Abstract
Background and aims
There are no clear conclusions as to whether heart failure (HF) and coronary heart disease (CAD) increase the risk of erectile dysfunction (ED).In our study, we used Mendelian randomization (MR) analysis to discover a causal relationship between HF, CAD and ED.
Methods
Single nucleotide polymorphisms (SNPs) associated with HF, CAD and ED were obtained from the MRC IEU Open Genome-Wide Association Study (GWAS) database.After a series of screenings, the remaining SNPs were selected as instrumental variables (IVs) for HF and CAD for MR analysis to assess the relationship between genetically predicted HF or CAD and the pathogenesis of ED.Among them, we used the random-effects inverse variance weighted (IVW) method as the primary analysis method.Finally, Cochran’s q-test, funnel plots, MR-Egger regression, Leave-one-out method and MR-PRESSO were used for sensitivity analysis.
Results
In the IVW method, there was no significant causal relationship between genetically predicted HF and CAD and the incidence of ED.(HF: OR = 1.17, 95% CI 0.99–1.39; p = 0.074;CAD: OR = 1.08, 95% CI 0.99–1.17, p = 0.068)。The results of sensitivity analyses supported our conclusion that no horizontal pleiotropism was found.
Conclusion
This study did not find a causal relationship between HF or CAD and ED in European populations, which requires further in-depth research.
Similar content being viewed by others
Introduction
Erectile dysfunction (ED) is one of the most common disorders in men and is defined as the long-term inability to achieve or maintain a penile erection. Erectile dysfunction is very important for the well-being and health of men because it not only affects the individual but also causes stress on the lifestyle and relationship of the couple [1].In the United States, at least 12 million men between the ages of 40 and 79 have ED [2].There are many risk factors for ED, including age, coronary artery disease, obesity, smoking, depression, hypertension, previous pelvic surgery, and spinal cord injury, among other psychological factors [3].
Cardiovascular diseases are the largest contributor to the global burden of non-communicable diseases, accounting for 17.9 million deaths (one-third of all deaths) and 45 per cent of deaths due to non-communicable diseases.In Europe, CVD caused 3.9 million deaths (45% of deaths).With the current decline in CVD mortality and the increasing aging of the population, the number of patients with CVD is increasing [4].Among cardiovascular diseases, the relationship between heart failure (HF) and coronary artery disease (CAD) and ED has been more studied.Some foreign studies show that 74–84% of men with HF have ED [5,6,7,8].In addition, most CAD patients are often affected by ED [9,10,11].Therefore, we often think that there is a strong correlation between HF and CAD and ED.
To the best of our knowledge, the available studies are primarily based on observational epidemiological designs and are susceptible to reverse causation and unmeasured confounding factors,failure to correctly understand the causal relationship between the two diseases [12]。To avoid this, Mendelian randomization (MR) has the advantage of using genetic variation as a tool variable, addressing bias in observational studies and thus providing an alternative way to explore causality [13, 14].In this study, we used the MR approach to investigate the causal relationship between the occurrence of ED in HF and CAD.
Materials and methods
Design and participants
This study used a two-sample MR design to detect a potential causal relationship between HF and CAD and ED risk.The hypotheses of the MR study include three conditions:(i)Tool variables (IVs) should be associated with exposure to HF and CAD;(ii)There was no clear correlation between IVs and confounders;(iii)IVs affect the risk of ED only through exposure (HF or CAD) and not through other means [15].Only when all three conditions are met can MR design control for potential confounding factors and provide reliable causal impact estimates, proving causal relationships between the two [16].Data on SNPs’ association with HF, CAD, and ED comes from publicly available large-scale genome-wide association studies (Gwas) and can be downloaded from the MRC IEU Open Gwas dataset.As aggregated data on exposure HF, download at GWAS ID: ebi-a-GCST009541 [17], This GWAS study included 47,309 cases and 930,014 controls;As summary statistics for exposed CAD, download them at GWAS ID: ebi-a-GCST005195 [18], The GWAS study included 122,733 cases and 424,528 controls.Summary, ED-related data as outcome variables are available at GWAS ID: ebi-a-GCST006956 [19],The GWAS study collected 6175 cases and 217,630 controls. All patients and controls were European populations.
In addition, all data from MR is publicly accessible (https://gwas.mrcieu.ac.uk/; Last visited on November 7, 2022).The study waived ethical approval, and all subjects in the original genome-wide association study received informed consent.
Selection of genetic variants
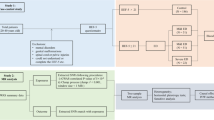
In this study, we obtained SNPs (p < 5 × 10 − 8) significantly correlated with CAD from GWAS aggregated data [20, 21], At the same time, we relaxed the GWAS p-value threshold for HF to 5 × 10 − 6,In order to obtain the appropriate number of SNPs for subsequent analysis [22].Then, we used the PLINK clumping method to calculate the LD through the two-sample MR package and selected independent SNPs with the following conditions (R2 < 0.001, window size = 10,000 kb) [23], to ensure that all the left IVs for HF and CAD are not in linkage disequilibrium (LD). We estimate the strength of the IVs on the basis of the F statistic. The formula is as follows: F = R2(N-2) (1-R2) (R2: variance of exposure explained by selected instrumental variables; N:sample size) [24]; R2 = 2×EAF× (1-EAF)× beta^2/((2×EAF× (1-EAF)× beta^2) + 2× EAF× (1-EAF)× se× N× beta^2) (beta: effect size for SNP; se: standard error for SNP; N:sample size) [25]. IVs were selected whose F > 10. After harmonizing the SNPs in the data source by effector alleles [26], we discoveryed each instrument SNP in the PhenoScanner GWAS database [27] to assess any prior association (P < 5 × 10 − 8) with possible confounding factors (that is Body mass index and Cardiovascular diseases other than the current study diseases) [28,29,30] to avoid potential confounding. Finally, the SNPs left were selected as IVs for the following MR test.
Statistical analysis
In the study, we applied the random-effects inverse-variance weighted (IVW) method as the main analysis to evaluate the casual relation of genetically predicted HF and CAD with the risk of ED [31]. Other methods including MR Egger [32], weighted-median [33], weighted mode [34] and simple mode [35] were also applied. Besides, several sensitivity analyses were carried out to evaluate the strength of the association. First, Cochran’s Q test and funnel plots were performed to assess the heterogeneity [36]. Second, we applied MR Egger regression to recognize the existence of directional pleiotropy by calculating whether the intercept was statistically away from zero [32]. Third, we used the Leave-one-out method to verify the robustness of the findings [37]. Fourth, in order to detect possible outliers, we apply the MR pleiotropy residual sum and outlier (MR-PRESSO) test [38]. We used odds ratios (ORs) with their 95% confidence intervals (CIs) to present the associations between HF and CAD and risk of ED and applied RStudio (version 2022.02.3) with ‘TwoSampleMR’ and ‘MRPRESSO’ to perform MR analyses. In this study, p < 0.05 was considered a statistically significant difference.
Results
Genetically predicted HF on ED
After the above selection (the specific flow chart is shown in Figs. 1), 30 IVs were left, accounting for approximately 2.6% of the observed variance of hf (the F-statistics range from 53.0 to 262.5) (Supplementary Table 1). Genetically predicted HF was not related to ED (OR = 1.17, 95% CI 0.99–1.39; p = 0.074) in the IVW analyses (Fig. 2A). The consistent results were obtained in the weighted median approaches (OR = 1.19, 95% CI = 0.93–1.51, p = 0.164), weighted mode approaches (OR = 1.51, 95% CI = 0.87–2.62, p = 0.154), simple mode (OR = 0.99, 95% CI = 0.56–1.73, p = 0.963) and MR-Egger regression (OR = 1.31, 95% CI = 0.76–2.26, p = 0.333) (Fig. 2B). There was no heterogeneity found by a Cochran Q test (P = 0.731 of MR-Egger; p = 0.766 of IVW) (Table 1) and funnel plots (Supplementary Fig. 1). The MR-Egger intercept did not deviate significantly from zero with a p-value of 0.661(Table 1). The leave-one-out test showed that there were no significant differences (Supplementary Fig. 2) and the MR-PRESSO test did not find any outliers.
Genetically predicted CAD on ED
After the above selection (the specific flow chart is shown in Figs. 1), 61 IVs were left, accounting for approximately 10.9% of the observed variance of CAD and all the F-statistics were above 10, ranging from 66.6 to297.5 (Supplementary Table 2). Genetically predicted was not related to risk of ED (OR = 1.08, 95% CI 0.99–1.17, p = 0.068) in the IVW analyses (Fig. 2B). Meanwhile, similar results were discovered by weighted mode approaches (OR = 1.15, 95% CI = 0.99–1.33, p = 0.074), simple mode (OR = 1.09, 95% CI = 0.85–1.40, p = 0.481) and MR-Egger regression (OR = 1.15, 95% CI = 0.98–1.35, p = 0.101). No heterogeneity was found in the study with a Cochran Q-test (P = 0.728 of MR-Egger; p = 0.734 of IVW) (Table 1) and funnel plots (Supplementary Fig. 3). The MR Egger intercept did not deviate significantly from zero with a p-value of 0.387 (Table 1). The leave-one-out test also generally support our results while we removed a single SNP and applied the MR analysis again, demonstrating our results’ robustness (Supplementary Fig. 4). By using the MR-PRESSO test, Outliers are not found, verifying the absence of unknown pleiotropic effects of the genetic instruments.
Discussion
In this study, we used a two-sample MR analysis method to investigate the causal relationship between HF or CAD and ED risk.However, in our results, we did not find a significant causal relationship between HF or CAD and ED.
To our knowledge, the relationship between HF and ED is currently unclear.However, many scholars believe that HF patients are often accompanied by ED, and there is a certain correlation between the two.An observational study by Andrea Crafa et al. showed that arterial ED is strongly associated with cardiovascular risk [39].In a cross-sectional study, Zeighami Mohammadi et al. studied 100 men with systolic heart failure (HF), by filling in the International Erectile Function Index-5 item (IIEF-5), the Minnesota Heart Failure Living Questionnaire (MLHFQ) assesses the extent of its ED and HF, It was found that 80% of patients with HF had ED, of these, 36% suffered from severe erectile dysfunction [5]. In the study of Medina et al. [6], Schwarz et al. [7]and Rastogi et al. [8] also found that patients with HF had a higher prevalence of erectile dysfunction in 74%, 84%, and 75%, respectively.
Many researchers believe that the mechanisms of ED in HF patients are diverse, such as endothelial dysfunction, reduced exercise tolerance, heart drugs, and HF-related hypogonadism [40]. (1) Endothelial dysfunction.This pathology is closely related to HF, which may cause reduced NO production and limited vasodilation, which in turn causes ED [41, 42]. (2) Exercise tolerance disorders.Depending on the severity of cardiac function, exercise tolerance in people with HF decreases to varying degrees, from limitations in physical exertion to limitations in basic activities of daily living. Therefore, although the physical work associated with sexual activity is relatively moderate [43], but some people with HF, especially those with NYHA class III-IV, may not be able to afford the energy expenditure of sex.A study by Basile L et al. showed that the vasodilatory effect of sildenafil improved athletic performance. This also reflects the correlation between HF and ED [44]0.3. Cardiac medication:Because HF-modifying drugs have multiple vascular, metabolic, and neurohumoral effects, many cardiovascular drugs (such as thiazide diuretics, β blockers, and lipid-lowering drugs) have been found to negatively affect erectile function [45, 46]. 4. Anabolic disorders:Metabolic imbalances are typical of patients with HF and often lead to increased catabolism and cardiac cachexia.Anabolic hormones, including insulin-like growth factor-1 (IGF-1), dehydroepiandrosterone sulfate (DHEA-S), and total testosterone (TT), could enhance exercise tolerance in healthy men.A decrease in these hormones can lead to a decrease in exercise capacity.Antonio Aversa et al. reported that decreased androgen production leads to the development of late-onset hypogonadism, which is characterized by erectile dysfunction (ED) and hypogonadism [47].Many studies have shown an increased prevalence of anabolic disorders in HF. Jankowska et al. evaluated 208 patients with HF of various etiologies and found that TT and DHEA-S levels were inversely correlated with NYHA grades [48]. The common clinical manifestation of anabolic disorders and hypogonadism is ED.To further investigate the direct causal relationship of HF for ED, we used the Mendelian randomization method and found that HF did not directly contribute to the risk of developing ED in our study.Therefore, the relationship between HF and ED needs further study.
Similarly, the relationship between CAD and ED is not clear.In a cross-sectional observational study, Kałka et al. [9]recruited 751 rehabilitative CAD patients in five cardiac rehabilitation centers, and found that ED was present in 568 (75.63%) of the patients. In addition, many scholars believe that the coexistence of clinically obvious CVD and ED symptoms is a common phenomenon [10, 11]. Regarding the co-existence mechanism between the two, endothelial dysfunction is considered to be a common risk factor for both diseases, causing the occurrence of both diseases [10, 49]. However, no causal studies have been conducted on the relationship between the two.In our study, we further explored whether CAD directly causes ED.Finally, our results suggest that CAD does not directly cause ED.Further confirmation of the coexistence between the two diseases may be caused by common risk factors, but further confirmation is needed.
The MR study design is one of the greatest strengths of this study. This approach can reverse causality inherent and minimize residual confounding in observational studies. Besides, it can allow us to discovery potential causal relationships between erectile dysfunction and CAD or HF. The study can further support the results through other secondary analytical approaches and sensitivity analyses, increasing the reliability of our conclusions. In addition, we extracted the instrumental variables from the most recent GWAS available with confidence to minimize weak instrumental bias.
However, there were some several limitations. First, the data from GWASs of this study came from European, so that the similar study should be investigated in other populations. Second, there are different subtypes of ED (non-vascular or vascular), which were not distinguished in this study. Subsequent studies could be devoted to ED analysis of different subgroups. For example, ED was divided into two groups, non-vascular and vascular, and MR Was used for analysis. The conclusions of each group were compared to see whether there would be a positive result for vascular ED.Similarly, a targeted study on the different types of HF (high ejection fraction or low ejection fraction), could provide further information about the association between these conditions, to formulate predictive parameters of severity or even response to therapy.Thirdly, only 2.6% of the observed variance in HF was explained by IVs, so the statistical power may be insufficient. Therefore, for this negative result, we need to interpret it with caution to avoid drawing this conclusion due to insufficient power.
Conclusion
This is the first study to explore the causal relationship between HF, CAD and ED. We did not find a causal relationship between HF or CAD and ED in European populations, which requires further in-depth research to verify.
Data Availability
Data on heart failure and coronary artery disease was provided by Shah et al. (2020) and van der Harst et al. (2017). Data on erectile dysfunction was provided by Bovijn et al. (2019). The datasets generated and analysed during the current study are available in the IEU open gwas project [https://gwas.mrcieu.ac.uk/datasets/], and the GWAS ID are ebi-a-GCST009541, ebi-a-GCST005195 and ebi-a-GCST006956, respectively.
References
Krzastek SC, Bopp J, Smith RP, et al. Recent advances in the understanding and management of erectile dysfunction. F1000Res. 2019;8:F1000FacultyRev–102. https://doi.org/10.12688/f1000research.16576.1. Published 2019 Jan 25.
Sangiorgi G, Cereda A, Benedetto D, et al. Anatomy, pathophysiology, Molecular Mechanisms, and Clinical Management of Erectile Dysfunction in Patients affected by coronary artery disease: a review. Biomedicines. 2021;9(4):432. https://doi.org/10.3390/biomedicines9040432.
McCabe MP, Sharlip ID, Lewis R, et al. Risk factors for sexual dysfunction among women and men: a Consensus Statement from the Fourth International Consultation on sexual Medicine 2015. J Sex Med. 2016;13(2):153–67.
Wilkins E, Wilson L, Wickramasinghe K et al. European Cardiovascular Disease Statistics 2017 Brussels. Eur Heart Netw. 2017.
Zeighami Mohammadi S, Shahparian M, Fahidy F, et al. Sexual dysfunction in males with systolic heart failure and associated factors. ARYA Atheroscler. 2012;8(2):63–9.
Medina M, Walker C, Steinke EE, et al. Sexual concerns and sexual counseling in heart failure. Prog Cardiovasc Nurs. 2009;24(4):141–8.
Schwarz ER, Kapur V, Bionat S, et al. The prevalence and clinical relevance of sexual dysfunction in women and men with chronic heart failure. Int J Impot Res. 2008;20(1):85–91.
Rastogi S, Rodriguez JJ, Kapur V, et al. Why do patients with heart failure suffer from erectile dysfunction? A critical review and suggestions on how to approach this problem. Int J Impot Res. 2005;17(Suppl 1):25–S36.
Kałka D, Gebala J, Biernikiewicz M, et al. Erectile Dysfunction in Men burdened with the familial occurrence of coronary artery disease. J Clin Med. 2021;10(18):4046. https://doi.org/10.3390/jcm10184046.
Solomon H, Man JW, Wierzbicki AS, et al. Relation of erectile dysfunction to angiographic coronary artery disease. Am J Cardiol. 2003;91:230–1.
Montorsi F, Briganti A, Salonia A, et al. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur Urol. 2003;44:360–5.
Davey Smith G, Phillips AN. Correlation without a cause: an epidemiological odyssey. Int J Epidemiol. 2020;49(1):4–14. https://doi.org/10.1093/ije/dyaa016.
Burgess S, Foley CN, Zuber V. Inferring Causal Relationships between Risk factors and outcomes from genome-wide Association Study Data. Annu Rev Genomics Hum Genet. 2018;19:303–27. https://doi.org/10.1146/annurev-genom-083117-021731.
Davey Smith G, Holmes MV, Davies NM, et al. Mendelian randomization and causal inference in observational data: substantive and nomenclatural issues. Eur J Epidemiol. 2020;35(2):99–111. https://doi.org/10.1007/s10654-020-00622-7.
Lawlor DA, Commentary. Two-sample mendelian randomization: opportunities and challenges. Int J Epidemiol. 2016;45(3):908–15. https://doi.org/10.1093/ije/dyw127.
Lawlor DA, Harbord RM, Sterne JA, et al. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–63. https://doi.org/10.1002/sim.3034.
Shah S, Henry A, Roselli C, et al. Genome-wide association and mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. 2020;11(1):163. https://doi.org/10.1038/s41467-019-13690-5.
van der Harst P, Verweij N. Identification of 64 Novel genetic loci provides an expanded view on the Genetic Architecture of Coronary Artery Disease. Circ Res. 2018;122(3):433–43. https://doi.org/10.1161/CIRCRESAHA.117.312086.
Bovijn J, Jackson L, Censin J, et al. GWAS identifies risk locus for Erectile Dysfunction and implicates hypothalamic neurobiology and diabetes in etiology. Am J Hum Genet. 2019;104(1):157–63. https://doi.org/10.1016/j.ajhg.2018.11.004).
Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. https://doi.org/10.1038/nature11582.
Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979–86. https://doi.org/10.1038/ng.3359.
Zhu Z, Zheng Z, Zhang F, et al. Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat Commun. 2018;9(1):224. https://doi.org/10.1038/s41467-017-02317-2.
Li H, Wen Z. Effects of ulcerative colitis and Crohn’s disease on neurodegenerative diseases: a mendelian randomization study. Front Genet. 2022;13:846005. https://doi.org/10.3389/fgene.2022.846005.
Burgess S, Thompson SG, CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–64. https://doi.org/10.1093/ije/dyr036.
Papadimitriou N, Dimou N, Tsilidis KK, et al. Physical activity and risks of breast and colorectal cancer: a mendelian randomization analysis. Nat Commun. 2020;11(1):597. https://doi.org/10.1038/s41467-020-14389-8. Published 2020 Jan 30.
Hartwig FP, Davies NM, Hemani G, et al. Two-sample mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. 2016;45(6):1717–26. https://doi.org/10.1093/ije/dyx028.
Kamat MA, Blackshaw JA, Young R, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35(22):4851–3. https://doi.org/10.1093/bioinformatics/btz469.
Minhas S, Bettocchi C, Boeri L, et al. European Association of Urology Guidelines on male sexual and Reproductive Health: 2021 update on male infertility. Eur Urol. 2021;80(5):603–20. https://doi.org/10.1016/j.eururo.2021.08.014.
Wang M, Jian Z, Gao X et al. Causal Associations Between Educational Attainment and 14 Urological and Reproductive Health Outcomes: A Mendelian Randomization Study. Front Public Health. 2021;9:742952. Published 2021 Oct 28. https://doi.org/10.3389/fpubh.2021.742952.
Xiong Y, Zhang FX, Zhang YC, et al. Genetically predicted insomnia causally increases the risk of erectile dysfunction. Asian J Androl. 2022. https://doi.org/10.4103/aja202261.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–65. https://doi.org/10.1002/gepi.21758.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25. https://doi.org/10.1093/ije/dyv080.
Bowden J, Davey Smith G, Haycock PC, et al. Consistent estimation in mendelian randomization with some Invalid Instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14. https://doi.org/10.1002/gepi.21965.
Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–98. https://doi.org/10.1093/ije/dyx102.
Zhu G, Zhou S, Xu Y, et al. Mendelian randomization study on the causal effects of omega-3 fatty acids on rheumatoid arthritis. Clin Rheumatol. 2022;41:1305–12. https://doi.org/10.1007/s10067-022-06052-y.
Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. https://doi.org/10.1136/bmj.d4002.
Burgess S, Bowden J, Fall T, et al. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017;28(1):30–42. https://doi.org/10.1097/EDE.0000000000000559.
Verbanck M, Chen CY, Neale B, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8. https://doi.org/10.1038/s41588-018-0099-7.
Crafa A, Condorelli RA, Mongioì LM, Cannarella R, Barbagallo F, Aversa A, Izzo G, Perri A, Calogero AE, La Vignera S. Mean platelet volume as a marker of Vasculogenic Erectile Dysfunction and Future Cardiovascular Risk. J Clin Med. 2020;9(8):2513.
Alberti L, Torlasco C, Lauretta L, et al. Erectile dysfunction in heart failure patients: a critical reappraisal. Andrology. 2013;1(2):177–91. https://doi.org/10.1111/j.2047-2927.2012.00048.x.
Piatti PM, Monti LD, Galli L, et al. Relationship between endothelin-1 concentration and metabolic alterations typical of the insulin resistance syndrome. Metabolism. 2000;49(6):748–52. https://doi.org/10.1053/meta.2000.6257.
Costa C, Virag R. The endothelial-erectile dysfunction connection: an essential update. J Sex Med. 2009;6(9):2390–404. https://doi.org/10.1111/j.1743-6109.2009.01356.x.
Thorson AI. Sexual activity and the cardiac patient. Am J Geriatr Cardiol. 2003;12(1):38–40. https://doi.org/10.1111/j.1076-7460.2003.01755.x.
Basile L, Marino M, La Vignera S. Is sildenafil a doping drug in hypoxic conditions? Aging Male. 2022;25(1):156–8.
Grimm RH Jr, Grandits GA, Prineas RJ, et al. Long-term effects on sexual function of five antihypertensive drugs and nutritional hygienic treatment in hypertensive men and women. Treatment of mild hypertension study (TOMHS). Hypertension. 1997;29(1 Pt 1):8–14. https://doi.org/10.1161/01.hyp.29.1.8.
Karavitakis M, Komninos C, Theodorakis PN, et al. Evaluation of sexual function in hypertensive men receiving treatment: a review of current guidelines recommendation. J Sex Med. 2011;8(9):2405–14. https://doi.org/10.1111/j.1743-6109.2011.02342.x.
Aversa A, Duca Y, Condorelli RA, Calogero AE, La Vignera S. Androgen Deficiency and phosphodiesterase type 5 expression changes in Aging Male: therapeutic implications. Front Endocrinol (Lausanne). 2019;10:225.
Jankowska EA, Filippatos G, Ponikowska B, et al. Reduction in circulating testosterone relates to exercise capacity in men with chronic heart failure. J Card Fail. 2009;15(5):442–50. https://doi.org/10.1016/j.cardfail.2008.12.011.
La Vignera S, Condorelli RA, Cannarella R, Giacone F, Calogero AE. Arterial erectile dysfunction is an early sign of vascular damage: the importance for the prevention of cardiovascular health. Ann Transl Med. 2019;7(Suppl 3):124.
Acknowledgements
We thank this network for providing the main data (https://gwas.mrcieu.ac.uk/).
Author information
Authors and Affiliations
Contributions
Kaiyang Shao , Weikang Chen, Yaling Li and Ting Sun conceived and designed the analysis; Kaiyang Shao , Weikang Chen, Yaling Li and Huiyan Zheng performed the analysis; Kaiyang Shao and Weikang Chen wrote the manuscript; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shao, K., Chen, W., Li, Y. et al. Effects of heart failure and coronary artery disease on erectile dysfunction: a two-sample mendelian randomization study. BMC Urol 23, 163 (2023). https://doi.org/10.1186/s12894-023-01335-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-023-01335-1