Abstract
Background
Splenic lymphangiomas (SL) are very rare benign cystic lesions found in pediatric population. Their occurrence in adults is exceptional. Splenectomy is the common management of splenic lesions for diagnostic and/or therapeutic purpose. Our aim is to report additional cases of SL diagnosed on splenectomy specimens at our Pathology laboratory with literature review.
Methods
This is a retrospective study including all cases of splenectomy recorded at our Pathology laboratory (June 2020–August 2022). We performed a comparison of clinicopathological features between patients with SL and those with other benign splenic diseases.
Results
Sixteen cases of splenectomy were included. The mean age was 30.25 years (range of 6–70 years). The final histopathological diagnoses were congestive spleens in all cases of sickle cell disease (SCD) (5/16 patients, 31.25%), splenic cystic lymphangiomas (4/16 patients, 25%), capsular splenic infiltration by gastric and colic cancers (3/16 cases, 18.75%), splenic abscess (2/16 cases, 12.5%) and splenic rupture with subcapsular hematoma (1/16 patients, 6.25%). 12/16 patients (75%) had benign splenic conditions (4/12 with SL, 5/12 with SCD, 2/12 with abscess and 1/12 with splenic trauma). Patients with SL were older than those with other benign splenic conditions (mean age of 28.27 years versus 20.87 years). Also patients with SL presented with massive splenomegaly (mean splenic weight of 1675 g versus 418.75 g, mean splenic size of 19.62 cm versus 14.63 cm). Open surgery was performed in 15/16 patients (93.75%).
Conclusion
Unlike previous studies, our series shows that SL are a common indication for splenectomy and occur in older patients with massive cystic splenomegaly. Open splenectomy is still an usual surgical practice in our country.
Similar content being viewed by others
Background
Splenic lymphangioma (SL) is a very rare cystic lesion affecting usually children and less commonly reported in adult persons [1,2,3,4,5,6,7]. These lesions are benign and considered by some authors as vascular (lymphatics) malformations rather than true tumors as they are found mainly in children and young patients with some congenital malformative syndrome such as Klippel-Trenaunay syndrome (association of varicose veins, cutaneous capillary malformations and hypertrophy of bone and/or soft tissue) [7,8,9]. SL may be asymptomatic or present with upper left abdominal pain, splenomegaly, hypersplenism or splenic rupture with hemorragic shock [1, 2, 10, 11]. Clinical and radiological features of SL are not specific, usually they present as cystic splenic lesions that may correspond to a variety of splenic diseases: congenital epithelial cysts (CEC), neoplastic cysts, parasitic hydatid cysts, traumatic cysts or splenic abscess [6, 7, 12,13,14,15].
Splenectomy either total or partial, laparoscopic or open, is the common management of splenic lesions for diagnostic and/or therapeutic purpose [4, 16,17,18], without the need for preoperative histopathological diagnosis through biopsy or fine-needle aspiration cytology (FNAC) [19]. Splenectomy is also a common therapeutic options in splenic trauma or in many hematologic diseases like sickle cell disease (SCD), thalassemia, hemolytic anemia, immune thrombocytopenia, hereditary spherocytosis or certain types of leukemias [18, 20, 21].
As the current literature offers only some case reports and rare case-series of SL, our aim is to report additional cases of SL diagnosed on splenectomy specimens at our newly operative pathology laboratory (the unique functional pathology laboratory in a public hospital in our country), with comparison of clinicopathological features between SL and other spleen diseases that have been the indications for splenectomy.
Methods
Study design
This is a retrospective study including all cases of splenectomy recorded at the Pathology laboratory of the Niamey National Hospital (Hôpital National de Niamey) from June 2020 to August 2022. As the unique Pathology laboratory in public hospital (functional since June 2020), we receive specimens from all medical centers of the country. Our cases of splenectomy were from 3 main National hospitals of the capital city of our country (Hôpital National de Niamey, Hôpital Général de Référence de Niamey, and Hôpital National Amirou Boubacar Diallo de Niamey) and 1 regional hospital (Centre Hospitalier Régional de Tillabéry). We have collected patients data such as: age, sex, preoperative diagnosis (indication for splenectomy), spleen weight and size (measured at our Pathology laboratory after surgical resection), splenic gross features, the type of surgical resection (either splenectomy alone or associated with other organs resection) and histopathological final diagnosis.
Diagnoses
All cases have been diagnosed by routine histopathological techniques performed on surgical resected splenic specimens: formalin fixation, paraffin-embedding, microtome sectioning (4 microns thick) and hematoxylin–eosin (HE) staining. All cases have been analysed by using optic microscopy.
Data analysis
Continuous variables are presented as means and ranges, categorical variables as percentages. We have grouped benign splenic diseases into 2 groups: a group of splenic lymphangioma (SL) and a group of ther benign splenic conditions. We performed a comparison between these 2 groups. In the comparison, we have excluded spleens resected for malignancies as they had all normal gross features, the indications were not primary spleen malignancies and the splenic parenchyma was not involved by the tumors (only superficial involvement of the capsule in some cases).
All descriptive statistical analyses were performed by using IBM SPSS Statistics 23.0.
Results
Overral clinicopathological features of the entire series
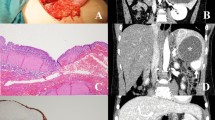
The Table 1 summerises the clinicopathological features of our series. From June 2020 to August 2022, we have registered 16 cases of splenectomy. The mean age was 30.25 years (range of 6–70 years), with a male to female ratio (M:F) of 1,66 (10:6). The preoperative indications were hypersplenism and abdominal pain in patients with SCD (5/16 cases, 31.25%), splenic cystic lesions (4/16 cases, 25%), intra-abdominal cancers with suspected extension to the spleen (4/16 cases, 25%), 2 cases of isolated left upper abdominal pain with fever in 1 patient (2/16 cases, 12.5%) and 1 patient with abdominal trauma (1/16 cases, 6.25%). The mean splenic weight and size were respectively 837.5 grammes (g) (range of 100–2300 g) and 14.96 cm (range of 10–24.5 cm). Mainly, the gross features were homogenous congestive spleen with smooth surface in 9 cases (56.25%) (Fig. 1A, B) and multiloculated cystic lesions in 4 cases (25%) (Fig. 2A–C). All cases, except one (case 6 who underwent laparoscopic splenectomy and cholecystectomy) have been resected by open surgery (15/16 patients, 93.75%). Four cases (25%) underwent complex organs resections due to locally advanced tumors (Fig. 1A), in the remaining cases (12/16 cases, 75%) total splenectomy was performed.
A Macroscopic view (after formalin fixation) of a splenic lymphangioma showing a well-encapsulated septated cyst with serous content (case 7). B Macroscopic view (after formalin fixation) of a splenic lymphangioma showing an entirely bosselated spleen (case 10). C Macroscopic view of a splenic lymphangioma cut surface showing multiple cysts occupying the entire spleen with mucoid and sero-hematic content (case 10)
The final histopathological diagnoses were congestive spleens in all cases of SCD (5/16 patients, 31.25%) (Fig. 3A, B), splenic cystic lymphangiomas in 4 patients (25%) (Fig. 4A, B), 3 cases of capsular splenic infiltration (18.75%) by a gastric GIST (gastrointestinal stromal tumor), colic adenocarcinoma and gastric adenocarcinoma, 2 cases of splenic abscesses (12.5%) (Fig. 5) and 1 patient with splenic rupture and subcapsular hematoma due to an abdominal trauma.
A Histological view of a splenic specimen in a patient with SCD showing congestive spleen with multiple dilated small vessels in the red and white pulp (hematoxylin–eosin × 40) (case 16). B At higher magnification, the splenic parenchyma is dissociated by numerous sickled, spindle-shaped red blood cells characteristic of the SCD (black arrows) (hematoxylin–eosin × 400) (case 16)
A Histological view of a splenic lymphangioma showing variable-sized cystic spaces containing amorphous eosinophilic material (hematoxylin–eosin × 100) (case 10). B At higher magnification, splenic lymphangioma is made of cystic spaces lined by flattened endothelial cells without atypias (hematoxylin–eosin × 400) (case 10)
Benign splenic diseases (conditions) versus splenic lymphangiomas
Our series included 4 cases (25%) of spleen resections associated with malignant tumors of adjacent organs (stomach, colon and pancreas) that superficially invaded the spleen capsule. The gross features of the spleen (weight and size particularly) in these cases were quite normal. The remaining cases (12/16 cases, 75%) had benign splenic conditions (4 patients with SL, 5 patients with SCD, 2 patients with abscesses and 1 case of splenic trauma).
The Table 2 shows the differential clinical and gross features between patients with SL and those with other benign conditions. Patients with SL were older than those with other benign splenic conditions (mean age of 28.27 years versus 20.87 years). Also cases of SL presented with massive splenomegaly with larger size and heavier spleens (mean splenic weight of 1675 g versus 418.75 g and mean splenic size of 19.62 cm versus 14.63 cm). There was a male predominance in patients with benign conditions, as well as in the 2 subgroups of SL and other benign diseases other than lymphangiomas.
Discussion
We report from a Subsaharan African country a series of patients that underwent total splenectomy for a variety of diseases. Open surgery was performed in almost all cases (15/16 patients, 93.75%); SCD (5/16 cases, 31.25%), SL (4/16 cases, 25%) and malignant intra-abdominal tumors with suspected extension to the spleen (4 cases, 25%), were the most indications for the splenectomy. Benign splenic conditions were the most frequent indications for surgery (12/16 patients, 75%), among them SCD (5/12 cases, 41.66%) and SL (4/12 cases, 33.33%) were the leading causes of surgery. Hematologic diseases have been reported to be the major indication for splenectomy in the literature [17, 21,22,23,24,25,26,27,28], however we are not aware of SL as a common indication for surgery even in selected series (especially in pediatric population). The small sample and the short period of our study could be the reason of these discrepancies with the previous reports in the literature. Hematologic diseases such as idiopathic thrombocytopenia purpura (ITP) and hereditary spherocytosis are the major indication for splenectomy in series especially from Western countries [21,22,23, 25] while splenic complications of SCD represent a common cause of splenectomy in African and Middle-East countries [16, 29, 30]. Splenectomy is usually performed in patients with SCD complications like acute splenic sequestration crisis, hypersplenism, splenic abscess, and massive splenic infarction, with good outcomes and this surgery also reduces requirements for repeated blood transfusions and prevents mechanical complications of an enlarged spleen [16, 30]. However, a recent meta-analysis challenged these beneficial effects of splenectomy by concluding that there is a lack of evidence from trials showing that splenectomy improves survival and decreases morbidity in people with SCD [31]. Malignant tumors either primary or secondary affect very rarely the spleen, and represent a less usual indication for splenectomy [23, 32]. In our series there were no primary splenic tumors, however we have registered 3 cases with splenic capsular invasion by cancers from adjacent organs (stomach and left colon).
Since its introduction in surgical clinical practice in early 1990 s, laparoscopic splenectomy (LS) has become a gold standard in the management of patients with splenic diseases either benign or malignant [21, 23, 25]. Many reports shows that LS has lower morbidity with cosmetic advantages, less perioperative bleeding, short hospital stay and less conversion rate to open surgery [23, 33]. Even in patients with massive splenomegaly (> 1000 g), LS could be safely performed [33, 34]. However, this minimally invasive surgery requires experience and acquisition of more technical skills over time (learning curve), with longer operative time especially in patients with massive splenomegaly [23, 25, 35]. In our series, open splenectomy (OS) was widely performed (15/16 cases, 93.75%) even in patients with benign diseases and mild splenomegaly (< 500 g). The reason for this classic surgical approach was the lack of adequate laparoscopic materials in our hospitals, with rare surgeons that have skills in laparoscopic surgery.
In our study, patients with SL were older than patients with other benign splenic conditions (mean age of 28.25 versus 20.87 years), because of many children with SCD in this subgroup of benign splenic conditions. Also, patients with SL presented with heavier spleens and massive splenomegaly (mean weight and size of 1675 g and 19.62 cm) whereas patients with other benigh splenic conditions had mild splenomegaly (mean weight and size of 418.75 g and 14.43 cm). In fact in this subgroup, the indications for splenectomy were acute symptoms such as abdominal pain, fever and hypersplenism, rather than the importance of the splenomegaly and abdominal distension in comparison with patients that had SL.
In the literature, SL are usually reported in children as asymptomatic or rarely symptomatic lesions, with few case reports in adults [2, 4, 6, 7, 15, 36]. Our current series challenged these classic features as all patients presented with massive splenomegaly with abdominal distension (mean splenic weight of 1675 g, range of 1300–2300 g). Also, in our series patients were older (mean age of 28.25 years, range of 17–37 years) with 3 young adults and 1 adolescent patient of 17 years. As in our cases, SL present as cystic lesions of variable size with honeycombing appearance and compact pale, yellowish content (lymphatic fluid) on resected specimens [2, 7]. The preoperative imaging techniques are not specific, they show only the cystic aspect of the lesion, that could correspond to a variety of cystic splenic lesions [12]. The Table 3 summerises the main characteristics of splenic cystic lesions. Several classifications of splenic cystic lesions have been proposed [6, 13, 15, 36]. Splenic cystic lesions are classified as: neoplastic cysts, congenital epithelial cysts (CEC), parasitic cysts (mainly hydatid cysts), and other cysts as a results of infarction (traumatic cysts) or inflammation/infection (abscess). Neoplastic cysts include vascular lesions (hemangiomas, lymphangiomas or angiosarcomas) or any malignant solid splenic tumors with cystic degeneration. Vascular cystic lesions are lined by regular flat endothelial cells, with luminal content made of red blood cells (hemangioma) or proteinaceous amorphous eosinophilic material (lymphangioma) [36]. The histological aspects of our 4 cases of SL were typically consistent with the later aspect. In angiosarcomas, the lining endothelial cells present atypias, mitoses and necrosis [37]. Congenital epithelial cysts (CEC) are common in pediatric population, the cysts are lined by epithelial cells with mesothelial, transitional or squamous differenciation. All these types of epithelial cells could be found in combination in a single lesion. These lesions are supposed to derive from mesothelial invagination through the splenic parenchyma [6, 15]. Splenic hydatid cysts are caused by Echinococcus granulosus infection and are endemic in certain areas of the World [38]. On histopathological analysis, the parasit’s structures are readily observed in the splenic cyst. The remaining causes of splenic cysts are the result of previous trauma, infarction or infection. They present histologically as pseudo-cystic lesions without epithelial or endothelial lining. Previously, splenic pseudo-cysts were believed to be common, however a careful histological analysis usually find focal endotheilal or epithelial lining in what may be misdiagnosed as a pseudo-cyst [14].
As a retrospective analysis, our study has some limitations, especially a small number of patients. The data about imaging techniques and follow-up are not available. However some provisional conclusions could be drawn, until the availability of larger studies on SL in the future.
Conclusion
Unlike previous literature reports, our series shows that splenic lymphangiomas (SL) are a common indication for splenectomy. They occur in older patients and present with massive cystic splenomegaly. Open splenectomy is still an usual surgical practice in poor countries. The definitive diagnosis of SL relies on the histopathological analysis of the resected splenic specimens.
Availability of data and materials
All data generated or analysed during this study are included in this article.
Abbreviations
- SL:
-
Splenic lymphangioma
- SCD:
-
Sickle cell disease
- HE:
-
Hematoxylin–eosin
- FNAC:
-
Fine needle aspiration cytology
- GIST:
-
Gastrointestinal stromal tumor
- LS:
-
Laparoscopic splenectomy
- ITP:
-
Idiopathic thrombocytopenia purpura
- CEC:
-
Congenital epithelial cysts
References
Duvvada S, Senapati D, Challa SR, Kalluri T. Cystic lymphangioma of spleen in adults. BMJ Case Rep. 2017;2017:bcr2016216267. https://doi.org/10.1136/bcr-2016-216267.
Efared B, Atsame-Ebang G, Zabeirou A, Hammas N, Mazaz K, El Fatemi H, et al. Isolated splenic lymphangioma presenting as a huge mass causing anemia and abdominal distension in an adult patient: a case report. J Med Case Rep. 2018;12:97. https://doi.org/10.1186/s13256-018-1664-5.
Kakaje A, Mujahed R, Hamdan O. Isolated Splenic Lymphangiomas presenting in an infant with isolated anaemia. Case Rep Med. 2020;2020:8919424. https://doi.org/10.1155/2020/8919424.
Kimura K, Kurashima Y, Tanaka K, Nakanishi Y, Asano T, Ebihara Y, et al. Laparoscopic partial splenectomy for splenic lymphangioma: a case report. Surg Case Rep. 2020;6:140. https://doi.org/10.1186/s40792-020-00882-1.
Xu G-P, Shen H-F, Yang L-R, Ma Q, Gao B-L. Splenic cystic lymphangioma in a young woman: case report and literature review. Acta Gastroenterol Belg. 2011;74:334–6.
Hodge MG, Ricketts RR, Simoneaux SF, Abramowsky CR, Elawabdeh N, Shehata BM. Splenic cysts in the pediatric population: a report of 21 cases with review of the literature. Fetal Pediatr Pathol. 2012;31:54–62. https://doi.org/10.3109/15513815.2011.648725.
Ioannidis I, Kahn AG. Splenic lymphangioma. Arch Pathol Lab Med. 2015;139:278–82. https://doi.org/10.5858/arpa.2013-0656-RS.
Yamazaki M, Kawamura Y, Ohka T, Katada S, Morita K, Nakagawa M, et al. Cavernous lymphangioma of the spleen in a patient with Klippel-Trenaunay-Weber syndrome. Intern Med. 1994;33:574–7. https://doi.org/10.2169/internalmedicine.33.574.
Withana M, Rodrigo C, Shivanthan MC, Warnakulasooriya S, Wimalachandra M, Gooneratne L, et al. Klippel-Trenaunay syndrome presenting with acanthocytosis and splenic and retroperitoneal lymphangioma: a case report. J Med Case Rep. 2014;8:390. https://doi.org/10.1186/1752-1947-8-390.
Evola G, Mazzone G, Corsaro A, Brancato G, Evola FR, Basile G. Hemorrhagic shock from post-traumatic rupture of microcystic splenic lymphangioma: a case report and review of the literature. Int J Surg Case Rep. 2020;75:376–9. https://doi.org/10.1016/j.ijscr.2020.09.045.
Perez A, Perez MEC, Yuga AC, Viray BAG. Splenic lymphangioma in adulthood: a case report. Int J Surg Case Rep. 2020;67:250–3. https://doi.org/10.1016/j.ijscr.2020.01.061.
Kim N, Auerbach A, Manning MA. Algorithmic Approach to the splenic lesion based on radiologic-pathologic correlation. Radiographics. 2022;42:683–701. https://doi.org/10.1148/rg.210071.
Morgenstern L. Nonparasitic splenic cysts: pathogenesis, classification, and treatment. J Am Coll Surg. 2002;194:306–14. https://doi.org/10.1016/s1072-7515(01)01178-4.
Vajda P, Kereskai L, Czauderna P, Schaarschmidt K, Kalman A, Koltai J, et al. Re-evaluation of histological findings of nonparasitic splenic cysts. Eur J Gastroenterol Hepatol. 2012;24:316–9. https://doi.org/10.1097/MEG.0b013e32834ea639.
Shabtaie SA, Hogan AR, Slidell MB. Splenic cysts. Pediatr Ann. 2016;45:e251-6. https://doi.org/10.3928/00904481-20160523-01.
Al-Salem AH. Indications and complications of splenectomy for children with sickle cell disease. J Pediatr Surg. 2006;41:1909–15. https://doi.org/10.1016/j.jpedsurg.2006.06.020.
Wang Z, Peng C, Wu D, Wang K, Xu J, Sun J, et al. Surgical treatment of benign splenic lesions in pediatric patients: a case series of 30 cases from a single center. BMC Surg. 2022;22:295. https://doi.org/10.1186/s12893-022-01745-2.
Weledji EP. Benefits and risks of splenectomy. Int J Surg. 2014;12:113–9. https://doi.org/10.1016/j.ijsu.2013.11.017.
Gonzalez-Urquijo M, Rodarte-Shade M, Gil-Galindo G. Splenic primary solid tumors: does a preoperative histopathology diagnosis really Matter? Am Surg. 2021;87:316–20. https://doi.org/10.1177/0003134820951480.
Chopra R, Al-Mulhim AR, Al-Baharani AT. Fibrocongestive splenomegaly in sickle cell disease: a distinct clinicopathological entity in the Eastern province of Saudi Arabia. Am J Hematol. 2005;79:180–6. https://doi.org/10.1002/ajh.20380.
Corcione F, Pirozzi F, Aragiusto G, Galante F, Sciuto A. Laparoscopic splenectomy: experience of a single center in a series of 300 cases. Surg Endosc. 2012;26:2870–6. https://doi.org/10.1007/s00464-012-2272-x.
Murawski M, Patkowski D, Korlacki W, Czauderna P, Sroka M, Makarewicz W, et al. Laparoscopic splenectomy in children–a multicenter experience. J Pediatr Surg. 2008;43:951–4. https://doi.org/10.1016/j.jpedsurg.2007.11.040.
Pattenden CJ, Mann CD, Metcalfe MS, Dyer M, Lloyd DM. Laparoscopic splenectomy: a personal series of 140 consecutive cases. Ann R Coll Surg Engl. 2010;92:398–402. https://doi.org/10.1308/003588410X12664192076133.
Rescorla FJ, West KW, Engum SA, Grosfeld JL. Laparoscopic splenic procedures in children: experience in 231 children. Ann Surg. 2007;246:683–7. https://doi.org/10.1097/SLA.0b013e318155abb9. discussion 687–688.
Fraser SA, Bergman S, Garzon J. Laparoscopic splenectomy: learning curve comparison between benign and malignant disease. Surg Innov. 2012;19:27–32. https://doi.org/10.1177/1553350611410891.
Fu X, Yang Z, Tu S, Xin W, Chen H, Li X, et al. Short- and long-term outcomes of 486 consecutive laparoscopic splenectomy in a single institution. Med (Baltim). 2021;100:e25308. https://doi.org/10.1097/MD.0000000000025308.
Frasier LL, Malani PN, Diehl KM. Splenectomy in older adults: indications and clinical outcomes. Int J Hematol. 2013;97:480–4. https://doi.org/10.1007/s12185-013-1300-5.
Kouadio KG, Kouassi JC, Ehua SF, Kanga-Miessan JB, Turquin TH. Splenectomy for splenomegaly in Ivory Coast. Indications and short term results. Mali Med. 2006;21:23–6.
Tang X-F, Zhang W-Y, Li G, Jiang L-L, Liu W-P. Splenic lymphangioma: clinicopathologic and immunohistochemical study of 18 cases and review of literature. Zhonghua Bing Li Xue Za Zhi. 2007;36:98–101.
Ghmaird A, Alnoaiji MM, Al-Blewi S, Zaki S, El-Lewi A, Ahmad N. Splenectomy in patients with Sickle Cell Disease in Tabuk. Open Access Maced J Med Sci. 2016;4:107–11. https://doi.org/10.3889/oamjms.2016.034.
Owusu-Ofori S, Remmington T. Splenectomy versus conservative management for acute sequestration crises in people with sickle cell disease. Cochrane Database Syst Rev. 2017;11:CD003425. https://doi.org/10.1002/14651858.CD003425.pub4.
Efared B, Mazti A, Atsame-Ebang G, Tahiri L, El Bouhaddouti H, Hammas N, et al. An unusual site of metastasis: splenic metastastasis from a colon cancer. J Surg Case Rep. 2016;2016:rjw175. https://doi.org/10.1093/jscr/rjw175.
Rodríguez-Luna MR, Balagué C, Fernández-Ananín S, Vilallonga R, Targarona Soler EM. Outcomes of laparoscopic splenectomy for treatment of splenomegaly: a systematic review and Meta-analysis. World J Surg. 2021;45:465–79. https://doi.org/10.1007/s00268-020-05839-x.
Targarona EM, Espert JJ, Cerdán G, Balagué C, Piulachs J, Sugrañes G, et al. Effect of spleen size on splenectomy outcome. A comparison of open and laparoscopic surgery. Surg Endosc. 1999;13:559–62. https://doi.org/10.1007/s004649901040.
Shin RD, Lis R, Levergood NR, Brooks DC, Shoji BT, Tavakkoli A. Laparoscopic versus open splenectomy for splenomegaly: the verdict is unclear. Surg Endosc. 2019;33:1298–303. https://doi.org/10.1007/s00464-018-6394-7.
Yocum BP, Hwang M, Mesa H, Collins K. Differential diagnosis of cystic lesions of the spleen: a review of Clinical, Imaging and pathological findings. Int J Surg Pathol. 2022;2022:10668969221107080. https://doi.org/10.1177/10668969221107080.
Sangiorgio VFI, Arber DA. Vascular neoplasms and non-neoplastic vascular lesions of the spleen. Semin Diagn Pathol. 2021;38:154–8. https://doi.org/10.1053/j.semdp.2020.07.001.
Ozogul B, Kisaoglu A, Atamanalp SS, Ozturk G, Aydinli B, Yıldırgan M, et al. Splenic hydatid cysts: 17 cases. Indian J Surg. 2015;77:257–60. https://doi.org/10.1007/s12262-012-0788-x.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
BE, wrote the article and made substantial contributions to conception and design of the article; ABAB, HY, IB, AZ, HHK, HSB, SA, ISK, JDL and HN have been involved in drafting the manuscript and revising it critically for important intellectual content. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the declaration of Helsinki and with approval from the Ethics Committee of the Niamey National Hospital (Anapath, département-DAMTE, HNN). As a retrospective study reporting anonymous, de-identified data, the need for informed consent is deemed unnecessary by the ethics commitee (DAMTE, Hôpital National de Niamey, Niger).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Efared, B., Bako, A.B.A., Younssa, H. et al. Splenic lymphangiomas as a common indication for splenectomy: a case series with literature review. BMC Surg 22, 446 (2022). https://doi.org/10.1186/s12893-022-01898-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-022-01898-0