Abstract
Background
Germline mutations represent a high risk of hereditary cancers in population. The landscape and characteristics of germline mutations in genitourinary cancer are largely unknown, and their correlation with patient prognosis has not been defined.
Methods
Variant data and relevant clinical data of 10,389 cancer patients in The Cancer Genome Atlas (TCGA) database was downloaded. The subset of data of 206 genitourinary cancer patients containing bladder urothelial carcinoma (BLCA), kidney chromophobe carcinoma (KICH), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP) and prostate adenocarcinoma (PRAD) cancer with germline mutation information was filtered for further analysis. Variants were classified into pathogenic, likely pathogenic and non-pathogenic categories based on American College of Medical Genetics and Genomics (ACMG) guidelines. Genome Aggregation Database (gnomAD) database was used to assist risk analysis.
Results
There were 48, 7, 44, 45 and 62 patients with germline mutations identified in BLCA, KICH, KIRC, KIRP and PRAD, respectively. Pathogenic germline mutations from 26 genes and likely pathogenic mutations from 33 genes were revealed. GJB2, MET, MUTYH and VHL mutations ranked top in kidney cancers, and ATM and CHEK2 mutations ranked top for bladder cancer, while ATM and BRCA1 mutations ranked top for prostate cancer. Frameshift, stop gained and missense mutations were the predominant mutation types. BLCA exhibited the highest ratio of stop gained mutations (22/48 = 45.8%). No difference in patient age was found among pathogenic, likely pathogenic and non-pathogenic groups for all cancer types. The number of male patients far overweight female patients whether PRAD was included (P = 0) or excluded (P < 0.001). Patients with pathogenic or likely pathogenic germline mutations exhibited significantly worse overall survival rate than the non-pathogenic group for all genitourinary cancers. More important, analyses assisted by gnomAD database revealed that pathogenic or likely pathogenic germline mutations significantly increased the risk for genitourinary cancer in population, with the odds ratio at 14.88 (95%CI 11.80–18.77) and 33.18 (95%CI 24.90–44.20), respectively.
Conclusions
The germline mutational status for genitourinary cancers has been comprehensively characterized. Pathogenic and likely pathogenic germline mutations increased the risk and indicated poor prognosis of genitourinary cancers.
Similar content being viewed by others
Introduction
Genitourinary cancers include bladder carcinoma (BLCA), kidney chromophobe carcinoma (KICH), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KRIP) and prostate adenocarcinoma (PRAD). The age-standardized rate (ASR) of bladder cancer, renal cell cancers and prostate cancer was reported to be 9.0 [1] 15.80 [2] and 29.3 [3] per 100,000 people in men, respectively, and 2.2 [1] and 7.56 [2] per 100,000 people in women, respectively. People in North American and Europe generally exhibited higher incidence than people in Asia and Africa [1,2,3].
Approximately 10–15% of cancer patients belong to hereditary cancer, characterized by strong hereditary background known as pathogenic germline mutations [4,5,6]. These patients generally inherit pathogenic mutations from their parents and have high risk of cancer than people without germline mutations. Many of them show cancer phenotypes at earlier stage of life than average risk population who may have sporadic cancers at elder ages. They may pass germline mutations to the next generation, thus increasing the cancer risk of their children. Genitourinary cancer with germline mutations represents a specific type of cancers with strong hereditary background. Reports on individual genitourinary cancer types showed strong link between the onset of cancer with pathogenic germline mutations, including prostate cancer [7] and urothelial cancer [8]. Genes involved in germline alterations of genitourinary cancer included those in DNA damage and repair (DDR) pathways, such as ATM, BRCA1, BRCA2, MLH1 and MSH2 [9, 10].
Although there are some reports available on germline mutations in certain types of genitourinary cancers, the full profile and characteristics of germline alterations in all genitourinary cancers have not been investigated in detail. It is also unclear on the correlation between germline alterations and patient phenotypes and prognosis. The risk caused by germline alterations in genitourinary cancers has not been quantified. Here we performed a database study and characterized the profile of germline mutations and their links with patient phenotypes, risk and prognosis for five individual genitourinary cancers. We aimed to provide useful information for future prevention, early intervention and treatment of genitourinary cancer patients with germline alterations.
Methods and materials
Germline variants and relevant clinical data of 10,389 cancer patients corresponding to 33 cancer types were downloaded from the TCGA database (generated by Huang et al.[11]) as the input dataset (https://www.sciencedirect.com/science/article/pii/S0092867418303635). A subset of 206 genitourinary cancer patients containing BLCA, KICH, KIRC, KIRP and PRAD were filtered for further analysis. All variants were classified into pathogenic, likely pathogenic and non-pathogenic subgroups based on American College of Medical Genetics and Genomics (ACMG) guidelines [12]. A summary of the patient demographic and clinicopathological information is presented as Table 1.
Information on variants from different variant categories was collected, and was grouped by gene names or cancer types, and was ranked in descending order to identify the high-frequency variants. The distribution of mutational categories and pathogenicity was plotted by R software. Variants in representative genes were displayed as lollipop plots by the R software. Variants located outside the exon regions were not displayed in plots. Wilcoxon tests were performed to compare the age among groups with different pathogenicity. Chi-square test or fisher exact test was used to determine the significance among rates or percentage. The Kaplan–Meier analysis and log-rank test were used to analyze and compare the overall survival rate among different groups. Variant frequency data in population from the gnomAD database was used to calculate the risk of germline mutations. The odds ratio (OR) values were calculated based on the variant frequency from the database and in this study. The significance of OR values was assessed by Fisher’s exact test and P values were adjusted by the Benjamini and Hochberg (BH) method. P < 0.05 was regarded as statistical significant difference.
Results
The landscape and characteristics of germline mutations in genitourinary cancer
The mutation data of a total of 206 patients with genitourinary cancers were collected. The demographic and clinicopathological information of all patients is summarized in Table 1. The number of male patients far overweight that of the female patients (P = 0.013). The race of patients was mainly white with cancer stage information invalid for the majority of patients. No difference in age was found among the pathogenic, likely pathogenic and non-pathogenic groups. Five cancers were involved in this study, including BLCA, KICH, KIRC, KIRP and PRAD.
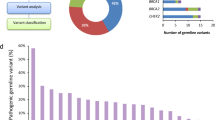
The distribution of germline mutations in genitourinary cancers was investigated first. All germline mutations were divided into pathogenic, likely pathogenic and non-pathogenic based on ACMG guidelines. The numbers and types of pathogenic and likely pathogenic mutations for kidney, bladder and prostate cancers are shown in Fig. 1A. It can be observed that for pathogenic mutations, GJB2, MET, MUTYH and VHL ranked top in kidney cancer, and ATM and CHEK2 ranked top for bladder cancer, while ATM and BRCA1 ranked top for prostate cancers. For likely pathogenic mutations, FANCM and FH ranked top for kidney cancer, RAD51C ranked top for bladder cancer, and ATM and PMS2 ranked top for prostate cancer. Pathogenic germline mutations from 26 genes and likely pathogenic mutations from 33 genes are shown in Fig. 1B. ATM germline mutations ranked the first in number in all pathogenic mutations, followed by BRCA1, CHEK2, VHL and BLM. ATM also ranked the highest in likely pathogenic mutations, followed by FANCM, FH and PMS2 (Fig. 1B). The distribution of mutation types for all involved genes is shown in Fig. 1B, grouped by mutation pathogenicity (pathogenic or likely pathogenic). The main mutation types included frameshift, stop gained and missense mutations, while other less frequent types, such as splicing and start lost mutations were also present (Fig. 1B). The mutational distribution of representative genes is shown in Fig. 1C, including ATM, BRCA1, BRCA2, PMS2, BLM and VHL. The mutational distribution appeared to be random and no obvious hotspot mutations were found.
The germline mutational status and distribution for kidney, bladder and prostate cancer. A the number of pathogenic and likely pathogenic mutations for each individual cancers; B the mutation types for genes with pathogenic or likely pathogenic mutations in all cancers. C schemes show the distribution of individual germline mutations for representative genes. Please be noted that variants located outside the exon region, mainly splicing variants, were not plotted in the schemes
The mutational distribution of the five types of genitourinary cancers is shown in Fig. 2A. There were 48, 7, 44, 45 and 62 patients with mutations found in BLCA, KICH, KIRC, KRIP and PRAD, respectively (Fig. 2A, Table 2). ATM, BRCA1, PMS2 and BRCA2 were among those with highest number of mutations. The distribution of mutation types for each cancer type is shown in Fig. 2B. BLCA had higher ratio of stop gained mutations (22/48 = 45.8%) compared with other four types of cancers (44/158 = 27.8%) (P = 0.019), suggesting a preference of the specific type of germline mutation for BLCA (Table 2). The status of pathogenicity for each gene is shown in Fig. 2C. Pathogenic mutations were mainly found in high frequency mutated genes while non-pathogenic mutations were also present in large majority of genes. This can also be observed in Fig. 2D, in which the status of pathogenicity for each cancer type is shown. No difference in the distribution of pathogenicity status across the five cancer types was found (P = 0.74) (Table 2).
Germline mutational status in five genitourinary cancers. A the distribution of germline mutations for each gene in BLCA, KICH, KIRC, KIRP and PRAD; B landscape of mutation types for each individual cancer types; C distribution of mutation pathogenicity across all involved genes; D landscape of mutation pathogenicity for each individual cancer types
The influences of germline mutations on patient phenotypes and prognosis
We further investigated the correlation between germline mutations and patient age and sex. It can be clearly seen from Fig. 3 that no difference in patient age was found among pathogenic, likely pathogenic and non-pathogenic groups for all genitourinary cancer patients or five individual cancer types (Fig. 3, as labeled), suggesting that the germline mutations had no influence on cancer onset age. The number of male patients far overweight that of the female patients (male:female = 160:46) for all patients across the five cancer types (P < 0.001), and the observation was also true even if PRAD patients were excluded (male:female = 98:46) (P = 0.00039) (Table 2). Interestingly, the number of female overweight that of the male in KIRC (female:male = 25:19), exhibiting significant difference to other genitourinary cancer (P < 0.001), even if PRAD was excluded (P = 0.00002) (Table 2).
The influence of germline mutations on patient overall survival was investigated in detail (Fig. 4). Kidney, bladder and prostate cancer patients with no pathogenic germline mutations (non-pathogenic group, green lines) exhibited significantly better overall survival than those with pathogenic (orange lines) or likely pathogenic mutations (blue lines) (P values are shown as indicated; Fig. 4A). Specifically, we found P < 0.001 when pathogenic or likely pathogenic group was compared with non-pathogenic group in kidney cancer and prostate cancer, and P < 0.01 was found in the above comparisons in bladder cancer. For subtypes of kidney cancers, patients with non-pathogenic mutations exhibited significantly better overall survival rate than those with pathogenic or likely pathogenic mutations in KIRC and KIRP (P values are shown as indicated, Fig. 4B). Specifically, we found P < 0.01 when pathogenic or likely pathogenic group was compared with non-pathogenic group in both KIRC and KIRP. However, no significant difference was found between non-pathogenic and likely pathogenic group in KICH (P > 0.05), possibly due to the limited number of patients in this cancer type. No significant difference in overall survival rate between pathogenic or likely pathogenic groups was found in all cancers (P > 0.05).
The prognosis of kidney, bladder and prostate cancer patients was affected by the pathogenicity of germline mutations. Significantly better overall survival was observed in non-pathogenic group (green lines) compared with pathogenic (orange lines) or likely pathogenic group (blue lines) in kidney, bladder and prostate cancer (A), and in subtypes of kidney cancer, including KIRC and KIRP (B). P values for each cancer are labeled. The P values between any two subgroups are listed below: kidney cancer (P vs. LP = 0.18; P vs. Non-P < 0.001; LP vs. Non-P < 0.001); bladder cancer (P vs. LP = 0.2024; P vs. Non-P = 0.0015; LP vs. Non-P = 0.0011); prostate cancer (P vs. LP = 0.81; P vs. Non-P < 0.001; LP vs. Non-P < 0.001); KICH (P vs. LP = 1.00; P vs. Non-P = 0.27; LP vs. Non-P = 1.00); KIRC (P vs. LP = 0.1108; P vs. Non-P = 0.0014; LP vs. Non-P < 0.001); KIRP (P vs. LP = 0.7304; P vs Non-P = 0.0004; LP vs. Non-P = 0.0004)
The impact of germline mutations on genitourinary cancer risk
To investigate the risk for genitourinary cancers in individuals carrying pathogenic or likely pathogenic germline mutations, the mutation prevalence of all germline mutations in general population was determined by searching the gnomAD (Table 3 for pathogenic and Table 4 for likely pathogenic mutations). By comparing the germline mutation frequency found in this study with the variant prevalence in general population, the overall OR was calculated for germline mutations in this study to avoid the bias from individual mutations. Table 3 presents a summary of demographic information, mutational status and OR for those with pathogenic mutations. The OR for individual mutations in each type of cancer is listed. The overall OR value of all pathogenic mutations was 14.88 (95% CI 11.80–18.77), when compared with the general population. Similarly, Table 4 summarizes the demographic information, mutational status and OR for those with likely pathogenic mutations, and the overall OR value was 33.18 (95% CI 24.90–44.20) when compared with the general population. The significance of each OR was assessed by the adjusted P value and was presented in both tables. These analyses suggested that the pathogenic and likely pathogenic germline mutations were significant risk factors for genitourinary cancer.
Discussion
In this study, we systematically investigated the characteristics of germline mutations and relevant phenotypes in five types of genitourinary cancers, and found a series of features, including highly cancer type-dependent top mutated genes, predominant mutation types with large fragment alterations, male-dominant patient distribution and age-irrelevant cancer onset. We also revealed significant correlation between pathogenic/likely pathogenic mutations and patient prognosis and the risk of genitourinary cancers in population, suggesting them as prognostic and risk factors. Our study established the clinical relevance of these mutations and highlighted the importance of early detection and intervention in population with pathogenic and likely pathogenic germline mutations.
Cancer patients with pathogenic or likely pathogenic germline mutations are a special group of patients with characteristic phenotypes, including early onset cancers, familial aggregation, multiple organ involvement, high level of malignancy, poor therapeutic response and poor prognosis [13,14,15,16]. The most commonly seen cancers with definite causes of germline mutations include Lynch syndrome and hereditary breast and ovarian cancer (HBOC) [17, 18], while recent evidence suggested that a subset patients with pathogenic germline mutations were also predisposed to higher lung cancer risk and familial aggregation [19,20,21]. It was reported that genes responsible for DNA damage repair (DDR) were mainly involved in germline mutations in hereditary cancers [8, 22]. This includes a series of genes, such as MLH1, MSH2, MSH6, PMS2, ATM, BLM, BRCA1, BRCA2, POLE and POLD1 [8, 22, 23]. Germline mutations of these genes may greatly enhance the risk of cancer, and certain group of mutations may correspond to certain cancer types. For example, genes in mismatch repair (MMR) (MLH1, MSH2, MSH6, PMS2, etc.) are predominantly linked to Lynch syndrome, and genes in homologous recombination repair (HRR) (BRCA1 and BRCA2) are mainly involved in HBOC. Other less-frequent germline mutations are less cancer type-specific and may be found in any cancer.
Although hereditary cancers such as Lynch syndrome and HBOC have been widely studied, the germline mutational status in genitourinary cancers and their correlation with prognosis and cancer risk have been largely uninvestigated. This is possibly due to the low incidence of germline mutation-induced genitourinary cancer and the fact that there have been few definite links between certain germline mutations and certain type of genitourinary cancer [24]. We therefore performed a database research and revealed interesting characteristics of germline mutations in genitourinary cancer and established their correlation with patient prognosis. It was not surprising to find that ATM, BRCA1, PMS2 and BRCA2 were among genes with highest number of mutations. As mentioned above, these genes belong to DDR and are sensitive to DNA damage. DNA damage is a common process happened during carcinogenesis, and factors including chronic inflammation, virus infection, carcinogen or toxin can all lead to DNA damage which initiate repair [25,26,27]. In normal tissues of subjects without germline mutations, repetitive damage and repair alter the microenvironment and the normal cellular cycle controlled by a series of epigenetic and genetic mechanisms. Abnormal gene regulation under repetitive damage and repair ultimately leads to accumulation of somatic mutations, and key mutations at driver genes result in malignant transformation of cells [28,29,30]. In contrast, for subjects with germline mutations at DDR genes, the DNA repair mechanism is impaired congenitally, cellular malignant transformation may therefore happen at early stage of life and lead to tumor growth. This is the reason for high cancer incidence and low onset age for people with Lynch syndrome or HBOC-related germline mutations [25,26,27].
ATM gene mutations were also reported in recent studies on germline mutations in non-small cell lung cancer (NSCLC) [21, 31, 32]. It was reported to be the gene with highest germline mutation frequency in western population [32]. Similarly, BRCA1, BRCA2 and PMS2 were also reported as germline mutations in cancers other than Lynch syndrome and HBOC [21, 31, 32]. BLM and VHL genes also contained germline mutations leading to Bloom syndrome [33] and Von Hippel-Lindau (VHL) disease [34], respectively. However, they were also found in other cancers with germline mutations. It is possible that DDR gene germline mutations can cause various types of cancers, with MMR genes preferentially found in Lynch syndrome and HRR genes preferentially found in HBOC. Functional subgroups of DDR genes may differentially affect carcinogenesis of different tissues.
It appeared from our study that bladder cancer and prostate cancer shared some common top mutated genes, while the top mutated genes were quite different in kidney cancer. In kidney cancer, two KIRC and one KIRP patients carried GJB2, three KIRP patients carried MET, one KIRC and two KRIP patients carried MUTYH and three KIRC patients carried VHL germline mutations. GJB2 germline mutations have been previously reported in congenital hearing loss [35] and rarely been reported in cancer [36]. Our study revealed GJB2 germline pathogenic mutations in KIRC and KIRP for the first time, providing new evidence for the pathogenicity of the gene in kidney cancers. In contrast, MET germline mutations have been implicated in many cancers, including KRIP [37], and it was not surprising that we also found MET germline mutations in this study. Similarly, MUTYH germline mutations have also been reported in many cancers, including kidney cancer [38, 39]. The germline VHL mutations have been linked to VHL disease, which is an inheritable condition leading to retinal and central nervous system hemangioblastomas, clear cell renal cell carcinomas, pheochromocytomas, pancreatic neuroendocrine tumors and endolymphatic sac tumors [40]. From our observations,.these top mutated genes were kidney-specific and distinguish themselves from those in bladder and prostate cancers, although they all belong to genitourinary cancer. Therefore, the mechanism of aberrancies in kidney cancers with germline mutations may be largely different from that of bladder and prostate cancer.
Determination of pathogenicity of germline mutations is crucial for establishing the link between mutations and phenotypes. Here in this study we interpreted the pathogenicity of all reported mutations based on ACMG guidelines. Frameshift and stop gained mutations were highly possible pathogenic or likely pathogenic mutations, indicating the inherit property of mutations. The interpretation would be more meaningful if the pathogenic mutations happened to key DDR genes related to known phenotypes. In contrast, missense mutations are more difficult to interpret, unless sufficient evidence is available to link single amino acid change with phenotypes. This is more likely to occur in single-gene related hereditary diseases, such as VHL disease [34, 41, 42]. In our study, pathogenic mutations of VHL gene were all missense mutations, reflecting the intrinsic properties of mutations in this disease. In contrast, the ratio of missense mutations was low in other highly mutated genes, such as ATM, BRCA1 and PMS2. It was interesting to find that nearly half of the mutations found in BLCA were stop gained mutations. These mutations spread many genes including both high and low frequency genes. This observation demonstrated characteristic mutational change in BLCA, suggesting high ratio of truncated DDR related proteins in the specific cancer.
It is widely known that male overweight female in patient number with a rough ratio of 2:1 in sporadic kidney and urothelial cancer [43]. We found similar trend in genitourinary cancer with germline mutations, suggesting that male is possibly more susceptible to genitourinary cancer if the chance of mutation heredity is similar for both sexes. It is also possible that male may have higher penetrance than female. KIRC is the most common type of kidney cancer, and it was interesting that female overweight male in the number of KIRC patients with germline mutations, although male overweight female in sporadic KIRC [43]. The reason for this discrepancy may include the manner of heredity, mutation penetrance and environmental factors. Previous studies on Lynch syndrome and HBOC revealed significantly lower onset age compared with sporadic colorectal, breast and ovarian cancer patients [44, 45]. However, we did not find age difference between those with pathogenic or likely pathogenic mutations and those with non-pathogenic mutations. Since non-pathogenic mutations were comprehensive found in sporadic cancer patients and normal subjects, our observation suggested that germline mutations of genitourinary cancers did not affect the onset age.
Previous studies reported that untreated patients with Lynch syndrome or HBOC exhibited significantly worse prognosis than sporadic patients [13,14,15,16]. Our study with genitourinary cancer patients also showed identical trend, in which patients with pathogenic or likely pathogenic mutations exhibited much worse overall survival rate than those with non-pathogenic mutations, suggesting that pathogenic and likely pathogenic germline mutations were risk factors for the prognosis of genitourinary cancer patients. Furthermore, the OR values we calculated strongly supported the notion that those with pathogenic or likely pathogenic germline mutations were in much higher risk for developing all types of genitourinary cancers than those without the germline mutations (general population). Our observation of higher overall OR in likely pathogenic mutations than pathogenic mutations suggested that some likely pathogenic mutation may be essentially pathogenic, although limited available evidence does not support the pathogenic interpretation currently. These observations provided strong evidence for the necessity of early detection of germline mutations for those with strong family history and continuous surveillance and early intervention for those with confirmed pathogenic or likely pathogenic germline mutations. On the other hand, it appeared from our study that no difference was found in overall survival rate between patients with pathogenic mutations and those with likely pathogenic mutations. This suggested that some likely pathogenic mutations may actually be pathogenic, although clinical evidence may be absent for interpretation of pathogenic for many likely pathogenic mutations, especially for frameshift and stop gained mutations.
Genitourinary cancer patients with pathogenic or likely pathogenic mutations may be treated with corresponding targeted drugs based on the availability of matched drugs for certain germline mutations. For example, locally advanced or metastatic genitourinary cancer patients with BRCA1/2 mutations may be treated with poly ADP-ribose polymerase (PARP) inhibitors. Although it has long been known that PARP inhibitors were effective for prostate cancer with pathogenic or likely pathogenic BRCA1/2 mutations [46, 47], it was not until recently that evidence started to emerge that PARP inhibitors were also effective in other genitourinary malignancies [47,48,49,50,51], except in renal cell carcinoma, since no evidence on the presence of BRCA1/2 mutations has been available in the cancer [52]. Similarly, locally advanced or metastatic cancer patients with germline mutations of MMR genes may be treated with immune checkpoint inhibitors such as PD-l inhibitors, as these cancers generally exhibit high tumor mutational burden and/or high microsatellite instability [53]. Future development of targeted drugs for DDR pathway may open the door for new treatment strategies for genitourinary cancer patients with germline mutations.
This study had some limitations. First, the number of genitourinary cancer patients was limited since data was available from only 206 patients, which led to the limited number of patients in each individual cancer. Secondly, due to the lack of ethnic diversity and predominant male population, the current findings could be non-generalizable to non-White and female patients. Thirdly, the prognosis of patients may be influenced by therapeutic strategies, however, the information of therapy is not available in the TCGA database.
Conclusions
In this study, the germline mutational characteristics for genitourinary cancers have been comprehensively investigated. A series of pathogenic and likely pathogenic germline mutations have been defined and their mutational landscape in several genitourinary cancers has been studied. Pathogenic and likely pathogenic germline mutations increased the risk and indicated poor prognosis of genitourinary cancers.
Availability of data and materials
Data are available for download by visiting the following website, and can be downloaded through the link of supplementary information: https://www.sciencedirect.com/science/article/pii/S0092867418303635. Alternatively, data can be downloaded directly from the link below: https://ars.els-cdn.com/content/image/1-s2.0-S0092867418303635-mmc2.xlsx. For original dataset source of the germline mutations, the raw data can be accessed at ISB cancer genome cloud (ISB-CGC) at the link below for qualified applicants: http://isb-cancer-genomics-cloud.readthedocs.io/en/latest/sections/webapp/Gaining-Access-To-Contolled-Access-Data.html.
References
Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96–108. https://doi.org/10.1016/j.eururo.2016.06.010.
Saad AM, Gad MM, Al-Husseini MJ, Ruhban IA, Sonbol MB, Ho TH. Trends in renal-cell carcinoma incidence and mortality in the United States in the last 2 decades: a SEER-based study. Clin Genitourin Cancer. 2019;17(1):46–57. https://doi.org/10.1016/j.clgc.2018.10.002.
Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020;77(1):38–52. https://doi.org/10.1016/j.eururo.2019.08.005.
Lynch HT, Smyrk T. Hereditary nonpolyposis colorectal cancer (Lynch syndrome). An updated review. Cancer. 1996;78(6):1149–67. https://doi.org/10.1002/(SICI)1097-0142(19960915)78:6%3c1149::AID-CNCR1%3e3.0.CO;2-5.
Buys SS, Sandbach JF, Gammon A, et al. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer. 2017;123(10):1721–30. https://doi.org/10.1002/cncr.30498.
Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–16.
Giri VN, Knudsen KE, Kelly WK, et al. Implementation of germline testing for prostate cancer: Philadelphia prostate cancer consensus conference 2019. J Clin Oncol. 2020;38(24):2798–811. https://doi.org/10.1200/JCO.20.00046.
Carlo MI, Ravichandran V, Srinavasan P, et al. Cancer susceptibility mutations in patients with urothelial malignancies. J Clin Oncol. 2020;38(5):406–14. https://doi.org/10.1200/JCO.19.01395.
Cheng HH, Sokolova AO, Schaeffer EM, Small EJ, Higano CS. Germline and somatic mutations in prostate cancer for the clinician. J Natl Compr Canc Netw. 2019;17(5):515–21. https://doi.org/10.6004/jnccn.2019.7307.
Nassar AH, Abou Alaiwi S, AlDubayan SH, et al. Prevalence of pathogenic germline cancer risk variants in high-risk urothelial carcinoma. Genet Med. 2020;22(4):709–18. https://doi.org/10.1038/s41436-019-0720-x.
Huang KL, Mashl RJ, Wu Y, et al. Pathogenic germline variants in 10,389 adult cancers. Cell. 2018;173(2):355-370.e14. https://doi.org/10.1016/j.cell.2018.03.039.
Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. https://doi.org/10.1038/gim.2015.30.
Risbridger GP, Taylor RA, Clouston D, et al. Patient-derived xenografts reveal that intraductal carcinoma of the prostate is a prominent pathology in BRCA2 mutation carriers with prostate cancer and correlates with poor prognosis. Eur Urol. 2015;67(3):496–503. https://doi.org/10.1016/j.eururo.2014.08.007.
Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169–80. https://doi.org/10.1016/S1470-2045(17)30891-4.
Hahnen E, Lederer B, Hauke J, et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: secondary analysis of the geparsixto randomized clinical trial. JAMA Oncol. 2017;3(10):1378–85. https://doi.org/10.1001/jamaoncol.2017.1007.
Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7(3):153–62. https://doi.org/10.1038/nrclinonc.2009.237.
Jiang W, Cai MY, Li SY, et al. Universal screening for Lynch syndrome in a large consecutive cohort of Chinese colorectal cancer patients: high prevalence and unique molecular features. Int J Cancer. 2019;144(9):2161–8. https://doi.org/10.1002/ijc.32044.
Gao X, Nan X, Liu Y, et al. Comprehensive profiling of BRCA1 and BRCA2 variants in breast and ovarian cancer in Chinese patients. Hum Mutat. 2020;41(3):696–708. https://doi.org/10.1002/humu.23965.
Lu S, Yu Y, Li Z, et al. EGFR and ERBB2 germline mutations in Chinese lung cancer patients and their roles in genetic susceptibility to cancer. J Thorac Oncol. 2019;14(4):732–6. https://doi.org/10.1016/j.jtho.2018.12.006.
Clamon GH, Bossler AD, Abu Hejleh T, Furqan M. Germline mutations predisposing to non-small cell lung cancer. Fam Cancer. 2015;14(3):463–9. https://doi.org/10.1007/s10689-015-9796-x.
Liu M, Liu X, Suo P, et al. The contribution of hereditary cancer-related germline mutations to lung cancer susceptibility. Transl Lung Cancer Res. 2020;9(3):646–58. https://doi.org/10.21037/tlcr-19-403.
Brown JS, O’Carrigan B, Jackson SP, Yap TA. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 2017;7(1):20–37. https://doi.org/10.1158/2159-8290.CD-16-0860.
Yao J, Gong Y, Zhao W, et al. Comprehensive analysis of POLE and POLD1 gene variations identifies cancer patients potentially benefit from immunotherapy in Chinese population. Sci Rep. 2019;9(1):15767. https://doi.org/10.1038/s41598-019-52414-z.
Chang DW, Gu J, Wu X. Germline prognostic markers for genitourinary bladder cancer: obstacles and opportunities. Urol Oncol. 2012;30(4):524–32. https://doi.org/10.1016/j.urolonc.2012.04.003.
O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60(4):547–60. https://doi.org/10.1016/j.molcel.2015.10.040.
Pilié PG, Tang C, Mills GB, Yap TA. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat Rev Clin Oncol. 2019;16(2):81–104. https://doi.org/10.1038/s41571-018-0114-z.
Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481(7381):287–94. https://doi.org/10.1038/nature10760.
Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non-small-cell lung cancer. N Engl J Med. 2017;376(22):2109–21. https://doi.org/10.1056/NEJMoa1616288.
Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. https://doi.org/10.1038/nature14169.
Shoshani O, Brunner SF, Yaeger R, et al. Chromothripsis drives the evolution of gene amplification in cancer. Nature. 2021;591(7848):137–41. https://doi.org/10.1038/s41586-020-03064-z.
Slavin TP, Banks KC, Chudova D, et al. Identification of incidental germline mutations in patients with advanced solid tumors who underwent cell-free circulating tumor DNA sequencing. J Clin Oncol. 2018;36(35):1800328. https://doi.org/10.1200/JCO.18.00328.
Parry EM, Gable DL, Stanley SE, et al. Germline mutations in DNA repair genes in lung adenocarcinoma. J Thorac Oncol. 2017;12(11):1673–8. https://doi.org/10.1016/j.jtho.2017.08.011.
German J, Sanz MM, Ciocci S, Ye TZ, Ellis NA. Syndrome-causing mutations of the BLM gene in persons in the bloom’s syndrome registry. Hum Mutat. 2007;28(8):743–53. https://doi.org/10.1002/humu.20501.
Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361(9374):2059–67. https://doi.org/10.1016/S0140-6736(03)13643-4.
Chan DK, Chang KW. GJB2-associated hearing loss: systematic review of worldwide prevalence, genotype, and auditory phenotype. Laryngoscope. 2014;124(2):E34–53. https://doi.org/10.1002/lary.24332.
Zeng B, Huang P, Du P, et al. Comprehensive study of germline mutations and double-hit events in esophageal squamous cell cancer. Front Oncol. 2021;11:637431. https://doi.org/10.3389/fonc.2021.637431.
Tovar EA, Graveel CR. MET in human cancer: germline and somatic mutations. Ann Transl Med. 2017;5(10):205. https://doi.org/10.21037/atm.2017.03.64.
Santos M, Lanillos J, Roldan-Romero JM, et al. Prevalence of pathogenic germline variants in patients with metastatic renal cell carcinoma. Genet Med. 2021;23(4):698–704. https://doi.org/10.1038/s41436-020-01062-0.
Win AK, Reece JC, Dowty JG, et al. Risk of extracolonic cancers for people with biallelic and monoallelic mutations in MUTYH. Int J Cancer. 2016;139(7):1557–63. https://doi.org/10.1002/ijc.30197.
Chittiboina P, Lonser RR. Von Hippel-Lindau disease. Handb Clin Neurol. 2015;132:139–56. https://doi.org/10.1016/B978-0-444-62702-5.00010-X.
Mettu P, Agrón E, Samtani S, Chew EY, Wong WT. Genotype–phenotype correlation in ocular von Hippel-Lindau (VHL) disease: the effect of missense mutation position on ocular VHL phenotype. Invest Ophthalmol Vis Sci. 2010;51(9):4464–70. https://doi.org/10.1167/iovs.10-5223.
Fields FR, Suresh N, Hiller M, Freed SD, Haldar K, Lee SW. Algorithmic assessment of missense mutation severity in the Von-Hippel Lindau protein. PLoS ONE. 2020;15(11):0234100. https://doi.org/10.1371/journal.pone.0234100.
Lucca I, Klatte T, Fajkovic H, de Martino M, Shariat SF. Gender differences in incidence and outcomes of urothelial and kidney cancer. Nat Rev Urol. 2015;12(10):585–92. https://doi.org/10.1038/nrurol.2015.232.
Møller P, Seppälä TT, Bernstein I, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the prospective lynch syndrome database. Gut. 2018;67(7):1306–16. https://doi.org/10.1136/gutjnl-2017-314057.
Lynch HT, Watson P, Lynch JF, Conway TA, Fili M. Hereditary ovarian cancer. Heterogeneity in age at onset. Cancer. 1993;71(2 Suppl):573–81. https://doi.org/10.1002/cncr.2820710213.
Mateo J, Lord CJ, Serra V, et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019;30(9):1437–47. https://doi.org/10.1093/annonc/mdz192.
Crocetto F, Barone B, Caputo VF, Fontana M, de Cobelli O, Ferro M. BRCA germline mutations in prostate cancer: the future is tailored. Diagnostics (Basel). 2021;11(5):908. https://doi.org/10.3390/diagnostics11050908.
Rimar KJ, Tran PT, Matulewicz RS, Hussain M, Meeks JJ. The emerging role of homologous recombination repair and PARP inhibitors in genitogenitourinary malignancies. Cancer. 2017;123(11):1912–24. https://doi.org/10.1002/cncr.30631.
Yang H, Liu Z, Wang Y, et al. Olaparib is effective for recurrent urothelial carcinoma with BRCA2 pathogenic germline mutation: first report on olaparib response in recurrent UC. Ther Adv Med Oncol. 2020;12:1758835920970845. https://doi.org/10.1177/1758835920970845.
Brönimann S, Lemberger U, Bruchbacher A, Shariat SF, Hassler MR. Poly(ADP-ribose) polymerase inhibitors in prostate and urothelial cancer. Curr Opin Urol. 2020;30(4):519–26. https://doi.org/10.1097/MOU.0000000000000776.
Pletcher JP, Bhattacharjee S, Doan JP, et al. The emerging role of poly (ADP-Ribose) polymerase inhibitors as effective therapeutic agents in renal cell carcinoma. Front Oncol. 2021;11:681441. https://doi.org/10.3389/fonc.2021.681441.
Attalla K, DiNatale RG, Rappold PM, et al. Prevalence and landscape of actionable genomic alterations in renal cell carcinoma. Clin Cancer Res. 2021;27(20):5595–606. https://doi.org/10.1158/1078-0432.CCR-20-4058.
Luchini C, Bibeau F, Ligtenberg MJL, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. 2019;30(8):1232–43. https://doi.org/10.1093/annonc/mdz116.
Acknowledgements
Not applicable
Funding
This study was supported by the Applied Basic Research Joint Project of Yunnan Provincial Science and Technology Department and Kunming Medical University, General Project 202001AY070001-077, “Effect and mechanism of EZH2 inhibitor on GC chemotherapy in bladder cancer”.
Author information
Authors and Affiliations
Contributions
Yong Yang and Hong Yang designed the study and were responsible for project management and implementation. Yong Yang, Guoying Zhang, Chen Hu, Wei Luo, Haiyang Jiang and Shaoyou Liu were responsible for data collection and processing. Yong Yang, Guoying Zhang, Chen Hu, Wei Luo, Haiyang Jiang and Shaoyou Liu performed the data analysis and interpretation. Yong Yang and Guoying Zhang performed the final statistics and made the figures and tables. Yong Yang, Guoying Zhang, Chen Hu, Wei Luo, Haiyang Jiang, Shaoyou Liu and Hong Yang wrote the manuscript. Yong Yang and Hong Yang proof read the manuscript. Hong Yang submitted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research is a database study. Ethics approval and consent to participate are not required. Data from this study were all downloaded from available database or processed dataset. The written informed consent and consent to participate were obtained by authors of the original database or studies. Therefore, informed consent and consent to participate were not required for this study, as this study performed data mining without directly recruiting patients.
Consent for publication
Not applicable.
Competing interests
All authors declare no competing interests in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Y., Zhang, G., Hu, C. et al. The germline mutational landscape of genitourinary cancers and its indication for prognosis and risk. BMC Urol 22, 196 (2022). https://doi.org/10.1186/s12894-022-01141-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-022-01141-1