Abstract
Background
There is no validated gold-standard diagnostic support tool for LSS, and therefore an accurate diagnosis depends on clinical assessment. Assessment of the diagnostic value of the history of the patient requires an evaluation of the differences and overlap of symptoms of the radicular and cauda equina types; however, no tool is available for evaluation of the LSS category. We attempted to develop a self-administered, self-reported history questionnaire as a diagnostic support tool for LSS using a clinical epidemiological approach. The aim of the present study was to use this tool to assess the diagnostic value of the history of the patient for categorization of LSS.
Methods
The initial derivation study included 137 patients with LSS and 97 with lumbar disc herniation who successfully recovered following surgical treatment. The LSS patients were categorized into radicular and cauda equina types based on history, physical examinations, and MRI. Predictive factors for overlapping symptoms between the two types and for cauda equina symptoms in LSS were derived by univariate analysis. A self-administered, self-reported history questionnaire (SSHQ) was developed based on these findings. A prospective derivation study was then performed in a series of 115 patients with LSS who completed the SSHQ before surgery. All these patients recovered following surgical treatment. The sensitivity of the SSHQ was calculated and clinical prediction rules for LSS were developed. A validation study was subsequently performed on 250 outpatients who complained of lower back pain with or without leg symptoms. The sensitivity and specificity of the SSHQ were calculated, and the test-retest reliability over two weeks was investigated in 217 patients whose symptoms remained unchanged.
Results
The key predictive factors for overlapping symptoms between the two categories of LSS were age > 50, lower-extremity pain or numbness, increased pain when walking, increased pain when standing, and relief of symptoms on bending forward (odds ratio ≥ 2, p < 0.05). The key predictive factors for cauda equina type symptoms were numbness around the buttocks, walking almost causes urination, a burning sensation around the buttocks, numbness in the soles of both feet, numbness in both legs, and numbness without pain (odds ratio ≥ 2, p < 0.05). The sensitivity and specificity of the SSHQ were 84% and 78%, respectively, in the validation data set. The area under the receiver operating characteristic curve was 0.797 in the derivation set and 0.782 in the validation data set. In the test-retest analysis, the intraclass correlation coefficient for the first and second tests was 85%.
Conclusion
A new self-administered, self-reported history questionnaire was developed successfully as a diagnostic support tool for LSS.
Similar content being viewed by others
Background
Lumbar spinal stenosis (LSS) is a well-recognized spinal disorder and a term used to describe a complex set of symptoms, physical findings, and radiological abnormalities caused by a narrowed spinal canal. The presence of a narrow canal in radiographic imaging does not in itself define the syndrome, and a diagnosis of LSS is defined by symptoms and clinical findings that must be supported by radiographic evidence. Computed tomography and magnetic resonance imaging are often non-specific and there may be discrepancies between clinical symptoms and imaging findings in cases of LSS [1–3].
There is no validated gold-standard diagnostic support tool for LSS, and therefore an accurate diagnosis depends on clinical assessment. However, there are few scientific evaluations of the sensitivity and specificity of diagnoses based on clinical history and physical examinations, or appropriate correlations of these data with imaging and operative findings. Katz et al. used the opinion of two expert orthopedic surgeons to define the presence or absence of LSS [4], and found that the factors in the patient history that were most strongly associated with diagnosis of LSS were a higher age, severe lower-extremity pain, and the absence of pain when seated. The physical findings most strongly associated with the diagnosis were a wide-based gait, an abnormal Romberg test, thigh pain following 30 seconds of lumbar extension, and neuromuscular deficits.
There are two categories of leg symptoms caused by LSS [5]. One type of stenosis presents as unilateral radicular pain (the radicular type), with symptoms of pain, burning, numbness and paresthesia following a specific dermatome or dermatomes. The fifth lumbar nerve root associated with L5 stenosis is most commonly involved. The other type of LSS has symptoms with less dermatomal-specific neurogenic claudication, and nerve roots below L5 are most commonly involved. The typical patient presents with complaints of aching, cramping, or a burning sensation in the bilateral legs. Occasionally, numbness is also apparent and some patients complain of bladder dysfunction and sexual difficulties.
Full-blown cauda equina syndrome only occurs in rare instances, but the above symptoms can occur as a part of cauda equina syndrome [6, 7]. Therefore, we hypothesized that leg symptoms in LSS might be divided into two categories: a radicular type and a cauda equina type. There are significant differences between the symptoms of these types, but there is also significant overlap between the symptoms. Since both the central canal and foraminal dimensions increase in flexion and diminish in extension, patients with both types of LSS experience exacerbation of symptoms with extension and improvement with flexion.
Assessment of the diagnostic value of the history of the patient requires an evaluation of the differences and overlap of symptoms of the radicular and cauda equina types; however, no tool is available for evaluation of the LSS category. Therefore, we attempted to develop a self-administered, self-reported history questionnaire as a diagnostic support tool for LSS using a clinical epidemiological approach. The aim of the present study was to use this tool to assess the diagnostic value of the history of the patient for categorization of LSS.
Methods
Derivation study 1
A series of 137 patients with LSS and 97 with lumbar disc herniation who successfully recovered following surgical treatment in our department during 2000 and 2003 were included in this study (Table 1). Patients with cervical myelopathy, diabetic neuropathy, previous surgery, peripheral vascular disease, inflammatory disorders, and degenerative scoliosis (defined as lateral tilting of more than 10 degrees) were excluded. Each patient was evaluated by the study investigators using a standard protocol. Operative and follow-up visit notes were reviewed to determine if stenosis was confirmed intraoperatively and if symptoms improved following surgery. Nerve root compression resulting exclusively from a herniated nucleus pulposus was not considered as a symptom of LSS.
Assessment of history included questions on location, frequency and severity of pain, and on symptoms including numbness, tingling, and provocative factors. The physical examination included a gait-loading test to confirm neurogenic intermittent claudication; this test involves assessment of walking capacity and symptoms, and a neurological examination of motor, sensory, and reflex activity [8]. We investigated symptoms during gait loading and neurological findings just after gait loading. Reflexes were graded from 0 (no response) to 4 (clonus) at the Achilles tendon and patellar tendon, and strength was graded from 0 (no movement) to 5 (normal) at the knee flexors and extensors, ankle dorsiflexors and plantar flexors, and extensor hallucis longus. A pinprick sensation was graded as absent, decreased, or normal at the dorsomedial foot, dorsolateral foot, medial calf, and lateral calf. MRI radiographical reports were abstracted from patient records.
The LSS patients were categorized into radicular and cauda equina types based on history, physical examination, and MRI findings. The radicular type was characterized by symptoms of pain, burning, numbness, and paresthesias following a specific dermatome with radiological evidence of the responsible nerve root compression, which was confirmed if intermittent claudication was abolished following single nerve root infiltration. Patients of the cauda equina type presented some bilateral symptoms related to cauda equina compression syndrome with less dermatomal-specific neurogenic claudication and radiological evidence of cauda equina compression. The cauda euqina type spinal stenosis is distinct from the cauda equina syndrome. A full blown cauda equina syndrome occurs in rare instances in the cauda equina type spinal stenosis. Therefore, urgent surgery is not required in the cauda equina type spinal stenosis. Predictive factors for overlap symptoms between the two types of LSS were derived from the data and factors for predicting the cauda equina type were also determined. Based on the results of univariate analysis for predictors of LSS, a self-administered, self-reported history questionnaire (SSHQ) was developed as a diagnostic support tool for LSS.
Derivation study 2
This study was performed in six university hospitals, ten medical centers, and thirty one hospitals and clinics affiliated with university hospitals or medical centers during January and March in 2004. A series of 115 patients with LSS gave informed consent to participate in the study and answered the SSHQ before surgery. All these patients recovered following surgery. Patients with cervical myelopathy, diabetic neuropathy, previous surgery, inflammatory disorders, and degenerative scoliosis were excluded. All patients were evaluated by study investigators using the same protocol as that in derivation study 1. Operative and follow-up visit notes were reviewed to determine if stenosis was confirmed intraoperatively and if symptoms improved following surgery. Nerve root compression resulting exclusively from a herniated nucleus pulposus was not considered as a part of LSS syndrome. All LSS patients were categorized into radicular or cauda equina types based on history, physical examination, and MRI findings using the same criteria as those in derivation study 1. There were 55 patients with radicular type LSS and 60 patients with the cauda equina type (Table 2). A responsible nerve root was confirmed if intermittent claudication was abolished following single nerve root infiltration. The sensitivity of each question on the SSHQ was calculated and compared between the radicular and cauda equina types. To assess the cut-off point to distinguish between the types, one point was assigned to each question on the SSHQ, and the clinical prediction rule was defined based on the scores.
Validation study
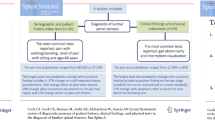
We prospectively evaluated the association between the diagnosis of LSS and clinical information, including the history and physical examination of patients with leg symptoms. This study was performed in six university hospitals, ten medical centers, and sixty eight hospitals and clinics affiliated with university hospitals or medical centers during July and September in 2004. We enrolled consecutive patients older than 20 years of age with primary symptoms of pain or numbness in the legs. We excluded patients who have been treated by some medical practices within one year before examination. Patients with cervical myelopathy, previous surgery, degenerative scoliosis (defined as lateral tilting of more than 10 degrees) and inflammatory disorders were also excluded. This study included 250 patients who complained of leg symptoms, including cases of LSS (n = 165), lumbar disc herniation (n = 61), diabetic neuropathy (n = 13), and peripheral vascular disease (n = 11) (Table 3). The study was approved by the institutional review board of each study institution as necessary. Written informed consent was obtained from the all patients. The patients gave informed consent and then answered the SSHQ. The following steps were taken to reach a final diagnosis for each of the enrolled patients (Figure 1). In the first step, at each institution the orthopedic physician who saw a patient made the clinical diagnosis based on the history, physical examination, and radiographic findings. In addition, to verify the diagnosis made by each physician, six board-certified spine surgeons approved by the Japanese Board of Spine Surgery also made a diagnosis for each patient based on the clinical information and findings of the MRI. The opinions of six board-certified spine surgeons approved by the Japanese Board of Spine Surgery were used as the gold standard for diagnosis of LSS. The radicular type was characterized by symptoms of pain, burning, numbness, and paresthesias following a specific dermatome with radiological evidence of the responsible nerve root compression, which was confirmed if intermittent claudication was abolished following single nerve root infiltration. Patients of the cauda equina type presented some bilateral symptoms related to cauda equina compression syndrome with less dermatomal-specific neurogenic claudication and radiological evidence of cauda equina compression.
The sensitivity, specificity, likelihood ratio, and area under the receiver operating characteristic (ROC) curve were estimated. 217 patients classified by investigators as suffering from lower back pain without a significant change in symptoms were given the SSHQ two weeks later during an outpatient visit, and the test-retest reliability over two weeks was investigated in these patients.
Statistical analysis
History and physical examination variables were dichotomized at clinically sensible cut-off values. Pinprick, strength, and Achilles reflexes were each classified as always normal or with at least 1 abnormal finding. Univariate analyses were performed to derive predictors of LSS using logistic regression analysis. Two-by-two contingency tables were prepared to calculate the sensitivity, specificity, and likelihood ratio of the SSHQ. The area under the ROC curve for the derivation data set was estimated to investigate the internal validity of the clinical prediction rule, and the area under the ROC curve for the validation data set was estimated to examine the external validity. Reliability was investigated based on the reproducibility in the test-retest method. Test-retest analysis was performed in 217 patients with a 14-day period between the first and second tests. Test-retest data were examined graphically by plotting the difference between tests against the mean of the 2 tests [9]. The intraclass correlation coefficient of the SSHQ score for the first and second tests was calculated to confirm reproducibility. The κ coefficient was calculated to examine conformity for each item, based on the following criteria: 0 to < 0.2, poor; 0.2 to < 0.4, fair; 0.4 to < 0.6, moderate; 0.6 to < 0.8, substantial; and > 0.8, almost perfect [10]. All the studies were approved by the ethics committee of Fukushima Medical University.
Results
Univariate analysis for predictors of LSS
Key factors for predicting overlapping symptoms between the two types of LSS are shown in Table 4. Five history findings had an odds ratio ≥ 2 or p < 0.05: age > 50, lower-extremity pain or numbness, increased pain when walking, increased pain when standing, and improvement of symptoms on bending forward. No physical examination finding had an odds ratio ≥ 2 or p < 0.05. There was no significant difference in the odds ratio of all the predictive factors except for age. Key factors for predicting the cauda equina type of LSS are shown in Table 5. Six history findings had an odds ratio ≥ 2 or p < 0.05: numbness around the buttocks, walking nearly causes urination, a burning sensation around the buttocks, numbness in the soles of both feet, numbness in both legs, and numbness without pain. Physical examination findings with an odds ratio ≥ 2 or p < 0.05 included the absence of or a weak Achilles reflex response.
A self-administered, self-reported history questionnaire (SSHQ)
Based on the results of univariate analysis for predictors of LSS, we developed the SSHQ as a diagnostic support tool for LSS (see Additional File 1 and 2). The SSHQ included the following questions:
Q1: Numbness and/or pain in the thighs down to the calves and shins.
Q2: Numbness and/or pain increase in intensity after walking for a while, but are relieved by taking a rest.
Q3: Standing for a while brings on numbness and/or pain in the thighs down to the calves and shins.
Q4: Numbness and/or pain are reduced by bending forward.
The key questions for diagnosis of cauda equina symptoms were as follows:
Q5: Numbness is present in both legs.
Q6: Numbness is present in the soles of both feet.
Q7: Numbness arises around the buttocks.
Q8: Numbness is present, but pain is absent.
Q9: A burning sensation arises around the buttocks.
Q10: Walking nearly causes urination.
Clinical prediction rule
The sensitivity of each question in the derivation study was calculated for the radicular and cauda equina types of LSS. The sensitivity differed significantly between the categories (Figure 2). To assess the cut-off point to distinguish between the two types, each question was assigned one point. The scores of predictors of cauda equina symptoms (Q5-Q10) were significantly different between the categories, and the cut-off point was two (Figure 3). Based on these results, a clinical prediction rule was defined based on the total scores: a score of 4 points on Q1–Q4 indicates the presence of LSS; a score of 4 on Q1–Q4 and < 1 on Q5–Q10 indicates the radicular type of LSS; and a score of > 1 on Q1–Q4 and > 2 on Q5–Q10 indicates the cauda equina type of LSS.
Validity
It took respondents an average of about 1 minute to answer the 10 questions on the SSHQ. Performance indices for the clinical prediction rule are shown in Table 6. The area under the ROC curve was 0.797 in the derivation set and 0.782 in the validation data set (Figure 4). These findings indicate that the SSHQ has both internal and external validity as a diagnostic tool for LSS. The difference between tests plotted against the mean of the tests indicated no obvious relationship or bias (Figure 5). The intraclass correlation coefficient of the SSHQ score for the first and second tests was 0.85, which indicates sufficient reproducibility. One item of the κ coefficient was found to be "fair" (question 8), and all other items were rated as having a conformity of moderate or above.
Discussion
Spinal stenosis patients frequently present with few objective physical findings. Jonsson and Stromqvist found that about 65% of patients have decreased walking ability [11], but up to 95% of patients treated surgically have only subjective symptoms, principally pain [12, 13]. Furthermore, diagnostic imaging cannot be used reliably to diagnose LSS, since CT and MRI are often non-specific and do not prove that symptoms arise from nerve root compression [14, 15]. A systematic review of original diagnostic studies on LSS revealed that no firm conclusions about the diagnostic performance of the different tests could be drawn due to heterogeneity and overall poor quality[16].
We developed a simple clinical diagnostic support tool to identify patients with LSS[17]. Although further studies are needed to validate this tool in primary care settings, it has a sensitivity of 92.8% and a specificity of 72.0%. By asking patients, who presented with back and leg symptoms suggestive of LSS, to fill out a simple questionnaire, consisting of five questions on their medical history (age and history of diabetes) and symptoms (presence or absence of intermittent claudication, aggravation of symptoms by standing and relief of symptoms by forward bending) followed by a short clinical examination checking the postural changes in their leg symptoms, Achilles' tendon reflex, SLR test and the measurement of ABI, the diagnosis of LSS can be established by high sensitivity and spcificity without obtaining MRI. Therefore, misdiagnosis or underdianosis of LSS at the primary care levels can be minimized and patients have a greater chance to get access to appropriate medical service by a referral to a spine specialist. A self-administered, self-reported history questionnaire as a diagnostic support tool for LSS might be more useful for clinician or patients. Therefore, we attempted to develop a self-administered, self-reported history questionnaire as a diagnostic support tool for LSS using a clinical epidemiological approach. To make an accurate diagnosis of LSS from history findings, we categorized the condition into radicular and cauda equina types. A comparison of the sensitivity of each question on the SSHQ showed both overlap and differences between the two categories (Figure 1). These findings suggest that the category of LSS requires consideration to make an accurate diagnosis. The scoring system includes a cut-off point to distinguish the radicular type from the cauda equina type (Figure 2). About 50% of radicular-type cases show relief of symptoms at six months after nerve root block and a further 17% improve with more time after nerve root block. In the cauda equina type, nerve root block is not efficient in relieving the symptoms and therefore surgical intervention is recommended [18]. Therefore, we consider that it is important to define the type of neurogenic intermittent claudication before selecting the therapeutic method, since surgery may be avoidable in certain cases.
The 10 items on the SSHQ for diagnosis of LSS require answers of either "yes" or "no" to minimize any difficulty with responses. As noted above, it took respondents an average of about 1 minute to answer the questions, which indicates that the questionnaire was easy to understand. However, the study has several limitations. First, there is no gold standard for diagnosis of LSS, but in the absence of valid objective criteria we believe that expert opinion is a reasonable strategy for making a diagnosis of a clinical syndrome, and this approach has been used for a variety of disorders. Therefore, we used LSS diagnoses made by six board-certified spine surgeons in our validation study. Second, we did not use logistic regression and multivariate models. Based on the results of this paper, we are planning to use logistic regression and multivariate models in the next project according to STARD checklist for reporting diagnostic accuracy studies [19]. We also note that a larger prospective derivation and validation studies might reveal additional independent factors that correlate with diagnosis of LSS.
Conclusion
The newly developed self-administered, self-reported history questionnaire can be used for diagnosis of LSS with high sensitivity, specificity, and reproducibility.
References
de Graaf I, Prak A, Bierma-Zeinstra S, Thomas S, Peul W, Koes B: Diagnosis of lumbar spinal stenosis: a systematic review of the accuracy of diagnostic tests. Spine. 2006, 31: 1168-1176. 10.1097/01.brs.0000216463.32136.7b.
Beattie PF, Meyers SP, Stratford P, Millard RW, Hollenberg GM: Associations between patient report of symptoms and anatomic impairment visible on lumbar magnetic resonance imaging. Spine. 2000, 25: 819-828. 10.1097/00007632-200004010-00010.
Amundsen T, Weber H, Lilleas F, Nordal HJ, Abdelnoor M, Magnaes B: Lumbar spinal stenosis. Clinical and radiologic features. Spine. 1995, 20: 1178-1186. 10.1097/00007632-199505150-00013.
Katz JN, Dalgas M, Stucki G, Katz NP, Bayley J, Fossel AH, Chang LC, Lipson SJ: Degenerative lumbar spinal stenosis Diagnostic value of the history and physical examination. Arthritis & Rheumatism. 1995, 38: 1236-1241. 10.1002/art.1780380910.
Mirkovic S, Lybulski G, Montgomery DM, Wang AM, Wesolowski DP, Garfin SR: Spinal stenosis Clinical Evaluation and Different Diagnosis. Rothman-Simeone, the spine. Edited by: Herkowitz HN et al. 1999, 796-806. 4
Rauschning W: Pathoanatomy of lumbar disc degeneration and stenosis. Acta Orthop Scand. 1993, 64: 3-12.
Yoshida M, Shima K, Taniguchi Y, Tamaki T, Tanaka T: Hypertrophied ligamentum flavum in lumbar spinal canal stenosis: Pathogenesis and morphologic and immunohistochemical observation. Spine. 1992, 17: 1353-1360.
Konno S, Kikuchi S: Prospective study of surgical treatment of degenerative spondylolisthesis. Comparison between decompression alone and decompression with Graf system stabilization. Spine. 2000, 25: 1533-1537. 10.1097/00007632-200006150-00012.
Bland JM, Altman DG: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986, 1: 307-10.
Chalmers I: Underreporting research is scientific misconduct. JAMA. 1990, 263: 1405-1408. 10.1001/jama.263.10.1405.
Jonnson B, Stromqvist B: Symptoms and signs in degeneration of the lumbar spine : a prospective, consecutive study of 300 operated patients. J Bone Joint Surg Br. 1993, 75: 381-384.
Pheasant HC, Dyck P: Failed lumbar disc surgery: cause, assessment, treatment. Clin Orthop Relat Res. 1982, (164): 93-109.
Ray CD: Extensive lumbar decompression: Patient selection and results. Lumbar spine surgery. Edited by: White AH, Rothman RH, Ray CD. 1987, St. Louis CV Mosby Co
Boden SD, Davis DO, Dina TS, Partronas NJ, Wiesel SW: Abnormal magnetic resonance scans of the lumbar spine in asymptomatic subjects: a prospective investigation. J Bone Joint Surg [Am]. 1990, 72: 403-408.
Wiesel SW, Tsourmas N, Feffer HL, Citrin CM, Patronas N: A study of computer-assisted tomography. The incidence of positive CAT scans in an asymptomatic group of patients. Spine. 1984, 9: 549-551. 10.1097/00007632-198409000-00003.
de Graaf I, Prak A, Bierma-Zeinstra S, Thomas S, Peul W, Koes B: Diagnosis of lumbar spinal stenosis: a systematic review of the accuracy of diagnostic tests. Spine. 2006, 31: 1168-1176. 10.1097/01.brs.0000216463.32136.7b.
Konno S, Hayashino Y, Fukuhara S, Kikuchi S, Kaneda K, Seichi A, Chiba K, Satomi K, Nagata K, Kawai S: Development of a clinical diagnosis support tool to identify patients with lumbar spinal stenosis. Eur Spine J. 2007, [Epub ahead of print],
Konno S, Kikuchi S: Pathomechanism of spinal canal stenosis. The therapeutic effects of nerve root infiltration, selective spinal angiography, and sympathetic nerve block. Proceeding of the 30th congress of the international college of surgeons. Bologna. Edited by: Abe O, Inokuchi K, Takahashi K Monduzzi. 1996, 1309-1313.
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC: Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003, 326: 41-4. 10.1136/bmj.326.7379.41.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2474/8/102/prepub
Acknowledgements
This study was supported by Grants from the Fukushima Medical Research Promotion Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
SKonno, SKikuchi, SKokuban, YT, YS, KY, MO, TY and HT conceived the study and participated in the study design. SK performed the statistical analysis. SK drafted the initial manuscript for journal submission and participated in revisions. SKonno, SKikuchi, SKokuban, YT, YS, KY, MO, TY and HT coordinated data collection at each site. All authors read and approved the final manuscript.
Electronic supplementary material
12891_2007_379_MOESM1_ESM.doc
Additional file 1: Japanese version of the SSHQ. A copy of the Japanese version of the SSHQ for Japanese readers (DOC 20 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Konno, Si., Kikuchi, Si., Tanaka, Y. et al. A diagnostic support tool for lumbar spinal stenosis: a self-administered, self-reported history questionnaire. BMC Musculoskelet Disord 8, 102 (2007). https://doi.org/10.1186/1471-2474-8-102
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2474-8-102