness of how diseases manifest differently in men and women, trends in age-standardized cancer incidence rates differ by sex, being stable in men and increasing slightly among women during the most recent five years [38, 39].
The mechanism of incident of RCC, immunosuppressive agents could induce carcinogenesis by reducing immunosurveillance and mechanisms involved in the immunologic control of oncogenic viral infection or direct deoxyribonucleic acid (DNA) damage. However, the therapeutic effect of each drug on RCC development remains controversial because of the use of multi-agent therapy regimens. The increased risk of RCC may be mediated by the overall or cumulative exposure to immunosuppression more than by the agent itself [35, 36].
There are also some limitations among our study. First, only 16 publications have been included into meta-analysis, and for the incident risk of RCC after heart and lung transplantation, only 6 and 3 articles have been included, the results from meta-analysis may not be accurate enough. Second, the subgroup analysis only grouped by geographic location (Europe, Asia, North America) and research type (retrospective study, cohort study). As for the incidence rate of RCC, the baseline difference in follow-up time is huge, and some original studies do not report the follow-up time, so it is difficult for us to conduct subgroup analysis. Third, we only did sensitivity analysis for renal transplantation which with higher heterogeneity. After sensitivity analysis, the heterogeneity among the studies was still large, which may be the impact of clinical heterogeneity of incidence RCC rate.
In summary, the results of this systematic review and meta-analysis suggest that liver, kidney, heart and lung transplantation patients have an increased risk of processing RCC compared to the general population and most subgroups, especially in geographic location of European subgroup. We addressed the issue of post-transplant tumor incidence and performed geographic risk stratification analysis suggesting that European patients should pay more attention to early tumor screening after transplantation. Future patients-based retrospective and cohort study are required to explore the potential clinical risk factors associated with incident risk of RCC.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Black CK, Termanini KM, Aguirre O, Hawksworth JS, Sosin M. Solid organ transplantation in the 21st century. Ann Transl Med. 2018;6(20):409. https://doi.org/10.21037/atm.2018.09.68.
Sarier M, Duman I, Callioglu M, et al. Outcomes of Conservative management of asymptomatic live donor kidney stones. Urology. 2018;118:43–6. https://doi.org/10.1016/j.urology.2018.04.035.
Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005;80(2 Suppl):254–S264. https://doi.org/10.1097/01.tp.0000186382.81130.ba.
Muller E, Botha FCJ, Barday ZA, Manning K, Chin-Hong P, Stock P. Kidney transplantation in HIV-positive patients: current practice and management strategies. Transplantation. 2021;105(7):1492–501. https://doi.org/10.1097/TP.0000000000003485.
Sampaio MS, Cho YW, Qazi Y, et al. Posttransplant malignancies in solid organ adult recipients: an analysis of the U.S. National Transplant Database. Transplantation. 2012;94:990–8.
Schulz TF. Cancer and viral Infections in immunocompromised individuals. Int J Cancer. 2009;125(8):1755–63.
Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. https://doi.org/10.1016/S0140-6736(07)61050-2.
Wan SS, Ying TD, Wyburn K, Roberts DM, Wyld M, Chadban SJ. The treatment of antibody-mediated rejection in kidney transplantation: an updated systematic review and Meta-analysis. Transplantation. 2018;102(4):557–68. https://doi.org/10.1097/TP.0000000000002049.
Zhou Q, Chen J, Pan W, Chen Y, Wen L, Liu K. Incidence of kidney cancer after liver transplantation: a meta-analysis. Eur J Gastroenterol Hepatol. 2020;32(10):1273–8. https://doi.org/10.1097/MEG.0000000000001747.
Chewcharat A, Thongprayoon C, Bathini T, Aeddula NR, Boonpheng B, Kaewput W, Watthanasuntorn K, Lertjitbanjong P, Sharma K, Torres-Ortiz A, Leeaphorn N, Mao MA, Khoury NJ, Cheungpasitporn W. Incidence and mortality of renal cell carcinoma after kidney transplantation: a Meta-analysis. J Clin Med. 2019;8(4):530. https://doi.org/10.3390/jcm8040530. PMID: 30999706; PMCID: PMC6517974.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71. PMID: 33782057; PMCID: PMC8005924.
University of York Centre for Reviews and Dissemination, Jinatongthai P, Kongwatcharapong J, Phrommintikul A, Nathisuwan S, Chaiyakunapruk N. Comparative efficacy and safety of reperfusion therapy with fibrinolytic agents in patient with ST-segment elevation Myocardial Infarction: a systematic review and network meta-analysis. 2022. PROSPERO 2019:CRD42019161406.https://www.crd.york.ac.uk/PROSPERO/#recordDetails.
Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90(8):1067–76. https://doi.org/10.1097/ACM.0000000000000786.
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. https://doi.org/10.1136/bmj.39489.470347.AD.
Yang C, Xu C, Li X, et al. Could Camrelizumab Plus Chemotherapy improve clinical outcomes in Advanced Malignancy? A systematic review and network Meta-analysis. Front Oncol. 2021;11:700165. https://doi.org/10.3389/fonc.2021.700165. Published 2021 Aug 9.
Feng F, Jiang Q, Jia H, et al. Which is the best combination of TACE and Sorafenib for advanced hepatocellular carcinoma treatment? A systematic review and network meta-analysis. Pharmacol Res. 2018;135:89–101. https://doi.org/10.1016/j.phrs.2018.06.021.
Hui F, Xu C, Xu X, et al. What is the most suitable Agent Combined with Apatinib for Transarterial Chemoembolization Treatment in Advanced Hepatocellular Carcinoma patients? A systematic review and network Meta-analysis. Front Oncol. 2022;12:887332. https://doi.org/10.3389/fonc.2022.887332. Published 2022 May 25.
Friman TK, Jäämaa-Holmberg S, Åberg F, et al. Cancer risk and mortality after solid organ transplantation: a population-based 30-year cohort study in Finland. Int J Cancer. 2022;150(11):1779–91. https://doi.org/10.1002/ijc.33934.
Leon-Rodriguez E, Armengol-Alonso A, Rivera-Franco MM, Alberú-Gómez J, Merchan-Alvear P. Malignancies after renal transplantation: experience of a Mexican referral center. Clin Transl Oncol. 2020;22(10):1796–801. https://doi.org/10.1007/s12094-020-02318-6.
Yeh CC, Khan A, Muo CH, et al. De Novo Malignancy after Heart, kidney, and liver transplant: a nationwide study in Taiwan. Exp Clin Transplant. 2020;18(2):224–33. https://doi.org/10.6002/ect.2019.0210.
Lengwiler E, Stampf S, Zippelius A, et al. Solid cancer development in solid organ transplant recipients within the Swiss transplant cohort study. Swiss Med Wkly. 2019;149:w20078. https://doi.org/10.4414/smw.2019.20078. Published 2019 May 19.
O’Neill JP, Sexton DJ, O’Leary E, et al. Post-transplant malignancy in solid organ transplant recipients in Ireland, the Irish transplant Cancer Group. Clin Transpl. 2019;33(10):e13669. https://doi.org/10.1111/ctr.13669.
Heo J, Noh OK, Oh YT, Chun M, Kim L. Second primary cancer after liver transplantation in hepatocellular carcinoma: a nationwide population-based study. Hepatol Int. 2017;11(6):523–8. https://doi.org/10.1007/s12072-017-9824-z.
Kaneko J, Sugawara Y, Tamura S, et al. De novo malignancies after adult-to-adult living-donor liver transplantation with a malignancy surveillance program: comparison with a Japanese population-based study. Transplantation. 2013;95(9):1142–7. https://doi.org/10.1097/TP.0b013e318288ca83.
Krynitz B, Edgren G, Lindelöf B, et al. Risk of Skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008–a Swedish population-based study. Int J Cancer. 2013;132(6):1429–38. https://doi.org/10.1002/ijc.27765.
Piselli P, Serraino D, Segoloni GP, et al. Risk of de novo cancers after transplantation: results from a cohort of 7217 kidney transplant recipients, Italy 1997–2009. Eur J Cancer. 2013;49(2):336–44. https://doi.org/10.1016/j.ejca.2012.09.013.
Schrem H, Kurok M, Kaltenborn A, et al. Incidence and long-term risk of de novo malignancies after liver transplantation with implications for prevention and detection. Liver Transpl. 2013;19(11):1252–61. https://doi.org/10.1002/lt.23722.
Cheung CY, Lam MF, Chu KH, et al. Malignancies after kidney transplantation: Hong Kong renal registry. Am J Transplant. 2012;12(11):3039–46. https://doi.org/10.1111/j.1600-6143.2012.04209.x.
Engels EA, Pfeiffer RM, Fraumeni JF Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891–901. https://doi.org/10.1001/jama.2011.1592.
Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10(8):1889–96. https://doi.org/10.1111/j.1600-6143.2010.03181.x.
Aberg F, Pukkala E, Höckerstedt K, Sankila R, Isoniemi H. Risk of Malignant Neoplasms after liver transplantation: a population-based study. Liver Transpl. 2008;14(10):1428–36. https://doi.org/10.1002/lt.21475.
Jiang Y, Villeneuve PJ, Fenton SS, Schaubel DE, Lilly L, Mao Y. Liver transplantation and subsequent risk of cancer: findings from a Canadian cohort study. Liver Transpl. 2008;14(11):1588–97. https://doi.org/10.1002/lt.21554.
Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. 2007;7(4):941–8. https://doi.org/10.1111/j.1600-6143.2007.01736.x.
Au E, Wong G, Chapman JR. Cancer in kidney transplant recipients. Nat Rev Nephrol. 2018;14(8):508–20. https://doi.org/10.1038/s41581-018-0022-6.
Acuna SA. Etiology of increased cancer incidence after solid organ transplantation. Transpl Rev (Orlando). 2018;32(4):218–24. https://doi.org/10.1016/j.trre.2018.07.001.
Cheung CY, Tang SCW. An update on cancer after kidney transplantation. Nephrol Dial Transplant. 2019;34(6):914–20. https://doi.org/10.1093/ndt/gfy262.
Acuna SA, Sutradhar R, Kim SJ, Baxter NN. Solid Organ Transplantation in patients with Preexisting malignancies in Remission: a propensity score matched Cohort Study. Transplantation. 2018;102(7):1156–64. https://doi.org/10.1097/TP.0000000000002178.
Henley SJ, Ward EM, Scott S, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2020;126(10):2225–49. https://doi.org/10.1002/cncr.32802.
Buxeda A, Redondo-Pachón D, Pérez-Sáez MJ, Crespo M, Pascual J. Sex differences in cancer risk and outcomes after kidney transplantation. Transpl Rev (Orlando). 2021;35(3):100625. https://doi.org/10.1016/j.trre.2021.100625.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
WY, LYN, ZYS: Conceptualization. WY, LYN, GHF, SF, SHW: Data curation; Formal analysis; Methodology. LYN and SF: Supervision. WY, LYN, ZYS: Writing - original draft. WY, LYN, GHF, SF, SHW, ZYS: Writing - review & editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
: Table S1 Search strategies
Supplementary Material 2
: Table S2 Risk of bias summary from NOS scale
Supplementary Material 3
: PRISMA(2020) checklist
Supplementary Material 4
: PRISMA Flow Diagram
Supplementary Material 5
: CRD4202022343633
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, C., Geng, H., Li, Y. et al. Incidence of renal cell carcinoma after solid organ transplantation: a systematic review and meta-analysis. BMC Urol 24, 11 (2024). https://doi.org/10.1186/s12894-023-01389-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-023-01389-1