Abstract
Background
Venous thromboembolism (VTE) is a serious and preventable postoperative complication. However, the predictive significance of perioperative biochemical parameters for VTE after minimally invasive colorectal cancer surgery remains unclear.
Methods
A total of 149 patients undergoing minimally invasive colorectal cancer surgery were collected between October 2021 and October 2022. Biochemical parameters related to preoperative and postoperative day 1, day 3, and day 5 were collected, including D-Dimer, mean platelet volume (MPV), and maximum amplitude (MA) of thromboelastography (TEG). Receiver operating characteristic (ROC) curves were used to explore the predictive powers of meaningful biochemical parameters for postoperative VTE, and calibration curves were used to assess predictive accuracy.
Results
The overall cumulative incidence of VTE was 8.1% (12/149). The preoperative and postoperative day 3 D-Dimer, postoperative day 3, and day 5 MPV, and postoperative day 1, day 3, and day 5 TEG-MA was significantly higher in the VTE group than in the non-VTE group (P < 0.05). The results of both the ROC curve and the calibration curve indicated that these meaningful D-Dimer, MPV, and TEG-MA had moderate discrimination and consistency for postoperative VTE.
Conclusions
D-Dimer, MPV, and TEG-MA may predict postoperative VTE in patients undergoing minimally invasive surgery for colorectal cancer at specific times in the perioperative period.
Similar content being viewed by others
Introduction
Globally, colorectal cancer (CRC) is one of the most common malignant neoplasms, and it poses a serious threat to human life and health [1]. Treatment for CRC is limited, and surgery combined with chemotherapy and radiotherapy has been considered a pivotal approach for patients with resectable tumors [2]. With the increasing development of technology, laparoscopic and robotic surgery has been widely used in the treatment of CRC patients. Laparoscopic and robotic surgery is known as minimally invasive surgery because it causes less trauma compared to traditional open surgery. However, regardless of the type of surgery, complications can occur after the surgery.
Venous thromboembolism (VTE) is the second leading cause of death among cancer patients undergoing medical and surgical treatment [3]. VTE is composed of deep vein thrombosis (DVT) and its complication pulmonary embolism (PE) [4, 5]. Due to the high risk of recurrent thromboembolism and bleeding, the treatment of venous thromboembolism is challenging in cancer patients [6]. Moreover, surgery is considered to have a proinflammatory effect on the occurrence of VTE [7]. The patient’s hypercoagulable state, venous blood pooling and vessel wall injury are all likely to occur during the surgery. Tissue factor exposure at the surgical site is also an important driver for the occurrence of VTE after surgery [8]. Previous studies have shown that red blood cell transfusions are associated with an increased risk of VTE in patients undergoing surgical resection of the colorectal [9]. Furthermore, platelets are essential for hemostasis and contribute to venous thrombosis through platelet G protein-coupled (GPCR) and immunoreceptor tyrosine-based activation motif (ITAM) receptor signaling [10]. It has been shown that platelet-related biochemical parameters are predictive of DVT in breast cancer patients [11], while thromboelastography (TEG)-related biochemical parameters are also predictive of VTE in gynecologic oncology patients [12]. However, previous studies lacked a focus on the effect of surgery on VTE and ignored the changes in biochemical parameters after surgery. The incidence of VTE events remains high in patients undergoing colorectal surgery [13, 14], and VTE also poses a serious threat to the health of cancer patients undergoing surgical treatment [3, 15]. Hence, there is a need to explore the predictive role of relevant biochemical parameters on the occurrence of postoperative VTE in patients with colorectal cancer treated with minimally invasive surgery.
In this study, we first compared the general data of VTE patients and non-VTE patients after minimally invasive colorectal cancer surgery. Next, we collected biochemical parameters related to the perioperative period in CRC patients. Finally, the predictive role of biochemical parameters on the occurrence of postoperative VTE was assessed by constructing ROC curves and calibration curves.
Materials and methods
Study Population
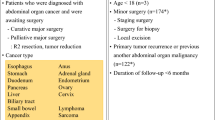
A retrospective observational study was conducted on consecutive patients who underwent minimally invasive colorectal cancer resection at the Second Hospital of Chongqing Medical University from October 2021 to October 2022. Inclusion criteria of the study: All patients had been diagnosed with colorectal adenocarcinoma by pathology preoperatively and no distant metastases were present; all patients underwent laparoscopic or robot surgery for colorectal cancer (All robotic surgery in this study were performed by the da Vinci Surgical System); all patients had no VTE confirmed by color Doppler ultrasound before the surgery. 202 patients were evaluated for inclusion in the study.
Exclusion criteria of the study: Patients who had received any thromboprophylaxis in the perioperative period (n = 24); patients with a previous history of thromboembolism (n = 1); patients who were converted to open surgery during the operation because minimally invasive surgery could not be performed (n = 2); patients with a previous history of malignancy of other organs (n = 3); patients with a previous history of preoperative chemotherapy or radiation therapy for malignancy (n = 15); patients who refuse to sign informed consent (n = 8). The final analysis included 149 patients. This study was approved by the Ethics Committee of the Second Hospital of Chongqing Medical University (Chongqing, China, Approval date: September 20, 2021, Ethical approval number: (2021) 507), and written informed consent was obtained from all patients.
Biochemical parameters
D-dimer was a biomarker of fibrinolysis and coagulation and was analyzed using HemosIL D-dimer HS 500 (Lexington, MA, USA). Mean platelet volume (MPV) was a measure of platelet size and was a potential marker of platelet activation [16]. A whole blood autoanalyzer (Sysmex XN-3000, Kobe, Japan) was used to measure platelet count and MPV. Thromboelastography (TEG) is a widely used coagulation test that allows dynamic monitoring of the clotting response from fibrin formation to clot lysis [17]. The TEG was analyzed on TEG 5000 Thromboelastograph Analyzer (Haemonetics Corporation, MA, USA), and the parameters of TEG in this study included reaction time (R, min), solidification time (K, min), alpha angle (α, degrees), and maximum amplitude (MA, mm). D-Dimer, platelet count, MPV, and TEG were collected from patients’ preoperative and postoperative day 1, day 3, and day 5 through medical record data.
Diagnosis of venous thromboembolism
Compression ultrasonography was performed according to standard procedures (grey scale, B-mode, color Doppler), and all VTE occurrences in this study were confirmed using a high-end ultrasound scanner (LOGIQ 9; GE, CA, USA) on day 8±2 after the surgery.
Statistical analysis
Continuous variables were compared using Student’s t-test and presented as mean ± standard deviation (SD). Categorical variables were tested by chi-square (χ2) test and expressed as percentages. Receiver operating characteristic (ROC) curves were used to assess the discrimination of the venous thromboembolism prediction model, where the area under the curve (AUC) of the ROC was used to quantify. Calibration curves and the Hosmer-Lemeshow test were used to assess the calibration performance of the venous thromboembolism prediction model.
All statistical analyses were conducted with SPSS version 25.0 (IBM, Armonk, NY, USA). Two-tailed P values < 0.05 were considered statistically significant. A P value > 0.05 for the Hosmer-Lemeshow test was considered as having no significant difference between the predicted and observed events.
Results
Overall, 202 patients were evaluated and a total of 149 patients were included in this study (Fig. 1). Among them, 97 cases underwent laparoscopic surgery, and 52 cases underwent robotic surgery. As shown in Table 1, postoperative VTE occurred in 12 of 149 patients, with a cumulative outcome incidence of 8.1%. All these VTE patients occurred with DVT, and one had a secondary PE due to DVT (8.3%). The mean age at surgery for VTE patients was 69.58 years, which was higher than the mean age at surgery for non-VTE patients, which was 60.40 years, although they were not statistically different. As shown in Table 1, the occurrence of VTE after surgery was associated with an increase in ASA score (P = 0.012). 9 of 12 (75%) patients with VTE had an ASA score of 3. In this study, we did not find any correlation between the occurrence of postoperative VTE and other general information such as gender, BMI, smoking status, history of diabetes mellitus, cardiac disease, history of chronic renal failure, operation time, operative procedure, type of resection, and pathological stage of patients.
In this study, we found that patients with VTE had significantly higher D-Dimer preoperatively and at postoperative day 3 than non-VTE patients (550.18 ± 357.71 vs. 279.58 ± 225.54, P = 0.028; 1564.77 ± 672.11 vs. 809.31 ± 443.35, P = 0.014). And MPV was significantly higher in the VTE patients than in the non-VTE patients at postoperative day 3 and day 5 (12.40 ± 1.85 vs. 10.58 ± 0.93, P < 0.001; 12.61 ± 1.43 vs. 10.56 ± 1.12, P = 0.046). Moreover, TEG-MA was significantly higher in the VTE patients than in the non-VTE patients at postoperative day 1, day 3, and day 5 (70.75 ± 0.90 vs. 68.81 ± 2.39, P = 0.015; 73.78 ± 3.90 vs. 70.21 ± 2.16, P = 0.021; 74.27 ± 2.69 vs. 71.24 ± 1.84, P = 0.029). However, no significant differences were seen in other relevant biochemical parameters (Table 2).
The ROC curves were constructed to assess the discrimination of meaningful biochemical parameters in predicting postoperative venous thromboembolism. As shown in Fig. 2A, the ROC curve areas of D-Dimer preoperatively and postoperative day 3 were 0.758 and 0.827, respectively. The ROC curve areas of MPV at postoperative day 3 and day 5 were 0.797 and 0.878, respectively (Fig. 2B). As shown in Fig. 2C, the ROC curve area of TEG-MA was 0.773, 0.849, and 0.807 at postoperative day 1, day 3, and day 5, respectively. Based on the ROC curves analysis, optimal cutoff values of these parameters in the prediction of postoperative VTE were identified (Table 3). Moreover, based on the Hosmer-Lemeshow test, our calibration curve results indicated that D-Dimer preoperative (P = 0.565) and postoperative day 3 (P = 0.439), MPV postoperative day 3 (P = 0.319) and day 5 (P = 0.172), and TEG-MA postoperative day 1 (P = 0.840), day 3 (P = 0.162), and day 5 (P = 0.459) all have good calibration (Fig. 3).
Discussion
In this study, we tried to investigate whether relevant biochemical parameters are associated with the occurrence of VTE after minimally invasive colorectal cancer surgery in a retrospective observational study. Our results showed that increased D-Dimer, MPV, and TEG-MA were positively associated with postoperative VTE in patients undergoing minimally invasive surgery for colorectal cancer at specific times in the perioperative period. Both our ROC curves and calibration curves confirmed the association. These results indicated that D-Dimer, MPV, and TEG-MA may be potential biochemical parameters to predict the occurrence of VTE after minimally invasive surgery for colorectal cancer.
In recent years, minimally invasive surgery has become more common and is considered to be the direction of surgical development [18]. In this study, our minimally invasive surgery included laparoscopic and robotic surgery, and 97 and 52 CRC patients were collected in the two groups, respectively. Regardless of the type of surgery performed, the potential risk for postoperative complications exists. In CRC surgery, in addition to complications such as anastomotic dehiscence and wound infection, the occurrence of postoperative VTE is equally life-threatening to patients. VTE is a serious complication of surgical procedures and is a preventable cause of death in patients hospitalized for surgery [19, 20]. Compared to non-VTE patients, VTE patients have significantly more complexity and financial burden of treatment [21]. Guidelines recommend antithrombotic therapy after surgery [22], and antithrombotic prophylaxis has been reported to reduce the incidence of venous thromboembolism by approximately 70% [23]. Despite the use of antithrombotic prophylaxis, the incidence of symptomatic venous thromboembolism within one month after major cancer surgery was reported to be 2.1% [24]. For CRC surgery, the risk of developing a significantly symptomatic VTE has been previously reported to be approximately 2–4% [25, 26]. In our study, the cumulative incidence of VTE after minimally invasive colorectal cancer surgery was 8.1%, and we not only collected patients with symptomatic VTE but also included patients with asymptomatic VTE. In our study center, 88 patients underwent rectal cancer surgery (59.1%), although our study showed no statistically significant difference between the type of resection and the occurrence of VTE. However, the rectal surgical procedure was performed in the lithotomy position, and previous studies have shown that surgery in the lithotomy position is a potential risk for venous thrombosis [27]. Furthermore, we found that the occurrence of postoperative VTE was associated with increased ASA scores, with the incidence of postoperative VTE occurring in ASA scores 2 and 3 being 3.4% and 14.8%, respectively.
Aside from antithrombotic therapy, it also appears essential to predict the risk of venous thromboembolism in cancer patients and to provide targeted treatment. Tian et al. found that after performing pulmonary surgery, patients with postoperative VTE had significantly higher D-Dimer preoperatively and at postoperative day 1 and day 3 than non-VTE patients [28]. In breast cancer, it has been shown that D-Dimer is significantly increased in patients with DVT after radical breast cancer surgery [29]. In our study, D-Dimer was significantly higher in VTE patients than in non-VTE patients at preoperative and postoperative day 3, which were 550.18 ± 357.71 vs. 279.58 ± 225.54, P = 0.028; 1564.77 ± 672.11 vs. 809.31 ± 443.35, P = 0.014, respectively. Previous studies have shown that MPV was an independent predictor of DVT and was significantly elevated in patients with DVT [30, 31]. Our results indicated that MPV was higher in VTE patients compared to non-VTE patients at postoperative day 3 and day 5, which were 12.40 ± 1.85 vs. 10.58 ± 0.93, P < 0.001, and 12.61 ± 1.43 vs. 10.56 ± 1.12, P = 0.046, respectively. Moreover, we also found that TEG-MA was also higher in VTE patients than in non-VTE patients at postoperative day 1, day 3, and day 5 (70.75 ± 0.90 vs. 68.81 ± 2.39, P = 0.015; 73.78 ± 3.90 vs. 70.21 ± 2.16, P = 0.021; 74.27 ± 2.69 vs. 71.24 ± 1.84, P = 0.029). Therefore, D-Dimer, MPV, and TEG-MA may play an important role in the prediction of postoperative VTE during specific periods of the perioperative period.
Then, we constructed ROC curves to assess the predictive power of D-Dimer, MPV, and TEG-MA for postoperative VTE occurrence. Our results showed that the area under the ROC curve for D-Dimer was 0.758 and 0.827 at preoperative and postoperative day 3, respectively. The area under the ROC curve for MPV was 0.797 and 0.878 at postoperative day 3 and day 5. The area under the ROC curve for TEG-MA at postoperative day 1, day 3, and day 5 were 0.773, 0.849, and 0.807. The results of ROC analysis showed that D-Dimer at preoperative, postoperative day 3, MPV at postoperative day 3, day 5, and TEG-MA at postoperative day 1, day 3, and day 5 were all discriminatory for postoperative VTE prediction. Finally, we constructed calibration curves based on the Hosmer-Lemeshow test. Our calibration curves showed good calibration of D-Dimer at preoperative, postoperative day 3, MPV at postoperative day 3, day 5, and TEG-MA at postoperative day 1, day 3, and day 5 for predicting VTE (all P-values > 0.05).
There are several limitations in our study. First, the sample size of this study is small and still needs to be confirmed by a large sample of multicenter studies. Second, the results of this study were only applicable to Chinese patients who underwent minimally invasive colorectal cancer surgery, and further studies are needed for other ethnic groups and surgical modalities. Third, our study excluded patients with a previous history of thromboembolism but failed to exclude patients who initially had subclinical VTE before surgery. Further studies are still needed for this group of patients.
In conclusion, D-Dimer at preoperative and postoperative day 3, MPV at postoperative day 3 and day 5, and TEG-MA at postoperative day 1, day 3, and day 5 may predict postoperative VTE in patients undergoing minimally invasive colorectal cancer surgery. Early prediction and diagnosis of postoperative VTE may provide important guidance for high-risk patients.
Data Availability
The data sets generated and analyzed during the current study are not publicly available due to restrictions on ethical approvals involving patient data and anonymity but can be obtained from the corresponding author at reasonable request.
References
Brody H. Colorectal cancer. Nature. 2015;521(7551):1.
Bai H, Wang J, Phan CU, Chen Q, Hu X, Shao G, et al. Cyclodextrin-based host-guest complexes loaded with regorafenib for colorectal cancer treatment. Nat Commun. 2021;12(1):759.
Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6(6):401–10.
Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585–93.
Huang W, Goldberg RJ, Anderson FA, Kiefe CI, Spencer FA. Secular trends in occurrence of acute venous thromboembolism: the Worcester VTE study (1985–2009). Am J Med. 2014;127(9):829–39e5.
Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484–8.
Gangireddy C, Rectenwald JR, Upchurch GR, Wakefield TW, Khuri S, Henderson WG, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335–. – 41; discussion 41 – 2.
Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451(7181):914–8.
Xenos ES, Vargas HD, Davenport DL. Association of blood transfusion and venous thromboembolism after colorectal cancer resection. Thromb Res. 2012;129(5):568–72.
Mwiza JMN, Lee RH, Paul DS, Holle LA, Cooley BC, Nieswandt B, et al. Both G protein-coupled and immunoreceptor tyrosine-based activation motif receptors mediate venous thrombosis in mice. Blood. 2022;139(21):3194–203.
Cui LN, Li N, Fu S, Zhang X, Wang X, Wang RT. Combination of preoperative D-dimer and mean platelet volume predicts postoperative deep venous thrombosis in breast cancer patients. Cancer Biomark. 2018;21(4):909–13.
Liu J, Wang N, Chen Y, Lu R, Ye X. Thrombelastography coagulation index may be a predictor of venous thromboembolism in gynecological oncology patients. J Obstet Gynaecol Res. 2017;43(1):202–10.
Wei Q, Wang Y, An YB, Yang ZY, Liu YS, Zhang X, et al. Rationale and design of a prospective, multicenter, cohort study on the evaluation of postoperative venous ThromboEmbolism incidence in patients with ColoRectal Cancer (CRC-VTE trial). Transl Cancer Res. 2022;11(5):1406–12.
Kimura Y, Oki E, Ando K, Saeki H, Kusumoto T, Maehara Y. Incidence of venous thromboembolism following laparoscopic surgery for gastrointestinal Cancer: a Single-Center, prospective cohort study. World J Surg. 2016;40(2):309–14.
Pannucci CJ, Fleming KI, Bertolaccini CB, Prazak AM, Huang LC, Pickron TB. Assessment of Anti-Factor Xa levels of patients undergoing colorectal surgery given Once-Daily Enoxaparin Prophylaxis: a clinical study examining enoxaparin pharmacokinetics. JAMA Surg. 2019;154(8):697–704.
Panova-Noeva M, Wagner B, Nagler M, Koeck T, ten Cate V, Prochaska JH et al. Comprehensive platelet phenotyping supports the role of platelets in the pathogenesis of acute venous thromboembolism – results from clinical observation studies.EBioMedicine. 2020;60.
Fan D, Ouyang Z, Ying Y, Huang S, Tao P, Pan X et al. Thromboelastography for the Prevention of Perioperative Venous Thromboembolism in Orthopedics. Clinical and Applied Thrombosis/Hemostasis. 2022;28.
Franklin ME Jr, Liang S, Russek K. Integration of transanal specimen extraction into laparoscopic anterior resection with total mesorectal excision for rectal cancer: a consecutive series of 179 patients. Surg Endosc. 2013;27(1):127–32.
Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA Jr, et al. Prevention of venous thromboembolism. Chest. 2001;119(1 Suppl):132s–75s.
Agnelli G. Prevention of venous thromboembolism in surgical patients. Circulation. 2004;110(24 Suppl 1):Iv4–12.
Cohoon KP, Ransom JE, Leibson CL, Ashrani AA, Petterson TM, Long KH, et al. Direct Medical costs attributable to Cancer-Associated venous thromboembolism: a Population-Based longitudinal study. Am J Med. 2016;129(9):1000e15–25.
Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and Prevention of thrombosis, 9th ed: american college of chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e419S–e96S.
Mismetti P, Laporte S, Darmon JY, Buchmüller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88(7):913–30.
Agnelli G, Bolis G, Capussotti L, Scarpa RM, Tonelli F, Bonizzoni E, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg. 2006;243(1):89–95.
Holwell A, McKenzie JL, Holmes M, Woods R, Nandurkar H, Tam CS, et al. Venous thromboembolism prevention in patients undergoing colorectal surgery for cancer. ANZ J Surg. 2014;84(4):284–8.
Nelson DW, Simianu VV, Bastawrous AL, Billingham RP, Fichera A, Florence MG, et al. Thromboembolic complications and prophylaxis patterns in colorectal surgery. JAMA Surg. 2015;150(8):712–20.
Gelder C, McCallum AL, Macfarlane AJR, Anderson JH. A systematic review of mechanical thromboprophylaxis in the lithotomy position. surgeon: J Royal Colleges Surg Edinb Irel. 2018;16(6):365–71.
Tian B, Song C, Li H, Zhang W, Chen Q, Chen S, et al. The significance of perioperative coagulation and fibrinolysis related parameters after lung surgery for predicting venous thromboembolism: a prospective, single center study. J Thorac Dis. 2018;10(4):2223–30.
Pang M, Zhao F, Yu P, Zhang X, Xiao H, Qiang W, et al. The significance of coagulation and fibrinolysis-related parameters in predicting postoperative venous thrombosis in patients with breast cancer. Gland Surg. 2021;10(4):1439–46.
Cil H, Yavuz C, Islamoglu Y, Tekbas E, Demirtas S, Atilgan ZA, et al. Platelet count and mean platelet volume in patients with in-hospital deep venous thrombosis. Clin Appl Thromb Hemost. 2012;18(6):650–3.
Canan A, Halıcioğlu SS, Gürel S. Mean platelet volume and D-dimer in patients with suspected deep venous thrombosis. J Thromb Thrombolysis. 2012;34(2):283–7.
Acknowledgements
Not applicable.
Funding
This work was supported by The General Project of Chongqing Natural Science Foundation, Chongqing Science and Technology Commission, China [cstc2021jcyj-msxmX0153 (Linglong Peng)], [cstc2021jcyj-msxmX0112 (Yaxu Wang)], and [CSTB2022NSCQ-MSX1005 (Haitao Gu)], and Kuanren Talents Project of the Second Affiliated Hospital of Chongqing Medical University in China [kryc-yq-2110 (Haitao Gu)].
Author information
Authors and Affiliations
Contributions
DBW, HTG, and YHT participated in the study conception and design. LLP, HL, and YHJ participated in data gathering, data analysis and interpretation. DBW, ZQX, QW, and YXW participated in manuscript drafting, and revising. All the authors approved the manuscript and agreed to publish the study.
Corresponding author
Ethics declarations
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Second Hospital of Chongqing Medical University, and written informed consent was obtained from all patients. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Authors’ information
1Department of Gastrointestinal Surgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing 400010, China.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, D., Gu, H., Tang, Y. et al. Predictive factors on postoperative venous thromboembolism after minimally invasive colorectal cancer surgery: a retrospective observational study. BMC Surg 23, 85 (2023). https://doi.org/10.1186/s12893-023-01992-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-023-01992-x