Abstract
Background
Systemic inflammation has been reported to be associated with cancer progression and metastasis. Systemic inflammation score (SIS), calculated from preoperative serum albumin level and lymphocyte-to-monocyte ratio, has been shown to be a novel prognostic factor for several types of tumors. This study aimed to evaluate the prognostic value of the SIS in patients with pT2–4 resectable gastric cancer (GC).
Methods
Total 97 patients with pT2–4 GC who underwent curative surgery from 322 cases between 2009 and 2015 in Fukushima Medical University Hospital were included. We performed univariate and multivariate analyses to evaluate the usefulness of preoperative SIS and other prognostic factors for relapse-free survival (RFS) and overall survival (OS).
Results
The higher SIS score was associated with undifferentiated cancer and recurrence. Univariate analysis of RFS identified deeper tumor invasion and higher SIS were significant risk factors and multivariate analysis revealed that both of them were independent prognostic factors for RFS. As for OS, age, tumor invasion, SIS and LNR were significantly correlated with RFS. In multivariate analysis, tumor invasion, SIS and LNR were independent prognostic factors for OS.
Conclusions
SIS was an independent prognostic factor for RFS and OS in pT2–4 resectable gastric cancer patients who underwent curative gastrectomy.
Similar content being viewed by others
Background
Gastric cancer (GC) is the third most common cause of cancer death worldwide [1]. Despite significant improvements in the therapies for GC patients, the survival of patients with advanced GC still remains poor [2, 3]. By far, several prognostic models have been used to predict prognosis for GC patients. However accurate prediction of individual survival remains controversial.
In addition to local inflammatory reaction, cancer patients often exhibit systemic inflammatory response, such as changes in peripheral blood cell counts and C-reactive protein (CRP), decreased hemoglobin and serum albumin (Alb) levels [4, 5]. Systemic inflammation has been reported to play a critical role in cancer progression and metastasis, and its interactions with host-tumor are currently recognized as the seventh hallmark of cancer [6,7,8,9]. Accordingly, prognostic factors based on the several ratios of the circulating blood cells, such as neutrophil-to-lymphocyte ratio (NLR) [10], lymphocyte-to-monocyte ratio (LMR) [11], Lymph node ratio (LNR) [12, 13], CRP-to-albumin ratio (CAR) [14, 15], modified Glasgow Prognostic Score (mGPS) [16, 17], have been developed and reported to be associated with poor survival in cancer patients.
Systemic inflammation score (SIS), based on the preoperative serum albumin level and LMR, was reported to be a prognostic marker for clear cell renal cell carcinoma (ccRCC) and colorectal cancer [18, 19]. Recently, some papers have reported on the association between SIS and poor prognosis in GC patients as well [20,21,22,23]. However, the prediction of GC prognosis by SIS is not yet common and further evidence needs to be accumulated.
The purpose of this study was to evaluate the clinical significance of preoperative SIS for relapse-free survival (RFS) and overall survival (OS) of GC patients with curative gastrectomy, compared to other prognostic biomarkers.
Methods
Patients
This retrospective study recruited GC patients who were curatively resected at Fukushima Medical University Hospital, based on 322 cases between January 2009 and September 2015. The inclusion criteria were the following: (a) pT2–4 advanced GC according to the Japanese Classification of Gastric Carcinoma, the 15th Edition [24] and (b) curatively resected with systemic lymphadenectomy. The exclusion criteria were the following: (a) neoadjuvant chemotherapy, (b) distant metastasis, (c) multiple cancer and (d) hematological disorder. Finally, 97 GC patients were included in this study (Fig. 1). All clinical data were retrospectively collected from medical records.
Definition of prognostic markers
The patients’ blood tests were performed before surgery. These included the lymphocyte count, monocyte count, platelet count, serum Alb and CRP levels.
LNR was defined as the ratio of positive divided by the total number of examined nodes [25]. mGPS was determined by serum Alb and CRP levels was defined based on the previously studies [17]. SIS was formulated according to serum Alb and LMR as follows: a score of 0 indicated patients with Alb ≥ 4.0 g/dL and LMR ≥ 4.44; a score of 1 indicated those with either Alb < 4.0 g/dL or LMR < 4.44; and a score of 2 indicated those with both Alb < 4.0 g/dL and LMR < 4.44 [18] (Table 1). CAR and LNR were divided into two groups by median, and the median (interquartile range) of each factor was 0.03 (0.01–0.09) and 0.067 (0.020–0.152). mGPS and SIS were also classified into two groups by scores.
Follow-up investigation
All patients received postoperative follow-up every 3 months up to 2 years and every 6 months during 3–5 years after surgery and annually afterward. The routine follow-up included physical examinations, laboratory tests, enhanced CT and annual upper gastrointestinal endoscopy. Relapse free survival (RFS) and Overall survival (OS) were defined as the interval from the date of surgery to the date of recurrence and death from any cause, respectively.
Statistical analysis
Fisher’s exact test was used to compare patient groups. Survival curves were plotted by the Kaplan–Meier method, and significance was determined by the log-rank test. Univariate and multivariate analysis were performed with the Cox proportional hazards model. ROC curves were used to evaluate their diagnostic ability.
A two-sided P-value less than 0.05 was considered to be statistically significant. All statistical analysis was performed using SPSS 26 (IBM Corporation) or GraphPad Prism v6.04 (Graphpad Software Inc.).
Results
Patient characteristics according to the SIS score
Totally, 97 GC patients were included in this study. The clinicopathological characteristics of the patients were shown in Table 2. There were 69 (71.1%) males and 28 (28.9%) females. The median age was 68.7 years (range 31–90 years). All parameters except patient’s age were divided into two groups. No significant differences between SIS 0–1 and SIS 2 were observed with respect to age, gender, tumor invasion, lymph node metastasis, lymphatic invasion, vascular invasion, administration of adjuvant therapy and surgical procedure. The high SIS score was significantly correlated with undifferentiated cancer and recurrence.
ROC curve analyses for representative prognostic factors of cancer
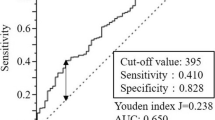
ROC curve analyses were used to evaluate the significance of representative prognostic factors. Area under the curves (AUC) of the LMR [AUC 0.7095, 95% CI 0.5955–0.8235], that is a component of SIS, was highest in RFS (Additional file 1: Fig. S1). In OS, LNR [AUC 0.7394, 95% CI 0.6201–0.8587] had the highest AUC, but LMR [AUC 0.6989, 95% CI 0.5958–0.8020] was also close to it (Additional file 1: Fig. S2).
Survival analysis for prognostic impact of SIS on RFS and OS
The median follow-up time of patients was 1825 days (range 33–1825 days). From the Kaplan–Meier survival curve, higher SIS scores was significantly associated with poorer RFS and OS (Fig. 2). Table 3 shows the results of univariate and multivariate analyses for RFS. Univariate analysis demonstrated that tumor invasion and SIS score, but not other scores, were significantly correlated with RFS. Also, in multivariate analysis, tumor invasion [HR 3.162, 95% CI 1.212–8.251, p = 0.019] and SIS [HR 2.847, 95% CI 1.172–6.919, p = 0.021] were significantly associated with RFS. Regarding OS, age, tumor invasion, SIS score and LNR were associated with OS in univariate analysis. Multivariate analysis revealed a significant association between OS and age [HR 3.537, 95% CI 1.406–8.901, p = 0.007], tumor invasion [HR 4.396, 95% CI 1.820–10.622, p = 0.001], SIS score [HR 3.558, 95% CI 1.562–8.107, p = 0.003] and LNR score [HR 4.734, 95% CI 1.844–12.153, p = 0.001] (Table 4). Whereas, other prognostic scores, including mGPS and CAR, were not shown to be independent predictive factor for OS.
Discussion
We analyzed 97 patients with pT2–4 advanced GC who underwent curative surgery. Preoperative SIS was correlated with histologic type and recurrence. In addition, we demonstrated that relapse-free and overall survival were significantly poorer in higher SIS score group in multivariate analysis.
Many studies have shown that systemic inflammation and nutrition are related to the outcomes of cancer patients [6, 26]. The SIS, which is based on the combination of preoperative LMR and serum Alb, was reported to have prognostic value in several malignancies. Low LMR levels means low lymphocyte and high monocyte counts in the blood. Low lymphocyte counts indicate the suppressed immune surveillance and can lead to cell proliferation, invasion and metastasis of cancer [27], whereas, tumor-associated macrophages, differentiated from monocyte, had been reported to contribute the tumor invasion, metastasis and therapeutic resistance in cancer [28, 29]. Therefore, several studies had reported that preoperative LMR was correlated with prognosis in various cancers [11, 30]. Serum albumin level is not an indicator of nutritional status, but also systemic inflammation response [26]. Low serum albumin level had also been reported to be related with poor prognosis in many types of cancer [31, 32].
Recently, several studies reported the usefulness of SIS in gastric cancer. Sato et al. showed that SIS can predict the incidence of postoperative complications and survival in pT2–4 GC patients after gastrectomy [22]. Ma et al. retrospectively calculated preoperative SIS in all Stage GC patients, in which SIS can predict 5-year OS better than NLR and maintained the predictive accuracy superiority throughout the observation period [23]. According to the report by Chen et al., preoperative SIS exceeded both mGPS and lymphocyte C-reactive protein score (LCS) in predicting the survival of Stage I–IV GC patients [20]. In the present study, we examined SIS score along with mGPS, CAR and LNR and demonstrated the correlation between systemic inflammation and the survival of advanced GC patients who underwent curative gastrectomy. We indicated that SIS was more significant than the other factors, including mSIS, CAR and LNR, in RFS and was significant along with LNR in OS. To our knowledge, this is the first report to show that SIS has a statistically significant difference in both RFS and OS.
There are some strengths of SIS as a prognostic factor. First, as it can be assessed prior to surgery, it may help in choosing the treatment for the individual patients. The study of Shoka et al. indicated that preoperative SIS was a significant predictor of postoperative pneumonia in GC patients [33]. Consideration of the need for preoperative nutritional management and complication control may reduce perioperative complications and improve prognosis. Second, SIS can be assessed readily and repeatedly because it is based on peripheral blood samples. In perioperative analysis, Hara et al. reported that SIS at 1 month after surgery could predict the tumor recurrence and survival of patients with Stage II–III GC [21]. Further studies are needed because SIS might be a predictor of recurrence and prognosis even during postoperative follow-up.
The strategies for perioperative chemotherapy for gastric cancer vary from country to country and still controversial. According to the Japanese gastric cancer treatment guidelines, adjuvant chemotherapy is recommended for Stage II/III GC patients [34]. In terms of neoadjuvant chemotherapy, several clinical trials have been conducted in Japan, but the evidence is limited and does not lead to clear recommendations [34, 35]. The advantages of neoadjuvant chemotherapy include possibility to administer chemotherapy more intensively, but the disadvantages include the difficulty of accurate preoperative diagnosis, the possibility of becoming unresectability due to cancer growth during chemotherapy and increased postoperative complications. Adjuvant chemotherapy can be given after accurate diagnosis by histopathological findings, but there is a possibility that adjuvant chemotherapy cannot be performed due to deterioration of the general condition or complications after surgery. Although further studies are needed to determine the optimal treatment strategy, prognostic factors such as SIS might be important to determine the treatment strategy.
Our study has several limitations. First, it was a retrospective and single-center study. Thus, it may have been subject to selection bias. Second, the number of cases in this study was small that the ability to detect significant difference might be low. Third, the occurrence of perioperative complications was not examined and may have acted as a confounding factor.
Conclusions
Our study showed that the preoperative SIS may be a significant prognostic factor for advanced GC.
Availability of data and materials
The datasets generated and analyzed in this study are not publicly available due to the protection of personal information of the patients, but they are available from the corresponding author on reasonable request.
Abbreviations
- SIS:
-
Systemic inflammation score
- GC:
-
Gastric cancer
- RFS:
-
Relapse free survival
- OS:
-
Overall survival
- CRP:
-
C-reactive protein
- Alb:
-
Albumin
- NLR:
-
Neutrophil-to-lymphocyte ratio
- LMR:
-
Lymphocyte-to-monocyte ratio
- LNR:
-
Lymph node ratio
- CAR:
-
CRP-to-albumin ratio
- mGPS:
-
Modified Glasgow Prognostic Score
- ccRCC:
-
Clear cell renal cell carcinoma
- LCS:
-
Lymphocyte C-reactive protein score
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-386.
Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;2010(3):Cd004064.
Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359(5):453–62.
Chechlinska M, Kowalewska M, Nowak R. Systemic inflammation as a confounding factor in cancer biomarker discovery and validation. Nat Rev Cancer. 2010;10(1):2–3.
Jagdev SP, Gregory W, Vasudev NS, Harnden P, Sim S, Thompson D, Cartledge J, Selby PJ, Banks RE. Improving the accuracy of pre-operative survival prediction in renal cell carcinoma with C-reactive protein. Br J Cancer. 2010;103(11):1649–56.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44.
Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457(7225):36–7.
Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493-503.
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81.
Saito H, Kono Y, Murakami Y, Shishido Y, Kuroda H, Matsunaga T, Fukumoto Y, Osaki T, Ashida K, Fujiwara Y. Prognostic significance of the preoperative ratio of C-reactive protein to albumin and neutrophil-lymphocyte ratio in gastric cancer patients. World J Surg. 2018;42(6):1819–25.
Hsu JT, Wang CC, Le PH, Chen TH, Kuo CJ, Lin CJ, Chou WC, Yeh TS. Lymphocyte-to-monocyte ratios predict gastric cancer surgical outcomes. J Surg Res. 2016;202(2):284–90.
Kim YS, Kim JH, Yoon SM, Choi EK, Ahn SD, Lee SW, Kim JC, Yu CS, Kim HC, Kim TW, et al. lymph node ratio as a prognostic factor in patients with stage III rectal cancer treated with total mesorectal excision followed by chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2009;74(3):796–802.
Zhu J, Xue Z, Zhang S, Guo X, Zhai L, Shang S, Zhang Y, Lu H. Integrated analysis of the prognostic role of the lymph node ratio in node-positive gastric cancer: a meta-analysis. Int J Surg. 2018;57:76–83.
Tominaga T, Nonaka T, Sumida Y, Hidaka S, Sawai T, Nagayasu T. The C-reactive protein to albumin ratio as a predictor of severe side effects of adjuvant chemotherapy in stage III colorectal cancer patients. PLoS ONE. 2016;11(12): e0167967.
Zhou L, Ma S, Balde AI, Han S, Cai Z, Li Z. A retrospective propensity score matched study of the preoperative C-reactive protein to albumin ratio and prognosis in patients with resectable non-metastatic breast cancer. Med Sci Monit. 2019;25:4342–52.
Hirashima K, Watanabe M, Shigaki H, Imamura Y, Ida S, Iwatsuki M, Ishimoto T, Iwagami S, Baba Y, Baba H. Prognostic significance of the modified Glasgow prognostic score in elderly patients with gastric cancer. J Gastroenterol. 2014;49(6):1040–6.
McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22(8):881–6.
Chang Y, An H, Xu L, Zhu Y, Yang Y, Lin Z, Xu J. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br J Cancer. 2015;113(4):626–33.
Suzuki Y, Okabayashi K, Hasegawa H, Tsuruta M, Shigeta K, Kondo T, Kitagawa Y. Comparison of preoperative inflammation-based prognostic scores in patients with colorectal cancer. Ann Surg. 2018;267(3):527–31.
Chen YR, Chen YL, Ouyang SS, Xu HW, Li P, He LJ, Zhu SL. Prognostic efficacy of preoperative mGPS, SIS and LCS in patients with gastric cancer. Clin Chim Acta. 2020;511:81–9.
Hara K, Aoyama T, Yamada T, Nakazono M, Nagasawa S, Shimoda Y, Kumazu Y, Numata M, Hayashi T, Tamagawa H, et al. The prognostic value of the perioperative systemic inflammation score for patients with advanced gastric cancer. Anticancer Res. 2020;40(3):1503–12.
Sato B, Kanda M, Tanaka C, Kobayashi D, Iwata N, Hattori N, Suenaga M, Hayashi M, Yamada S, Murotani K, et al. Significance of preoperative systemic inflammation score in short-term and long-term outcomes of patients with pathological T2–4 gastric cancer after radical gastrectomy. World J Surg. 2018;42(10):3277–85.
Ma M, Weng M, Chen F, Hu Y, Lai J, Wang Y, Zhou Y. Systemic inflammation score is a prognostic marker after curative resection in gastric cancer. ANZ J Surg. 2019;89(4):377–82.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 15th ed. Tokyo: Kanehara; 2017.
Ceelen W, Van Nieuwenhove Y, Pattyn P. Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol. 2010;17(11):2847–55.
McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–6.
Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228(7):1404–12.
Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27(4):462–72.
Aras S, Zaidi MR. TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer. 2017;117(11):1583–91.
Chan JC, Chan DL, Diakos CI, Engel A, Pavlakis N, Gill A, Clarke SJ. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg. 2017;265(3):539–46.
Matsuda S, Takeuchi H, Kawakubo H, Fukuda K, Nakamura R, Takahashi T, Wada N, Saikawa Y, Omori T, Kitagawa Y. Cumulative prognostic scores based on plasma fibrinogen and serum albumin levels in esophageal cancer patients treated with transthoracic esophagectomy: comparison with the Glasgow prognostic score. Ann Surg Oncol. 2015;22(1):302–10.
Eo WK, Chang HJ, Suh J, Ahn J, Shin J, Hur JY, Kim GY, Lee S, Park S, Lee S. The prognostic nutritional index predicts survival and identifies aggressiveness of gastric cancer. Nutr Cancer. 2015;67(8):1260–7.
Shoka M, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, Murai T, Asada T, Ishiyama A, Matsushita H, et al. Systemic inflammation score as a predictor of pneumonia after radical resection of gastric cancer: analysis of a multi-institutional dataset. Dig Surg. 2020;37(5):401–10.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2021. 6th ed. Tokyo: Kanehara; 2021.
Kakinuma D, Arai H, Yasuda T, Kanazawa Y, Matsuno K, Sakurazawa N, Watanabe M, Suzuki H, Yoshida H. Treatment of gastric cancer in Japan. J Nippon Med Sch. 2021;88(3):156–62.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conception and design: TM (Takuro Matsumoto), SO, KK. Acquisition of data, analysis and interpretation of data: TM (Takuro Matsumoto), SO, AK, AM, YM, LY, TT, HH, YW, SH, HO, WS, TM (Tomoyuki Momma), ZS, KK. Participated in drafting the article or revising it critically for important intellectual content: SO, HO, KK. Gave final approval of the version to be submitted and any revised version to be published: KK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Fukushima Medical University (No. 30170). In this study, written informed consent was obtained from all patients as approved by the Institutional Review Board of Fukushima Medical University. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
ROC curves of prognostic factors for RFS. Figure S2. ROC curves of prognostic factors for OS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Matsumoto, T., Ohki, S., Kaneta, A. et al. Systemic inflammation score as a preoperative prognostic factor for patients with pT2–T4 resectable gastric cancer: a retrospective study. BMC Surg 23, 8 (2023). https://doi.org/10.1186/s12893-023-01904-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-023-01904-z