Abstract
Background
People with nonspecific low back pain (NSLBP) can also experience overlapping symptoms of lumbar spinal stenosis (LSS), but the impact on treatment outcomes is unknown. This study investigated differences in treatment outcomes for disability, back pain intensity, and leg pain intensity following an education and exercise therapy program for NSLBP patients with and without comorbid LSS symptoms.
Methods
This was a longitudinal analysis of 655 Danish participants in the GLA:D® Back program; an education and exercise therapy program for people with persistent NSLBP. Participants were classified as having comorbid LSS symptoms based on self-report. Linear mixed models were used to assess differences in change in disability (Oswestry Disability Index [0-100]) and back and leg pain intensity (Numeric Rating Scale [0–10]) at 3-, 6-, and 12-months between those with and without LSS symptoms.
Results
28% of participants reported LSS symptoms. No certain differences in change in disability or back pain intensity improvement were observed at any time-point between those with and without LSS symptoms. Participants with LSS symptoms had slightly greater improvement in leg pain intensity at 6- (-0.7, 95% CI -1.2 to -0.2) and 12-months (-0.6, 95% CI -1.2 to -0.1).
Conclusion
Compared to those without LSS symptoms, patients with persistent NSLBP and LSS symptoms can expect similar improvements in disability and back pain intensity, and slightly greater improvements in leg pain intensity with treatment. Therefore, education and exercise therapy programs designed for NSLBP are likely helpful for those also experiencing LSS symptoms.
Similar content being viewed by others
Background
Like nonspecific low back pain (NSLBP), lumbar spinal stenosis (LSS) is a common condition in the aging population [1], including in primary care settings [2]. Also like NSLBP, most patients with LSS can be managed at the primary care level with interventions including patient education and exercise therapy [1, 3]. At present a significant proportion of people seeking care for NSLBP have symptoms typically attributed to LSS [4], even if they have no formal LSS diagnosis, due to the well-documented overlap in symptoms and diagnostic uncertainty between NSLBP and LSS symptoms [5,6,7,8,9,10] and lack of commonly accepted diagnostic standards for LSS [1]. It is therefore of interest to know whether primary care patients with LSS symptoms benefit from programs designed for patients with NSLBP, or if they require interventions tailored specifically for LSS [11].
The GLA:D® Back program and patient registry presents the unique opportunity to evaluate the impact of LSS symptoms on outcomes following education and exercise therapy for people with NSLBP. GLA:D® Back is a standardised care package delivered across Denmark comprised of group-based patient education and exercise therapy aimed at improving self-management abilities [12, 13]. An objective of the GLA:D® Back research program is to identify subgroups of people who do not benefit sufficiently from the intervention [12] and previous work has found that 71% of participants in GLA:D® Back report “sometimes having pain or numbness in one or both legs or buttocks”; a symptom commonly attributed to LSS [4]. Other common LSS symptoms ranged in prevalence from 11 to 58% in these participants [4], but the impact on GLA:D® Back treatment outcomes has not been evaluated.
The objective of this study was to investigate differences in treatment outcomes for disability, back pain intensity, and leg pain intensity following an education and exercise therapy program (GLA:D® Back) for NSLBP patients with and without comorbid LSS symptoms. We hypothesised that LSS symptoms would be associated with less improvement in all outcomes.
Methods
Study design
Longitudinal analysis of registry data from the GLA:D® Back program for NSLBP [12]. GLA:D® Back consists of two patient education sessions and 8 weeks of supervised group exercise sessions [12, 14]. Detailed information on program content is available elsewhere [12,13,14]. This report conforms to the STROBE statement for reporting observational studies. As part of the larger GLA:D Back research program [12], ethical approval for this analysis was not required according to the Regional Committees on Health Research Ethics for Southern Denmark (S-2017000-93), but was conducted in accordance with the Declaration of Helsinki. All participants provided informed consent prior to enrolment in GLA:D® Back.
Participants
People seeking care for persistent or recurrent NSLBP are eligible for GLA:D® Back if they are 18 years of age or older, understand Danish, and have a need for improved self-management based on shared decision-making by participant and enrolling clinician [12, 14]. Clinicians are given guidance in the GLA:D® Back training course on who may be best suited for the program, but are free to follow their best clinical judgement in a dialogue with the patient. Therefore, although the program was designed for NSLBP, clinicians can enrol patients with symptoms that overlap with symptoms of LSS and/or radiculopathy, when they deem the program relevant to these patients.
Self-report symptom items associated with LSS were included in the routinely collected electronic baseline survey in the GLA:D® Back registry. Participants with complete baseline LSS symptom data, who were enrolled between May and October 2019, were included in this analysis. This data collection period was selected to ensure only participants completing the treatment program (approximately 3 months) prior to the COVID-19 pandemic were included.
LSS symptoms (exposure)
Participants were classified as having LSS symptoms (yes/no) if they reported all of the following: (1) “sometimes feeling pain or numbness in one/both legs or buttocks”; (2) one or more symptom-worsening activities (walking, standing for a while); (3) one or more symptom-relieving activities (bending forwards, sitting, riding a bicycle, bending over a shopping cart); and (4) were aged 60 years or older. While not diagnostic, these items are commonly-used in the self-report of LSS and are able to adequately differentiate leg pain from LSS from other sources of back-related leg pain [5]. Similar LSS definitions have been used in recent LSS studies [15,16,17,18].
Outcomes
The primary outcome was the difference in mean change in disability between those with LSS symptoms and those without from baseline to 3-, 6-, and 12-months based on the Oswestry Disability Index (ODI) version 2.1 [19]. The ODI is scored from 0 (best) to 100 (worst) and is a valid and reliable measure in Danish people with NSLBP [19]. Although not specific to LSS, the ODI was selected as the primary outcome measure as it was relevant to both NSLBP and LSS.
Secondary outcomes were differences in mean change in back pain intensity and leg pain intensity, respectively, from baseline to 3-, 6-, and 12-months on the Numeric Rating Scale (NRS). The NRS is scored from 0 (best) to 10 (worst) for both back pain intensity and leg pain intensity [20].
Analysis
Multiple imputation
Under the assumption of data missing at random, missing data for baseline covariates (confounders described in main analyses) and outcomes at all follow-up times were imputed using multiple imputation with chained equations [21]. The imputation model included all outcomes at each time point, LSS symptom status (exposure), and all confounders. Predictive mean matching was used to impute all continuous data, logistic regression for binary data, and ordered logistic regression for ordered categorical data. 52 imputed data sets were generated to match the proportion of missing data for the primary outcome at 12-months [21].
Main analyses
The primary outcome (difference in mean change in disability) from baseline to 3-, 6-, and 12-month follow-up) was estimated using a linear mixed model (restricted maximum likelihood ratio), where LSS symptom status and follow-up time were entered as fixed effects and participants nested within clinics were random effects. Unadjusted and adjusted differences in change with 95% confidence intervals (CI) were combined across imputed data sets using Rubin’s rules [22]. Potential confounders in the adjusted model included age (continuous), sex (binary), BMI (continuous), education level (ordered categorical), STarT Back Screening Tool classification (ordered categorical), and episode duration (ordered categorical). The same analysis approach was used for the secondary outcomes (difference in mean change in back pain intensity and leg pain intensity, respectively) from baseline to 3-, 6-, and 12-month follow-up. All analyses were performed in Stata 17.0.
Sensitivity analyses
In the first sensitivity analysis, the impact of missing data was explored by conducting a complete-case analysis, where only participants with completed outcomes were included. Adjusted differences (same controlled covariates as main analyses) in mean change in all outcomes from baseline to 3-, 6-, and 12-month follow-up were estimated.
Second, the impact of the selected age cut-point (≥ 60 years) in the LSS symptom definition was evaluated by repeating the analysis using an alternate cut-point of 50 years or older. This cut-point was selected since some LSS diagnostic studies suggest a cut-point as low as > 48 years [8] and 50 years old has been used in an analysis with similar LSS symptom items [6]. Adjusted differences (same controlled covariates as main analyses) in mean change in all outcomes from baseline to 3-, 6-, and 12-month follow-up were estimated.
Results
Sample characteristics
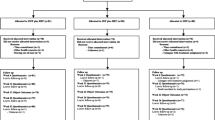
A total of 861 participants were enrolled in GLA:D® Back during the study period. A total of 206 participants were excluded due to missing baseline LSS symptom data. The excluded sample could not be compared with the analytic sample since the majority (99%) of excluded participants provided no baseline data. Accordingly, 655 participants were included in the analytic sample, of which 185 (28%) were classified as reporting LSS symptoms. Participants with LSS symptoms were substantially older, more often had a longer episode duration, and had worse disability, back pain, and leg pain scores when compared to those without LSS symptoms (Table 1).
Missing outcome data
The proportion of missing ODI data was 32%, 42%, and 52% at 3-, 6-, and 12-month follow-up for the ODI. Proportion of missing back and leg pain NRS data was slightly less at each time-point. Participants with missing primary outcome data (ODI) at 12-months were younger, and had: a higher STarT Back classification (i.e., greater risk of chronicity), a longer episode duration length, and worse ODI and back and leg pain intensity scores (Additional File 1). A smaller proportion of participants with missing primary outcome data were classified as having LSS symptoms (24% versus 33%).
Disability
In the adjusted analysis, we found disability scores slightly improved over time regardless of LSS symptom status, but those with LSS symptoms had slightly worse scores at baseline, 3-, and 6-months, but not at 12-months (Fig. 1). There was no certain difference between those with and without LSS symptoms in change in disability scores from baseline to 3- (0.4, 95% CI -1.4 to 2.3), 6- (0.1, 95% CI -2.0 to 2.2), or 12-month follow-up (-0.8, 95% CI -3.1 to 1.5) (Table 2). Unadjusted results were similar (Additional File 1).
Back pain intensity
The adjusted analysis found back pain intensity scores improved over time regardless of LSS symptom status and no certain differences in back pain intensity scores were found at any time-point (Fig. 2). There was no certain difference between those with and without LSS symptoms in change in back pain intensity scores from baseline to 3- (-0.1, 95% CI -0.5 to 0.4), 6- (-0.2, 95% CI -0.7 to 0.3), or 12-month follow-up (0.0, 95% CI -0.5 to 0.5) (Table 2). Unadjusted results were similar (Additional File 1).
Leg pain intensity
The adjusted analysis found leg pain scores intensity scores improved over time regardless of LSS symptom status, but those with LSS symptoms had worse leg pain intensity scores at all time-points (Fig. 3). There was no certain difference between those with and without LSS symptoms in change in leg pain intensity scores from baseline to 3-month follow-up (-0.3, 95% CI -0.8 to 0.2), but participants with LSS symptoms had slightly greater improvement from baseline to 6- (-0.7, 95% CI -1.2 to -0.2) and 12-month follow-up (-0.6, 95% CI -1.2 to -0.1) (Table 2). Unadjusted results were similar (Additional File 1).
Sensitivity analyses
Results of the complete case sensitivity analysis (Additional File 1) and the alternate age cut-point sensitivity analysis (Additional File 1) confirmed that no certain difference in change in disability scores was observed between those with and without LSS symptoms at any time-point. Likewise, both sensitivity analyses confirmed the main analysis results for between-group differences in change in back and leg pain intensity scores, respectively.
Discussion
Our findings suggest that regardless of LSS symptom status, patients with persistent or recurrent NSLBP can expect similar, small improvements in disability and moderate improvements in back pain intensity for up to a year following a structured education and exercise therapy program. However, patients with LSS symptoms can expect slightly greater improvement in leg pain intensity compared to those without LSS symptoms. Irrespective of differences in improvement, patients with LSS symptoms do experience slightly worse absolute disability and moderately worse absolute leg pain intensity scores both before and after treatment.
We found 28% of patients enrolled in primary care program for NSLBP also had LSS symptoms, which is in line with prevalence estimates in primary care settings [2]. These patients experienced similar magnitudes of improvements in all outcomes compared to patients without LSS symptoms, except for even greater leg pain intensity improvement from baseline to 6- and 12-months. However, it is unclear if the magnitude of these between-group differences (0.7 and 0.6 points on a 10-point scale, respectively) represent a clinically meaningful difference. While no studies have investigated what constitutes a meaningful between-group difference in leg pain intensity, previous studies suggest a minimum clinically important within-group change on the back pain NRS to range from 1.0- to 2.0-points in NSLBP populations [23, 24]. Since the 95% CI for both these between-group estimates include a 1.0-point change, there may be indication that a clinically meaningful difference in leg pain intensity improvement exists. The findings in both sensitivity analyses support this interpretation.
We are not aware of any previous studies that have investigated the impact of LSS symptoms in samples of patients participating in an education and exercise therapy program for persistent or recurrent NSLBP. Therefore, the findings of this study should be considered alongside relevant LSS literature [25] to guide treatment selection through a shared-decision making process by patients and clinicians. Our findings suggest that a program designed for patients with persistent or recurrent NSLBP can be helpful for patients with LSS symptoms, especially where primary care programs tailored for patients with LSS are not available. Treatment decision-making would benefit from trials making direct comparisons of programs like GLA:D® Back to LSS-specific programs that also include education and exercise, [16, 26] as well as other conservative treatment options for LSS [11].
The main study limitation is the LSS symptom case definition. There is no widely accepted diagnostic standard for LSS [1, 27] and varying definitions have been used in previous studies [8, 28]. We used symptom items identified in a review of self-report LSS screening items that found these items were able to differentiate leg pain due to LSS from other back-related causes of leg pain [5]. These items also include those most likely to identify LSS [8] and our definition resembles definitions used in a recent LSS trial and prevalence study [15, 16]. Confirmation of our results using the alternate age cut-point increases confidence in our findings. We were unable to include imaging confirmation of LSS since this is not collected in GLA:D® Back, but our approach is sufficient for preliminary estimates in this field [17, 18], considering LSS is a clinical diagnosis [27].
There was also a large proportion of missing baseline and outcome data, which is unavoidable in real-world implementation programs like GLA:D® Back. However, results were similar when analysing imputed outcomes and complete cases only, but the impact of differing baseline characteristics between the analytic and excluded samples are unknown. A selection bias in GLA:D® Back participants may also exist since enrolling clinicians are likely to recommend alternative treatments to patients with more severe clinical presentations of LSS, which may underestimate the impact of LSS symptoms on treatment outcomes. Conversely, between-group differences may be smaller than our observed results due to unmeasured confounding, for example from differences in comorbid pain sites and imaging characteristics such as LSS severity and number of affected levels.
Conclusion
Compared to those without LSS symptoms, patients with persistent or recurrent NSLBP and LSS symptoms can expect similar improvements in disability and back pain intensity, and slightly greater improvements in leg pain intensity with treatment. Therefore, education and exercise therapy programs designed for people with NSLBP are likely helpful for those also experiencing LSS symptoms.
Data Availability
The datasets generated during and/or analysed during the current study are not publicly available due to licensing restrictions on availability of data but are available from the corresponding author on reasonable request.
Abbreviations
- NSLBP:
-
Nonspecific low back pain
- LSS:
-
Lumbar spinal stenosis
- STROBE:
-
Strengthening the Reporting of Observational studies in Epidemiology
- ODI:
-
Oswestry Disability Index
- NRS:
-
Numeric Rating Scale
References
Jensen RK, Harhangi BS, Huygen F, Koes B. Lumbar spinal stenosis. BMJ. 2021;373:n1581. https://doi.org/10.1136/bmj.n1581.
Jensen RK, Jensen TS, Koes B, Hartvigsen J. Prevalence of lumbar spinal stenosis in general and clinical populations: a systematic review and meta-analysis. Eur Spine J. 2020;29:2143–63. https://doi.org/10.1007/s00586-020-06339-1.
Rousing R, Jensen RK, Fruensgaard S, Strøm J, Brøgger HA, Degn JDM, et al. Danish national clinical guidelines for surgical and nonsurgical treatment of patients with lumbar spinal stenosis. Eur Spine J. 2019;28:1386–96. https://doi.org/10.1007/s00586-019-05987-2.
Young JJ, Hartvigsen J, Roos EM, Ammendolia C, Kongsted A, Skou ST, et al. Symptoms of lumbar spinal stenosis in people with knee or hip osteoarthritis or low back pain: a cross-sectional study of 10,234 participants in primary care. Osteoarthritis Cartilage. 2021;29:1515–20. https://doi.org/10.1016/j.joca.2021.07.012.
Jensen RK, Lauridsen HH, Andresen ADK, Mieritz RM, Schiøttz-Christensen B, Vach W. Diagnostic screening for lumbar spinal stenosis. Clin Epidemiol. 2020;12:891–905. https://doi.org/10.2147/CLEP.S263646.
Konno S, Kikuchi S, Tanaka Y, Yamazaki K, Shimada Y, Takei H, et al. A diagnostic support tool for lumbar spinal stenosis: a self-administered, self-reported history questionnaire. BMC Musculoskelet Disord. 2007;8:102. https://doi.org/10.1186/1471-2474-8-102.
Konno S, Hayashino Y, Fukuhara S, Kikuchi S, Kaneda K, Seichi A, et al. Development of a clinical diagnosis support tool to identify patients with lumbar spinal stenosis. Eur Spine J. 2007;16:1951–7. https://doi.org/10.1007/s00586-007-0402-2.
de Schepper EIT, Overdevest GM, Suri P, Peul WC, Oei EHG, Koes BW, et al. Diagnosis of lumbar spinal stenosis: an updated systematic review of the accuracy of diagnostic tests. Spine. 2013;38:E469–81. https://doi.org/10.1097/BRS.0b013e31828935ac.
Tominaga R, Kurita N, Sekiguchi M, Yonemoto K, Kakuma T, Konno S. Diagnostic accuracy of the lumbar spinal stenosis-diagnosis support tool and the lumbar spinal stenosis-self-administered, self-reported history questionnaire. PLoS ONE. 2022;17:e0267892. https://doi.org/10.1371/journal.pone.0267892.
Aizawa T, Tanaka Y, Yokoyama T, Shimada Y, Yamazaki K, Takei H, et al. New diagnostic support tool for patients with leg symptoms caused by lumbar spinal stenosis and lumbar intervertebral disc herniation: a self-administered, self-reported history questionnaire. J Orthop Sci. 2016;21:579–85. https://doi.org/10.1016/j.jos.2016.07.012.
Ammendolia C, Hofkirchner C, Plener J, Bussières A, Schneider MJ, Young JJ, et al. Non-operative treatment for lumbar spinal stenosis with neurogenic claudication: an updated systematic review. BMJ Open. 2022;12:e057724. https://doi.org/10.1136/bmjopen-2021-057724.
Kongsted A, Ris I, Kjaer P, Vach W, Morsø L, Hartvigsen J. GLA:D® back: implementation of group-based patient education integrated with exercises to support self-management of back pain - protocol for a hybrid effectiveness-implementation study. BMC Musculoskelet Disord. 2019;20:85. https://doi.org/10.1186/s12891-019-2443-1.
Kjaer P, Kongsted A, Ris I, Abbott A, Rasmussen CDN, Roos EM, et al. GLA:D® back group-based patient education integrated with exercises to support self-management of back pain - development, theories and scientific evidence -. BMC Musculoskelet Disord. 2018;19:418. https://doi.org/10.1186/s12891-018-2334-x.
Kongsted A, Hartvigsen J, Boyle E, Ris I, Kjaer P, Thomassen L, et al. GLA:D® back: group-based patient education integrated with exercises to support self-management of persistent back pain — feasibility of implementing standardised care by a course for clinicians. Pilot Feasibility Stud. 2019;5:65. https://doi.org/10.1186/s40814-019-0448-z.
Williamson E, Sanchez Santos MT, Morris A, Garrett A, Conway O, Boniface G, et al. The prevalence of back and leg pain and the cross-sectional association with adverse health outcomes in community dwelling older adults in England. Spine. 2021;46:54–61. https://doi.org/10.1097/BRS.0000000000003719.
Williamson E, Boniface G, Marian IR, Dutton SJ, Garrett A, Morris A, et al. The clinical effectiveness of a physiotherapy delivered physical and psychological group intervention for older adults with neurogenic claudication: the BOOST randomised controlled trial. J Gerontol A Biol Sci Med Sci. 2022;glac063. https://doi.org/10.1093/gerona/glac063.
Young JJ, Kongsted A, Hartvigsen J, Roos EM, Ammendolia C, Skou ST, et al. Associations between comorbid lumbar spinal stenosis symptoms and treatment outcomes in 6,813 patients with knee or hip osteoarthritis following a patient education and exercise therapy program. Osteoarthr Cartil Open. 2022;4:100324. https://doi.org/10.1016/j.ocarto.2022.100324.
Young JJ, Kongsted A, Jensen RK, Roos EM, Ammendolia C, Skou ST, et al. Characteristics associated with comorbid lumbar spinal stenosis symptoms in people with knee or hip osteoarthritis: an analysis of 9,136 good life with osteoArthritis in Denmark (GLA:D®) participants. BMC Musculoskelet Disord. 2023;24:250. https://doi.org/10.1186/s12891-023-06356-3.
Lauridsen HH, Hartvigsen J, Manniche C, Korsholm L, Grunnet-Nilsson N. Danish version of the Oswestry Disability Index for patients with low back pain. Part 1: cross-cultural adaptation, reliability and validity in two different populations. Eur Spine J. 2006;15:1705–16. https://doi.org/10.1007/s00586-006-0117-9.
Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14:798–804. https://doi.org/10.1111/j.1365-2702.2005.01121.x.
White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–99. https://doi.org/10.1002/sim.4067.
Rubin D. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons, Ltd; 1987.
Lauridsen HH, Hartvigsen J, Manniche C, Korsholm L, Grunnet-Nilsson N. Responsiveness and minimal clinically important difference for pain and disability instruments in low back pain patients. BMC Musculoskelet Disord. 2006;7:82. https://doi.org/10.1186/1471-2474-7-82.
Ostelo RWJG, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine. 2008;33:90–4. https://doi.org/10.1097/BRS.0b013e31815e3a10.
Comer C, Ammendolia C, Battié MC, Bussières A, Fairbank J, Haig A, et al. Consensus on a standardised treatment pathway algorithm for lumbar spinal stenosis: an international Delphi study. BMC Musculoskelet Disord. 2022;23:550. https://doi.org/10.1186/s12891-022-05485-5.
Ammendolia C, Côté P, Southerst D, Schneider M, Budgell B, Bombardier C, et al. Comprehensive nonsurgical treatment versus self-directed care to improve walking ability in lumbar spinal stenosis: a randomized trial. Arch Phys Med Rehabil. 2018;99:2408–2419e2. https://doi.org/10.1016/j.apmr.2018.05.014.
Tomkins-Lane C, Melloh M, Lurie J, Smuck M, Battié MC, Freeman B, et al. Consensus on the clinical diagnosis of lumbar spinal stenosis: results of an international Delphi study. Spine. 2016;41:1239–46. https://doi.org/10.1097/BRS.0000000000001476.
Genevay S, Atlas SJ, Katz JN. Variation in eligibility criteria from studies of radiculopathy due to a herniated disc and of neurogenic claudication due to lumbar spinal stenosis: a structured literature review. Spine. 2010;35:803–11. https://doi.org/10.1097/BRS.0b013e3181bc9454.
Acknowledgements
The authors would like to thank the clinicians and patients involved in collecting data for GLA:D® Back.
Funding
No funding was received for conducting this study.
Open access funding provided by University Library of Southern Denmark
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection was performed by AK and JH. Analysis was performed by JJY, AK, and RKJ. The first draft of the manuscript was written by JJY and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
JJY is supported by postdoctoral funding from the Diana Kerbel Fellowship in Arthritis Integrated Care, Arthritis Society Canada, and the Danish Foundation for Chiropractic Research and Post-graduate Education and is the Assistant Project Manager for the GLA:D® International Network. AK is supported by an unrestricted grant from the Danish Foundation for Chiropractic Research and Post-graduate Education, is co-founder of GLA:D® Back, a not-for-profit initiative hosted at the University of Southern Denmark aimed at implementing clinical guidelines for back pain in clinical practice and has received personal fees from TrustMe-Ed and Physical Pod unrelated to this work. JH is co-founder of GLA:D® Back and has received personal fees from TrustMe-Ed and LearnPhysio.CA has no declarations.RKJ has no declarations.
Ethics approval and consent to participate
Ethics approval of GLA:D® studies are not needed under Danish legislation and according to the Regional Committees on Health Research Ethics for Southern Denmark (S-2017000-93), but all methods were carried out in accordance with the Declaration of Helsinki. The GLA:D® registry has been approved by the Danish Data Protection Agency (SDU; 10.084). According to the Danish Data Protection Act patient consent was not required for this analysis as personal data was processed exclusively for research and statistical purposes. Informed consent for the use of data in the GLA:D® Back registry for research was obtained for all participants when enrolling in GLA:D® Back.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Young, J.J., Kongsted, A., Hartvigsen, J. et al. Similar improvements in patient-reported outcomes for non-specific low back pain patients with and without lumbar spinal stenosis symptoms following a structured education and exercise therapy program. BMC Musculoskelet Disord 24, 839 (2023). https://doi.org/10.1186/s12891-023-06950-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-023-06950-5