Abstract
Background
Lateral ankle sprains are highly prevalent and result in tissue damage, impairments of muscle strength, instability, and muscle activation. Up to 74% will experience ongoing symptoms after a lateral ankle sprain. In healthy subjects, motor imagery might induce neural changes in the somatosensory and motor areas of the brain, yielding favourable enhancements in muscular force. However, during motor imagery, difficulties in building a motor image, no somatosensory feedback, and the absence of structural changes at the level of the muscle might explain the differences found between motor imagery and physical practice. In rehabilitation, motor imagery might be supportive in rebuilding motor networks or creating new networks to restore impairments in muscle activation and movement patterns. This systematic review was undertaken to summarize the current body of evidence about the effect on motor imagery, or action observation, on lower leg strength, muscle performance, ankle range of motion, balance, and edema in persons with, and without, a lateral ankle sprain compared to usual care, a placebo intervention, or no intervention.
Methods
A systematic review with meta-analysis of randomized controlled trials was conducted in healthy participants and participants with a lateral ankle sprain. Motor imagery or action observation in isolation, or in combination with usual care were compared to a placebo intervention, or no intervention. An electronic search of MEDLINE, EMBASE, Cinahl, Psychinfo, Sportdiscus, Web of Science, Cochrane and Google Scholar was conducted, and articles published up to 7th June 2023 were included. Two reviewers individually screened titles and abstracts for relevancy using the inclusion criteria. Variables related to muscle strength, muscle function, range of motion, balance, return to sports tests, or questionnaires on self-reported function or activities were extracted. A risk of bias assessment was done using the Cochrane Risk-of-Bias tool II by two reviewers. Meta-analysis using a random effects model was performed when two or more studies reported the same outcome measures. The Standardized Mean Difference (SMD) was calculated over the change from baseline scores. Review manager 5.4 was used to perform analysis of subgroup differences and test for statistically significant differences. Confidence intervals were visually checked for overlap between subgroups.
Results
Nine studies, six examining healthy participants and three examining participants with an acute lateral ankle sprain, were included. All studies were rated with moderate to high risk of bias overall. Quality of the motor imagery interventions differed largely between studies. Meta-analysis showed a large and significant effect of motor imagery on lower leg strength (SMD 1.47, 95% CI 0.44 to 2.50); however, the evidence was downgraded to very low certainty due to substantial heterogeneity (I2 = 73%), limitations in the studies (some concerns in risk of bias in all studies), and imprecision (n = < 300). Evidence showed no association with ankle range of motion (SMD 0.25, 95% CI -0.43 to 0.93), edema (SMD -1.11, 95% CI -1.60 to 3.81), the anterior reach direction of the Star Excursion Balance Test (SEBT) (SMD 0.73, 95% CI -0.62 to 2.08), the posterolateral direction (SMD 0.32, 95% CI -0.94 to 1.57), and the posteromedial direction (SMD 0.52, 95% CI -0.07 to 1.10). The certainty of evidence for the different comparisons was very low.
Conclusions
There is a low certainty, significant, positive effect for motor imagery being able to improve lower leg muscle strength in healthy participants. The effect on balance, range of motion and edema was uncertain and of very low certainty.
Systematic review registration
PROSPERO CRD42021243258.
Similar content being viewed by others
Introduction
Lateral ankle sprains are highly prevalent in the general population and during sports activities [1, 2]. It is estimated that 20% to 40% of all sports injuries are ankle sprains [3] resulting in considerable time loss from sports activities [4]. On average, 15 to 28 days being lost was reported in amateur and professional soccer players [5, 6]. In particular, athletes involved in basketball, volleyball and field sports are at increased risk of sustaining an ankle sprain [7]. Lateral ankle sprains are often classified as a one-time injury, yet evidence suggests that up to 74% of persons with a lateral ankle sprain will experience persistent symptoms [8]. There is evidence for a multifactorial contribution of impaired balance, reaction time and strength to the development of ankle instability [9]. Further, lateral ankle sprains might result in instability and impairments in strength, peroneal muscle activation, proprioception, range of motion, joint laxity and decreased self-reported functioning [10,11,12]. The initial sprain results in tissue damage, but additionally causes impairments in the sensory-perceptual and motor-behavioural systems [12]. For instance, participants with ankle instability were found to activate the muscles of the hip, knee and ankle later than control participants without ankle instability during a shift from double leg stance to single leg stance [13]. The impaired movement patterns have been related to changes within the central nervous system [14] due to functional reorganization of the cortex, increasing the contribution of secondary sensorimotor areas [15]. Brain areas responsible for generating movement are activated extensively during the execution of movement, but similarly also during observation and imagination of the same movement [16,17,18].
Movement representation techniques can be defined as “the representation of movement, especially observation and/or imagination of normal pain-free movement” [19]. Motor imagery and action observation are two extensively studied movement representation techniques [20] and are widely used in a sport and in a rehabilitation context [21]. Motor imagery is defined as a cognitive and dynamic ability involving the cerebral representation of an action, without its real motor execution. Action observation training is considered as the internal representation of a set of movements evoked by the observer during live visualization of the movements [22]. Positive effects on motor performance, such as movement speed, accuracy, variability, and muscle strength, have been demonstrated in athletes [23]. In addition, training-mode specific effects were observed in a study examining the effect of six weeks of motor imagery training in professional basketball players. A group mentally rehearsing back squat and bench press exercises at 85% one repetition maximum (1-RM) had greater maximal strength gains, whereas the optimum power loads group experienced greater improvements on lower limb jumping capacity and muscular power [24]. Moreover, motor imagery showed beneficial effects on maximal muscle voluntary strength in healthy adults, but when compared to physical practice, a small benefit in favour of physical practice was found [25]. Motor imagery might induce neural changes in the somatosensory and motor areas of the brain, yielding favourable enhancements in muscular force. However, during motor imagery, difficulties in building a motor image, no somatosensory feedback, and the absence of structural changes at the level of the muscle might explain the differences found between motor imagery and physical practice [24, 25].
In rehabilitation, motor imagery might be supportive in rebuilding motor networks or creating new networks to restore impairments in muscle activation and movement patterns [17] and is of specific interest in a setting where movement is impaired due to pain or injury and could provide a substitute to actual exercise [22, 26]. Positive effects on muscle activation, muscle strength and functional outcomes have been demonstrated in persons after a total knee arthroplasty [27] and anterior cruciate ligament reconstruction [28].
Over the last twenty years, several studies have looked at the effect of motor imagery on ankle function and performance in healthy participants [10, 29] and after an ankle injury [30,31,32]. However, there is a considerable heterogeneity in the methodology and interventions studied, for instance the type and duration of motor imagery. To date, no systematic review has been undertaken into the topic and there is a need for a clear overview of the effect of motor imagery on ankle function and performance. Therefore, the goal of this systematic review was to summarize the current body of evidence. Our research question was:
What is the effect of motor imagery or action observation on ankle function (muscle strength, range of motion, edema) and performance (balance, return to sport tests) in persons with, or without, a lateral ankle sprain?
Methods
This systematic review was performed in accordance with the guideline Preferred Reporting Items for Systematic reviews and Meta-Analysis: The PRISMA statement [33] and was prospectively registered (PROSPERO CRD42021243258).
Identification and selection of studies
An electronic search was conducted using the databases MEDLINE, EMBASE, Cinahl, Psychinfo, Sportdiscus, Web of Science, Cochrane and Google Scholar. The search strategy was designed in consultation with two experienced librarians [TP and DM]. Articles published up to 7th June 2023 were included in the search. The results were uploaded to Rayyan [34], and duplicate results were removed.
Medical Subject Headings (MeSH) and free-text words were used to design the search string. Boolean operator “AND” was used to combine keywords and “OR” was used to combine the keywords within each group. The following search terms were used and combined to search the databases: motor, mental, movement, mirror, imagery, ankle, injuries. The final searches for all databases can be found in Appendix 1. The electronic search was conducted by one librarian [TP] and two reviewers [LS and MW] screened titles and abstracts for relevancy using the inclusion criteria [33, 35] randomized controlled trials published in peer-reviewed journals. Selection of studies was limited to articles written in English, German or Dutch. Trials including healthy participants, or participants with a lateral ankle sprain were included. No restrictions to age were made. The intervention consisted of movement representation techniques (motor imagery or action observation) in isolation, or in combination with usual care. Whereas the control group received usual care, an intervention which differed from the movement representation techniques, a placebo intervention, or did not receive any intervention. Studies were included if measured outcome measures related to function or performance, such as muscle strength, muscle endurance, range of motion, balance, return to sports tests, or questionnaires on self-reported function or activities were reported.
If disagreement upon article relevancy existed between the two reviewers, consensus was achieved by discussion. Full texts of eligible articles were retrieved and evaluated individually by the two reviewers. Again, disagreements were resolved using discussion to reach consensus. A third reviewer [HN] was consulted to make the final decision if disagreement remained during the process. Additionally, reference lists of included articles were checked manually to identify additional literature. The Kappa statistic and percentage of agreement as a measure for interrater reliability were calculated and Kappa results were interpreted as: 0.01–0.20 none to slight, 0.21–0.40 fair 0.41–0.60 moderate, 0.61–0.80 substantial, and 0.81–1.00 almost perfect agreement.
Assessment of characteristics of studies
Study quality
A risk of bias assessment was conducted blinded and individually by two reviewers [LS and MW]. The Cochrane risk-of-bias tool 2 for randomized trials was used to judge the domains: bias arising from the randomization process; bias due to deviations from intended interventions; bias due to missing outcome data; bias in measurement of the outcome and bias in selection of the reported result [36]. Upon disagreement, consensus was reached through discussion. A third reviewer [HN] was available in case of persisting disagreement. The risk of bias judgement was presented in a table alongside the results of the systematic review [37].
Data analysis
The data collection process was performed using a data extraction form [38]. The items were discussed by the reviewer and colleagues [LS, MW, HN, NS, and JBS] in order to develop the form. The extraction form was pilot-tested by two reviewers on three randomly-articles and the extraction form were revised where needed. The primary reviewer [LS] extracted the information and the second reviewer [MW] checked the extracted results. Disagreement was settled using discussion. If agreement could not be reached, a third reviewer [HN] was consulted to provide the final decision [33]. The final version of the data extraction form can be found in Appendix 3. The following key information was extracted: author(s), year of publication, origin/country of origin, the aim of the study, inclusion and exclusion criteria, demographic description of the population (gender, age, type of injury, duration of injury) and sample size, the methodology, the intervention (detailed information about the design, the application and theory), duration of the intervention, the comparison group, the outcomes measures (i.e. muscle strength, muscle endurance, range of motion, edema, functional tests, questionnaires), the measurement instruments and their validity and reliability, the follow-up (and duration), and key findings.
Meta-analysis using a random effects model was performed when two, or more, studies reported the same outcome measures in a comparable study population. Statistical heterogeneity of the intervention effect was assessed using the I2 as it is preferred over the Chi-square test and was considered not important if I2 was between 0 and 30%; moderate between 30 and 50%, substantial between 50 and 75%, and considerable above 75% [39]. Review Manager (4.5) [40] and SPSS, version 28, were used to prepare and maintain the systematic review and conduct the meta-analysis. Outcomes on a continuous scale were presented as the weighted average using the mean difference (MD) and standard deviation. The standardized mean difference (SMD) and standard deviations were presented if studies used different instruments to measure the same construct. The MD or SMD were calculated over the change from baseline scores [41], authors were contacted if the published results were insufficient for the data analysis. When possible, subgroup analysis to explore whether the results differed between healthy subject and injured participants was performed. A statistical test (Borenstein and Higgins, 2013) to explore for subgroup differences was conducted using Review Manager 5.4 when a minimum of 10 studies were included in the analysis. Since there were only two subgroups, a visual exploration of differences was performed by checking if confidence intervals were overlapping.
The trial registers ClinicalTrials.gov, EU Clinical Trials Register, Netherlands Trial Register, WHO International Clinical Trials Registry Platform (ICTRP) and ISRCTN registry were checked to identify unpublished trials.
The qualitative analysis of the body of evidence was performed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework. The certainty of evidence was classified as high, moderate, low, or very low certainty. Evidence from randomized controlled trials began as high certainty evidence yet could be downgraded based on concerns in any of the following five categories: risk of bias, imprecision, inconsistency, indirectness, and publication bias [42,43,44,45].
Quality of the motor imagery intervention
The quality of the motor imagery interventions was analysed using fifteen predefined criteria by Schuster et al. [46]. Motor imagery interventions might differ in type, duration, and context. Differences in the administration of the intervention might produce different results, and these differences might be responsible for variation in the estimates of effect between the studies [47].
Motor imagery sessions were scored successful when: (1) performed individually; (2) added after physical practice; (3) were supervised; (4) not directed; (5) the location of the motor imagery and (6) position of the participants was task-specific; (7) accompanied by acoustic and (8) detailed MI instructions; (9) performed with the eyes closed; (10) the perspective used during MI was an internal view with (11) kinaesthetic mode and (12) MI interventions included primarily motor-focused activities. (13) The average duration of a study was around 34 days, (14) with a total of 3 MI training sessions per week, and (15) had an average duration of 17 min per training session (with a minimum of 178 min) [46].
Results
Flow of studies through the review
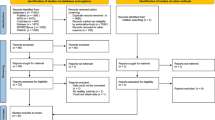
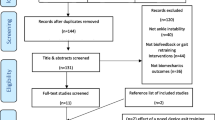
An electronic search of the databases identified 743 records (see Fig. 1; MEDLINE 83 records, EMBASE 119 records, Cinahl 32 records, Psychinfo 30 records, Sportdiscus 30 records, Web of Science 80 records, Cochrane 65 records, and Google Scholar 304 records). After removing duplicates, 496 records were screened for title and abstract. In total, 472 records were excluded based on study design, population, outcome, or intervention. The Kappa statistic for agreement between reviewers after screening titles and abstracts was 0.738 (substantial agreement) and the percentage of agreement was 97,7%. From the 24 included reports, 22 full text articles were retrieved, two reports led to ongoing studies and could therefore not be retrieved. Thirteen papers were excluded based on study design (no control group, n = 3) [10, 48, 49], outcomes (no outcomes on ankle function or performance, n = 5), [50,51,52,53,54] language (Korean, n = 2; Arabic, n = 2) [55,56,57,58], or publication type (not peer reviewed, n = 1) [59]. The Kappa statistic for agreement after screening full texts was 0.68 (substantial agreement), and the percentage of agreement was 98%. The third reviewer was consulted once to aid in decision making, further disputes were solved through careful deliberation and consensus was reached. In total, nine randomized controlled trials were included [29,30,31,32, 60,61,62,63,64]. Reference lists were checked manually for relevant studies, no additional reports were identified.
Characteristics of studies
Risk of bias
Risk of bias assessment revealed at least some concerns in six studies [29, 30, 60, 61, 63, 64] and high concerns in three [31, 32, 62] of the included studies (see Table 1). All studies used a random allocation sequence. Due to the nature of the intervention, in all studies participants and researchers delivering the intervention were probably aware of the assignment during the trial. In two studies a blinded assessor performed the pre-intervention measurements of range of motion, postural control, swelling and functional instability; however, the post-intervention measurements were not conducted by a blinded assessor [30, 63]. The other seven studies lacked information about the blinding of the assessor during the measurements [29, 31, 32, 60,61,62, 64]. Risk of bias assessments were conducted per outcome measure, as recommended by the Cochrane Handbook for Systematic Reviews [65]. Results of the risk of bias assessments were presented per study when the result for outcomes were equal. For the study of Nagar & Noohu [60] a small difference in risk of bias between the outcome measures was observed and these results were presented individually (see Table 1).
One trial protocol was identified, no further (published) pre-analysis plans were discovered, increasing risk of bias in the ‘selection of the reported result’ domain [30]. Five trial registers (ClinicalTrials.gov, EU Clinical Trials Register, Netherlands Trial Register, WHO International Clinical Trials Registry Platform (ICTRP) and ISRCTN registry) were checked to study non-reporting bias and in total, 334 results were located using the search criteria ‘ankle’ and ‘motor imagery’. Two trials in participants with lateral ankle sprain were identified, one recent trial is in the recruiting phase, and one has been included in the current systematic review. Publication bias was not assessed by testing for funnel plot asymmetry due to insufficient number of studies (n = < 10) included per meta-analysis [66].
Participants and sample sizes
The characteristics of the nine included studies can be found in Table 2. Three studies [30,31,32] recruited athletes with an acute ankle sprain and six studies recruited healthy participants [29, 60,61,62,63,64]. Participants in the studies were aged 13 to 15 years [63], 16 to 18 years [62], 16 to 20 years [30] and 18 to 30 years [29, 31, 32, 60, 61, 64]. The sample sizes in the studies varied from 18 [31, 61] to 400 participants [64]. The study of Deghan et al. [62] recruited 16 football squads (n = 400; 25 players per squad). The article does not provide detailed information about the intervention in both groups, the outcome measures and which measurement instruments were used. Data extraction was limited to participant information. Most studies divided participants into an intervention group and a control group, except for two studies [29, 64], one used a three-arm-design [29] and one used three intervention groups and one control group [64]. When assigned to the groups, sample sizes were between 7 [64] and 25 [62] participants per group.
Description of the intervention and control groups
In the studies using injured athletes, the control groups received normal physiotherapy and the intervention groups received normal physiotherapy and an additional motor imagery intervention [30,31,32]. The study with healthy dancers compared a motor imagery intervention with upper body exercises [63], and the study with healthy basketball players compared strength training, balance training and motor imagery to a control group receiving strength training and balance training [60]. In one study, motor imagery was compared to a control group which did not perform any exercise [61] and in the study of Sidaway & Trzaska [29], participants were randomly divided into a motor imagery group, an exercise group and a control group. The study using four groups compared differences between a motor imagery group, neuromuscular electrical stimulation group, a group combining motor imagery with neuromuscular electrical stimulation, and a control group [64]. One study performed “a proprioception training with mental imagery” and compared it to a control group. However, it is unclear what the intervention exactly encompassed and how it was delivered as there is no description provided in the article about the content of the mental imagery intervention, duration, or frequency of the intervention [62].
Quality and content of the motor imagery
An assessment of the quality of the motor imagery intervention based on the criteria of Schuster et al. [46] can be found in Table 3. Most of the studies directed the motor imagery using a verbal, read-aloud, prespecified protocol [29, 31, 32, 60, 63], one study combined it with an audio file [61], and in one study ankle–foot images were presented on a computer [30]. In some studies, the intervention took less than 17 min per session [60] or did not reach a total of 178 min during the intervention period [61] or failed to satisfy both criteria (volume per session and in total) [30]. Both studies from Christakou & Zervas and Christakou et al. included 12 motor imagery sessions lasting 45 min [31, 32]. The study of Nunes et al. [30] scored no, no information, or not applicable on 12 out of 15 criteria. This was largely due to the nature of the intervention: participants in the motor imagery intervention were shown 40 left–right images (lateralization) in contrast to the movement-related visualization given in most other studies. For the study of Dehghan et al. [62], no information was provided on any of the items in the article.
Motor imagery ability
Three studies used the Movement Imagery Questionnaire-Revised (MIQ-R) and one the Movement Imagery Questionnaire-Revised second version (MIQ-RS) to assess the individual’s ability to image four movements using internal visual imagery, and kinaesthetic imagery [60, 61, 63, 64]. In addition, one used a predetermined item score of ≥ 4 to include participants for their study [60]. The MIQ-R uses 8 items (4 visual and 4 kinaesthetic), and the MIQ-RS uses 14 items (7 visual and 7 kinaesthetic). Participants first form a visual image of a movement, and secondly feel what performing this movement is like. Their effort is rated on a 1 (very hard to see/feel) to 7 (very easy to see/feel) scale. The MIQ-R has a maximum of 56 points, where a higher score correlates with a better motor imagery ability, and the MIQ-RS a maximum of 49 points for both visual and kinaesthetic motor ability.
Grosprêtre et al. [61] reported 46.9 ± 3.5 points (maximum score: 56), Bouguetoch et al. [64] 46.1 ± 6.1 points and Nagar & Noohu [60] calculated the average for each item (5 ± 0.84 and 5.07 ± 0.91; maximum score: 7). Abraham et al. [63] calculated their scores per imagery type and participants scored 22.92 ± 3.14 points for visual and 20.31 (± 4.55) for kinaesthetic motor imagery.
In the two studies of Christakou et al. [31, 32] the Vividness of Movement Imagery Questionnaire (VMIQ) was used. The VMIQ uses 24-items on a 5-point scale from 1 (perfectly clear and vivid as normal vision to 5 (no image at all, you only “know” that you are thinking of the skill) to rate motor imagery ability for ‘doing it yourself’ and to image ‘somebody else’ do it. A score of 24 is the highest possibility and 120 the lowest. Christakou et al. [31, 32] report 66 ± 14.53 points for “watching somebody else” and 57.89 ± 14.24 points for “doing himself/herself” in one study and 65.80 ± 13.72 points for “watching somebody else” and 56.00 ± 14.70 points for “doing himself/herself” in their other study. Three studies did not assess, or report on the imagery ability of the participants [29, 30, 62].
Effect of intervention
Meta-analysis was performed for studies that compared motor imagery to control groups for lower leg strength, ankle range of motion, edema, and balance. One author [64] was contacted, and further information on the change from baseline scores was provided. Results of the meta-analysis can be found in Figs. 2, 3, 4 and 5. Results for the assessment of the certainty of the body of evidence are presented in Table 4.
a Standardized mean difference (95% CI) in the effect of motor imagery versus control groups on balance (m-SEBT, anterior direction). b Standardized mean difference (95% CI) in the effect of motor imagery versus control groups on balance (m-SEBT, posterolateral direction). c Standardized mean difference (95% CI) in the effect of motor imagery versus control groups on balance (m-SEBT, posteromedial direction)
Effect of motor imagery on strength
Meta-analysis of four studies (Fig. 2) with a total of 64 healthy participants showed very low certainty evidence of a large, significant effect of motor imagery on lower leg strength when compared to controls (SMD 1.47, 95% CI: 0.44 to 2.50; I2: 73%; p = 0.005). The evidence was downgraded to very low certainty due to substantial heterogeneity (I2 = 73%), limitations in the studies (some concerns in risk of bias in all studies), and imprecision (n = < 300) (Table 4).
Effect of motor imagery on range of motion
Meta-analysis of three studies (Fig. 3) with a total of 60 participants (35 subjects with a lateral ankle sprain, and 25 healthy subjects) demonstrated very low certainty evidence that motor imagery, when compared to control groups, had a small non-significant effect on ankle range of motion (SMD 0.25, 95% CI: -0.43 to 0.93; I2: 41%; p = 0.47). The evidence was downgraded to very low certainty due to moderate heterogeneity (I2 = 42%), limitations in the studies (high risk of bias in one study and some concerns for risk of bias in two studies), indirectness (due to differences in study populations and interventions), and imprecision (n = < 300).
Effect of motor imagery on edema
Meta-analysis of two studies (Fig. 4) with a total of 35 participants with a lateral ankle sprain provided very low certainty evidence of a non-significant effect on edema (SMD -1.11, 95% CI: -3.82 to 1.60; I2: 91%; p = 0.42). The evidence was downgraded to very low certainty due to considerable heterogeneity (I2 = 91%), limitations in the two studies (high risk of bias in one study and some concerns for risk of bias in the other study), indirectness (due to differences in the intervention), and imprecision (n = < 300).
Effect of motor imagery on balance
Meta-analysis of two studies (Fig. 5a) with a total of 47 participants (17 subjects with a lateral ankle sprain, and 30 healthy subjects) demonstrated very low certainty evidence of a moderate, non-significant effect of motor imagery when compared to controls on the anterior direction of the modified star balance excursion test (SMD 0.73, 95% CI: -0.62 to 2.08; I2: 79%; p = 0.29), very low certainty evidence of a small, non-significant effect of motor imagery on the posterolateral direction (SMD 0.32, 95% CI: -0.94 to 1.57; I2: 76%; p = 0.62) (Fig. 5b), and low certainty evidence of a moderate, non-significant effect of motor imagery on the posteromedial direction (SMD 0.52, 95% CI: -0.07 to 1.10; I2: 0%; p = 0.08) (Fig. 5c) of the modified Star Balance Excursion Test. The evidence was downgraded to very low certainty due to limitations in the studies (some concerns for risk of bias in both studies), imprecision (n = < 300), indirectness (due to differences in the study population and intervention), and considerable heterogeneity for the anterior and posterolateral direction (I2 = 79% and 76%).
Discussion
This systematic review with meta-analysis provides an overview of the evidence on the effect of motor imagery on ankle strength, range of motion, balance, and edema in persons with, or without, a lateral ankle sprain. Very low-certainty evidence for a significant, positive effect for motor imagery being able to improve lower leg muscle strength in healthy participants was found. The evidence for balance, ankle range of motion and edema in healthy and injured participants was uncertain, and of very low certainty.
Several methodological strengths for this systematic review can be defined: the protocol was designed using the Cochrane Handbook for Systematic Reviews [65] and Preferred Reporting Items for Systematic reviews and Meta-Analysis (The PRISMA statement) [33]; a focused review question was formulated; a thorough systematic literature search of multiple databases was conducted; only randomized controlled trials were included; the body of evidence was rated using the GRADE criteria; and the motor imagery interventions were rated using the criteria of a successful motor imagery intervention outcome [46]. The assessment of the quality of the motor imagery intervention provides a clear overview of the content and background of the motor imagery intervention (see Table 3). Furthermore, it might give an indication about the chance of finding a positive result.
This study has several limitations. A weakness of the current systematic review and meta-analysis is the small number of studies (n = 9) with heterogeneous backgrounds resulting in a limited number of studies and participants per outcome measure. The small number of studies could have underpowered the results. Moreover, certainty of evidence was low to very low because of moderate to high risk-of-bias in the included studies. The risk-of bias assessment showed problems in the blinding of the assessors performing the pre- and post-measurements which could have led to performance bias. Further, no placebo interventions were given to the control groups. A well-developed placebo is hard to construct in motor imagery study designs, however, several published randomized controlled trials in other areas than the ankle joint have used a placebo intervention [28, 67,68,69]. For instance, a study in persons with an anterior cruciate ligament reconstruction used a neutral task, e.g., mental calculation, or crosswords and showed increased muscle activation of the vastus medialis after five weeks of motor imagery when compared to the mental calculation task in the control group [28]. In a study in persons with non-specific chronic neck pain, mixed results for motor imagery on mobility tasks were found between a motor imagery group, an action observation group, and a placebo action observation group. The intervention groups imagined movement (motor imagery group) and watched a video of the same motor task (action observation group), and the placebo control group watched a video that showed nature landscapes, without any human motor actions [67].
Although caution must be exercised in interpreting the results of the current meta-analysis, the pooled results (n = 81, 4 studies) revealed a large, significant effect for motor imagery being able to improve lower leg strength in healthy participants (SMD 1.47; 95% CI: 0.44–2.50), i.e., the plantar and dorsal flexor muscles. The quality of the motor imagery intervention according to criteria of Schuster et al. [46] was rated 10 out of 15 points in two studies [61, 64], and 12 out of 15 points in the other two studies [29, 60]. To obtain a higher quality rating the studies of Grosprêtre et al., Bouguetoch et al., and Sidaway & Trzaska could have added physical practice sessions to their motor imagery intervention and lengthened the duration of the intervention [29, 61, 64]. However, it is questionable whether these modifications would have led to different results as it was previously noted that motor imagery might enhance strength after only a few sessions [25]. Only a small additional increase in strength is expected from a longer training period. In a 4-week training study in upper extremity strength, the major improvement in strength was observed in the first week after four motor imagery and strength training sessions, yet the increase in strength continued over the 4-week period [70].
Further, some differences in comparisons across the included studies were noted: one study added motor imagery to a strength training intervention [60], while the other three studies used a control group who did not participate in any physical or mental activity [29, 61, 64]. In addition, the sample of participants (n = 81) included in the meta-analysis for lower leg strength consisted entirely of young (18 to 26 years), healthy participants, and generalization towards conditions after an acute injury is not possible. Subgroup analysis to explore differences between the effect of motor imagery in healthy and injured participants would have been interesting as it was previously noted that injured persons sometimes suffer from an impaired possibility to generate motor images [16]. In addition, next to a greater difficulty in generating a visual and kinaesthetic motor image, participants with chronic low back pain were found to need more time to complete visualizing a movement [71]. It is therefore important to explore differences between healthy and injured participants. Unfortunately, due to the small number of studies in the meta-analysis, further exploration of subgroups with statistical tests was not possible.
It is important to check for the ability of participants to imagine movements, as the effectiveness of imagery is dependent on the individual capability to generate and control vivid images [72]. Over the last century, various assessment instruments have been developed to assess the imagery ability of an individual. According to a recent published systematic review, the MIQ-R offers sufficient psychometric properties to assess motor imagery ability [73]. In this study the MIQ-R has been used in half of the included articles, and the VMIQ in two others. The VMIQ-2 has the same level of psychometric properties as the MIQ-R, yet the first version of the instrument was used in the studies. The results indicate that for most studies, imagery ability was good. However, in three studies the imagery ability was not assessed, and the topic was not discussed.
The positive effect of motor imagery on lower leg strength found in the current systematic review is in line with results from several recent systematic reviews which studied the effect of motor imagery on strength, but in other body regions than the ankle [20, 74, 75]. In one of these systematic reviews, a large, positive effect with moderate certainty evidence of motor imagery increasing knee extensor strength was demonstrated in participants following a total knee arthroplasty [74].
The pooled results (n = 60, 3 studies) of the meta-analysis for ankle range of motion revealed no association with (SMD 0.25; 95% CI: -0.43–0.93) motor imagery improving range of motion of the ankle compared to a control group. An important difference between the three studies can be found in the content of the motor imagery interventions. Especially, the study of Nunes et al. [30] differed greatly from the other studies: a computer showed 40 left–right images (lateralization) of the ankle, the total duration was approximately 2:40 min, the motor imagery was not motor focused, and no detailed and acoustic instruction was given. Therefore only 3 out of 15 criteria for a successful motor imagery intervention were met (see Table 3). The study of Abraham et al. [63] (10/15 criteria) provided a group intervention, did not combine it with physical practice, participants were free to have their eyes open and was applied twice a week [63], despite evidence suggesting better effects when the exercise is done individually, combined with physical practice, with participants having their eyes closed in a quiet place and provided three times per week [46].
Other systematic reviews studying different populations in other regions of the body have found contradictory evidence for motor imagery [20, 74,75,76]. For instance, a meta-analysis in persons with various musculoskeletal conditions, such as shoulder, knee, and ankle disorders, found no significant effect in acute musculoskeletal conditions on range of motion [76]. Another review stated that the effect of adding motor imagery to standard therapy on active range of motion in patients with a total knee arthroplasty was unclear [75]. Both studies are in line with the results of the current systematic review and do not show a clear effect in acute injuries [75, 76].
With respect to balance, the body of evidence (n = 47, 2 studies) showed no association, with only a trend for the posteromedial direction on the Star Balance Excursion Test. When comparing the motor imagery interventions between the two included studies in the meta-analysis for balance, heterogeneity is observed. Nagar & Noohu [60] scored positive on 12 out of 15 criteria for the quality of the intervention, while Nunes et al. [30] scored positive on only 3/15 criteria (see Table 3). This large difference might explain some of the variation between the results of both studies. Other recent systematic reviews, yet directed at different populations, found low certainty, small to moderate effects of action observation and motor imagery on balance [20, 77]. However, those reviews did not use the Star Balance Excursion test as a measurement instrument for balance, but used the Tinetti test [20], Berg Balance Scale, Functional Reach Test, body sway, or rated balance functionally during an obstacle course [77]. Direct comparison of the results of the current meta-analysis with those reviews is therefore limited.
Regarding edema (n = 35, 2 studies), no association with motor imagery was found (SMD 1.11; 95% CI: -1.60 to 3.82). The results were classified as indicating very low- certainty evidence. Only two studies could be included in the meta-analysis, again the large effect in favour of the control group in the study of Nunes et al. [30] had a strong influence on the overall effect. A search for other studies evaluating the effect of motor imagery on edema in the field of musculoskeletal disorders resulted in no hits, therefore, a further exploration of this result was not possible.
Due to the very low certainty of the evidence, the effect of motor imagery on muscle strength, ankle range of motion, balance, and edema, in persons with and without a lateral ankle sprain is still uncertain. It is recommended that researchers undertake more high-quality studies with larger sample sizes. Studies should use a randomized controlled trial design with blinded assessors during pre- and post-intervention measurements to decrease the changes of performance bias. Placebo motor imagery intervention should be developed to at least blind the participants, and the motor imagery intervention should score positive on as many of the 15 criteria of Schuster et al. as possible [46]. The criteria from Table 3 provide a framework for the design of a successful motor imagery intervention, and it is likely that a high number of fulfilled criteria results in a higher-quality motor imagery intervention. Further, pre-specified analysis plans should be published to promote unbiased assessment of the data and studies should aim at recruiting athletes with lateral ankle sprains. Researchers, as well as practitioners, are encouraged to use the criteria in developing future motor imagery interventions. Clinicians could use the framework that is discussed in this study as a guideline.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Delahunt E, Bleakley CM, Bossard DS, et al. Clinical assessment of acute lateral ankle sprain injuries (ROAST): 2019 consensus statement and recommendations of the international ankle consortium. Br J Sports Med. 2018;52(20):1304–10. https://doi.org/10.1136/bjsports-2017-098885.
Tassignon B, Verschueren J, Delahunt E, et al. Criteria-based return to sport decision-making following lateral ankle sprain injury: a systematic review and narrative synthesis. Sports Med. 2019;49(4):601–19. https://doi.org/10.1007/s40279-019-01071-3.
Dubin JC, Comeau D, McClelland RI, Dubin RA, Ferrel E. Lateral and syndesmotic ankle sprain injuries: a narrative literature review. J Chiropr Med. 2011;10(3):204–19. https://doi.org/10.1016/j.jcm.2011.02.001.
Kristianslund E, Bahr R, Krosshaug T. Kinematics and kinetics of an accidental lateral ankle sprain. J Biomech. 2011;44(14):2576–8. https://doi.org/10.1016/j.jbiomech.2011.07.014.
Ekstrand J, Hägglund M, Kristenson K, Magnusson H, Waldén M. Fewer ligament injuries but no preventive effect on muscle injuries and severe injuries: an 11-year follow-up of the UEFA Champions League injury study. Br J Sports Med. 2013;47(12):732–7. https://doi.org/10.1136/bjsports-2013-092394.
D’Hooghe P, Cruz F, Alkhelaifi K. Return to play after a lateral ligament ankle sprain. Curr Rev Musculoskelet Med. 2020;13(3):281–8. https://doi.org/10.1007/s12178-020-09631-1.
Vuurberg G, Hoorntje A, Wink LM, et al. Diagnosis, treatment and prevention of ankle sprains: update of an evidence-based clinical guideline. Br J Sports Med. 2018;52(15):956. https://doi.org/10.1136/bjsports-2017-098106.
Wikstrom EA, Cain MS, Chandran A, et al. Lateral ankle sprain increases subsequent ankle sprain risk: a systematic review. J Athl Train. Published online November 5, 2020 https://doi.org/10.4085/168-20.
Thompson C, Schabrun S, Romero R, Bialocerkowski A, van Dieen J, Marshall P. Factors contributing to chronic ankle instability: a systematic review and meta-analysis of systematic reviews. Sports Med. 2018;48(1):189–205. https://doi.org/10.1007/s40279-017-0781-4.
Zijdewind I, Toering ST, Bessem B, Van der Laan O, Diercks RL. Effects of imagery motor training on torque production of ankle plantar flexor muscles. Muscle Nerve. 2003;28(2):168–73. https://doi.org/10.1002/mus.10406.
Willems T, Witvrouw E, Verstuyft J, Vaes P, De Clercq D. Proprioception and muscle strength in subjects with a history of ankle sprains and chronic instability. J Athl Train. 2002;37(4):487–93.
Hertel J, Corbett RO. An updated model of chronic ankle instability. J Athl Train. 2019;54(6):572–88. https://doi.org/10.4085/1062-6050-344-18.
Van Deun S, Staes FF, Stappaerts KH, Janssens L, Levin O, Peers KKH. Relationship of chronic ankle instability to muscle activation patterns during the transition from double-leg to single-leg stance. Am J Sports Med. 2007;35(2):274–81. https://doi.org/10.1177/0363546506294470.
Bruce AS, Howard JS, Van Werkhoven H, McBride JM, Needle AR. The effects of transcranial direct current stimulation on chronic ankle instability. Med Sci Sports Exerc. 2020;52:335–44. https://doi.org/10.1249/MSS.0000000000002129.
Needle AR, Lepley AS, Grooms DR. Central nervous system adaptation after ligamentous injury: a summary of theories, evidence, and clinical interpretation. Sports Med. 2017;47(7):1271–88. https://doi.org/10.1007/s40279-016-0666-y.
Cuenca-Martínez F, Suso-Martí L, León-Hernández JV, La Touche R. The role of movement representation techniques in the motor learning process: a neurophysiological hypothesis and a narrative review. Brain Sci. 2020;10(1):27. https://doi.org/10.3390/brainsci10010027.
Orr ELR, Lacourse MG, Cohen MJ, Cramer SC. Cortical activation during executed, imagined, and observed foot movements. NeuroReport. 2008;19(6):625–30. https://doi.org/10.1097/WNR.0b013e3282fbf9e0.
Mizuguchi N, Kanosue K. Changes in Brain Activity during Action Observation and Motor Imagery: Their Relationship with Motor Learning. Vol 234. 1st ed. Elsevier B.V.; 2017. https://doi.org/10.1016/bs.pbr.2017.08.008.
Thieme H, Morkisch N, Rietz C, Dohle C, Borgetto B. The efficacy of movement representation techniques for treatment of limb pain - a systematic review and meta-analysis. Journal of Pain. 2016;17(2):167–80. https://doi.org/10.1016/j.jpain.2015.10.015.
Cuenca-Martínez F, Angulo-Díaz-Parreño S, Feijóo-Rubio X, et al. Motor effects of movement representation techniques and crosseducation: a systematic review and meta-analysis. Eur J Phys Rehabil Med. Published online 2021. https://doi.org/10.23736/S1973-9087.21.06893-3.
MacIntyre TE, Madan CR, Moran AP, Collet C, Guillot A. Motor Imagery, Performance and Motor Rehabilitation. Vol 240. 1st ed. Elsevier B.V.; 2018. https://doi.org/10.1016/bs.pbr.2018.09.010.
Suso-Martí L, La Touche R, Angulo-Díaz-Parreño S, Cuenca-Martínez F. Effectiveness of motor imagery and action observation training on musculoskeletal pain intensity: a systematic review and meta-analysis. European J Pain (United Kingdom). 2020;24(January):1–16. https://doi.org/10.1002/ejp.1540.
Ruffino C, Papaxanthis C, Lebon F. Neural plasticity during motor learning with motor imagery practice: Review and perspectives. Neuroscience. 2017;341:61–78. https://doi.org/10.1016/j.neuroscience.2016.11.023.
Dello Iacono A, Ashcroft K, Zubac D. Ain’t Just Imagination! effects of motor imagery training on strength and power performance of athletes during detraining. Med Sci Sports Exerc. 2021;53(11):2324–32. https://doi.org/10.1249/MSS.0000000000002706.
Paravlic AH, Slimani M, Tod D, Marusic U, Milanovic Z, Pisot R. Effects and dose-response relationships of motor imagery practice on strength development in healthy adult populations: a systematic review and meta-analysis. Sports Med. 2018;48(5):1165–87. https://doi.org/10.1007/s40279-018-0874-8.
Mulder T. Motor imagery and action observation: Cognitive tools for rehabilitation. J Neural Transm. 2007;114(10):1265–78. https://doi.org/10.1007/s00702-007-0763-z.
Paravlic AH, Pisot R, Marusic U. Specific and general adaptations following motor imagery practice focused on muscle strength in total knee arthroplasty rehabilitation: a randomized controlled trial. PLoS ONE. 2019;14(8):1–19. https://doi.org/10.1371/journal.pone.0221089.
Lebon F, Guillot A, Collet C. Increased muscle activation following motor imagery during the rehabilitation of the anterior cruciate ligament. Appl Psychophysiol Biofeed. 2012;37(1):45–51. https://doi.org/10.1007/s10484-011-9175-9.
Sidaway S, Trzaska A. Can mental practice increase ankle dorsiflexor torque? Phys Ther. 2005;85(10):1053–60. https://doi.org/10.1093/ptj/85.10.1053.
Nunes G, de Noronha M. Motor imagery in the treatment of acute lateral ankle sprains in soccer athletes: a pilot study. Fisioterapia e Pesquisa. 2015;22(3):282–90. https://doi.org/10.590/1809-2950/13856722032015.
Christakou A, Zervas Y. The effectiveness of imagery on pain, edema, and range of motion in athletes with a grade II ankle sprain. Phys Ther Sport. 2007;8(3):130–40. https://doi.org/10.1016/j.ptsp.2007.03.005.
Christakou A, Zervas Y, Lavallee D. The adjunctive role of imagery on the functional rehabilitation of a grade II ankle sprain. Hum Mov Sci. 2007;26(1):141–54. https://doi.org/10.1016/j.humov.2006.07.010.
Page MJ, McKenzie JE, Bossuyt PM, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. The BMJ. 2020;2021:372. https://doi.org/10.1136/bmj.n71.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. https://doi.org/10.1186/s13643-016-0384-4.
Thomas J, Kneale D, McKenzie JE, Brennan SE, Bhaumik S. Determining the scope of the review and the questions it will address. Cochrane handbook for systematic reviews of Interventions. Wiley. 2019;23:13–31. https://doi.org/10.1002/9781119536604.ch2.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. The BMJ. 2019;366:1–8. https://doi.org/10.1136/bmj.l4898.
Boutron I, Page MJ, Higgins JP, Altman DG, Lundh A, Hróbjartsson A. Considering bias and conflicts of interest among the included studies. Cochrane Handbook Syst Rev Interv. 2019;23:177–204. https://doi.org/10.1002/9781119536604.ch7.
Li T, Higgins JPT, Deeks JJ. Chapter 5: Collecting data. Cochrane Handbook for Systematic Reviews of Interventions, Version 60. Published online 2019:1–39. www.training.cochrane.org/handbook.
Deeks J, Higgins J, Altman D. Chapter 10: Analysing data and undertaking meta-analyses | Cochrane Training. In: Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. Vol 1. ; 2020. https://training.cochrane.org/handbook/current/chapter-10.
The Cochrane Collaboration. Review Manager (RevMan). Published online 2014.
Higgins JP, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. Cochrane Handbook Syst Rev Interv. 2019;23:143–76. https://doi.org/10.1002/9781119536604.ch6.
Guyatt GH, Oxman AD, Kunz R, et al. GRADE: going from evidence to recommendations. Br Med J. 2008;9(3):257–9.
Guyatt GH, Oxman AD, Kunz R, et al. what is “quality of evidence” and why is it important to clinicians. Br Med J. 2008;336(may):995–8.
Guyatt GH, Oxman AD, Kunz R, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. Br Med J. 2008;55(5):853–5. https://doi.org/10.1373/clinchem.2008.117614.
Guyatt GH, Oxman AD, Kunz R, et al. Rating quality of evidence and strength of recommendations: Incorporating considerations of resources use into grading recommendations. Br Med J. 2008;336(7654):1170.
Schuster C, Hilfiker R, Amft O, et al. Best practice for motor imagery: a systematic literature review on motor imagery training elements in five different disciplines. BMC Med. 2011;9(1):75. https://doi.org/10.1186/1741-7015-9-75.
Herbert RD, Bø K. Education and Debate Analysis of Quality of Interventions in Systematic Reviews.; 2005. http://submit.bmj.com
Yoon M, Shim J, Kim SJ, Namkoong S. Effect of kinesthetic imagery training and visual imagery training on balance and foot pressure in adults with functional ankle instability. Indian J Public Health Res Dev. 2018;9(3):838–43. https://doi.org/10.5958/0976-5506.2018.00392.3 (https://www.embase.com/search/results?subaction=viewrecord&id=L624087334&from=export).
Reiser M. Strength gains by motor imagery with different ratios of physical to mental practice. Front Psychol. 2011;2:194. https://doi.org/10.3389/fpsyg.2011.00194.
Chiacchiero M, Cagliostro P. Motor imagery improves balance in older adults. Topics in Geriatric …. Published online 2015. https://www.ingentaconnect.com/content/wk/tgr/2015/00000031/00000002/art00012
Gim M, Choi J, Ga H. Effects of Motor Imagery Training and Balance Training on Static Balance: A Quasi-Experimental Study. Journal of International Academy of …. Published online 2020. https://www.koreascience.or.kr/article/JAKO202033248935436.page
Lebon F, Collet C, Guillot A. Benefits of motor imagery training on muscle strength. The Journal of Strength & …. Published online 2010. https://journals.lww.com/nsca-jscr/fulltext/2010/06000/Benefits_of_Motor_Imagery_Training_on_Muscle.34.aspx.
Natali F, Gonzalez-Rosa JJ, Velikova S, et al. Action observation and motor imagery in motor learning: evidence from EEG and kinematic analysis. Clin Neurophysiol. 2011;122:S29. https://doi.org/10.1016/S1388-2457(11)60093-X (https://www.embase.com/search/results?subaction=viewrecord&id=L70495817&from=export).
Vera MDA, Varner MDK. Design of an injury prevention program for professional ballet: a randomized controlled investigation. 26 th Annual Conference. Published online 2016.
Abdoli B, Shams A. The effect of practice type on static and dynamic balance in elderly 60–75 year old women with no history of falling. … Medicine: Basic and …. Published online 2020. http://daneshvarmed.shahed.ac.ir/article_1501_5af6ef95403f1637bf70e78cff42382d.pdf.
Lee D, Ji S. Effect of short foot exercise using an imagery technique on chronic ankle instability. Journal of The Korean Society of Integrative …. Published online 2017. https://www.koreascience.or.kr/article/JAKO201720861205662.page.
Niazi AF, Eslami R, Namdar S. The effect of 10 sessions mental training compared with physical training on foot joints and accuracy of soccer penalty kick. Research in Sport …. Published online 2018. https://jrsm.khu.ac.ir/browse.php?a_code=A-10-1408-3&slc_lang=en&sid=1.
Yang H, Kang H, Heo J. Effects of Ankle Exercise combined with Mental Practice on Postural Alignment. Journal of The Korean Society of …. Published online 2018. https://www.koreascience.or.kr/article/JAKO201819355173522.page.
Madison MK. The Effect of Internal Motor Imagery on Proprioceptive Function in Injured Athletes. Published online 2019.
Nagar V, Noohu MM. The effect of mental imagery on muscle strength and balance performance in recreational basketball players. Medicina Sportiva. 2014;3(3):2387–93.
Grosprêtre S, Jacquet T, Lebon F, Papaxanthis C, Martin A. Neural mechanisms of strength increase after one-week motor imagery training. Eur J Sport Sci. 2017;18(2):209–18. https://doi.org/10.1080/17461391.2017.1415377.
Dehghan M, Eydivandi K, Jaafarzadeh G. The effect of proprioceptive training associated with the mental imagery exercise programs on the prevention of ankle sprains in young soccer players. Eur J Exp Biol. 2013;3(2):576–9.
Abraham A, Dunsky A, Dickstein R. the effect of motor imagery practice on elevé performance in adolescent female dance students: a randomized controlled trial. J Imag Res Sport Phys Act. 2017;12(1):1–19. https://doi.org/10.1515/jirspa-2016-0006.
Bouguetoch A, Martin A, Grosprêtre S. Insights into the combination of neuromuscular electrical stimulation and motor imagery in a training-based approach. Eur J Appl Physiol. 2021;121(3):941–55. https://doi.org/10.1007/s00421-020-04582-4.
Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions . Vol 6.2. 6.2. Cochrane; 2021. www.training.cochrane.org/handbook. Accessed 20 Feb 2022.
Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins J, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; 2019:349–374. https://doi.org/10.1002/9781119536604.ch13
Cuenca-Martínez F, La TR, León-Hernández JV, Suso-Martí L. Mental practice in isolation improves cervical joint position sense in patients with chronic neck pain: a randomized single-blind placebo trial. PeerJ. 2019;2019(9):1–24. https://doi.org/10.7717/peerj.7681.
Bang DH, Shin WS, Kim SY, Choi JD. The effects of action observational training on walking ability in chronic stroke patients: A double-blind randomized controlled trial. Clin Rehabil. 2013;27(12):1118–25. https://doi.org/10.1177/0269215513501528.
Buccino G, Arisi D, Gough P, et al. Improving upper limb motor functions through action observation treatment: a pilot study in children with cerebral palsy. Dev Med Child Neurol. 2012;54(9):822–8. https://doi.org/10.1111/j.1469-8749.2012.04334.x.
Reiser M. Kraftgewinne durch Vorstellung maximaler Muskelkontraktionen. Z Sportpsychol. 2005;12(1):11–21. https://doi.org/10.1026/1612-5010.12.1.11.
La Touche R, Grande-Alonso M, Cuenca-Martínez F, Gónzález-Ferrero L, Suso-Martí L, Paris-Alemany A. Diminished Kinesthetic and Visual Motor Imagery Ability in Adults With Chronic Low Back Pain. PM and R. 2018;(July). https://doi.org/10.1016/j.pmrj.2018.05.025.
Williams SE, Cumming J, Ntoumanis N, Nordin-Bates SM, Ramsey R, Hall C. Further validation and development of the movement imagery questionnaire. J Sport Exercise Psychol. 2012;34:621–46.
Suica Z, Behrendt F, Gäumann S, et al. Imagery ability assessments: a cross-disciplinary systematic review and quality evaluation of psychometric properties. BMC Med. 2022;20(1):1–24. https://doi.org/10.1186/s12916-022-02295-3.
Paravlic AH, Tod D, Milanovic Z. Mental simulation practice has beneficial effects on patients’ physical function following lower limb arthroplasty a systematic review and meta-analysis. American Congress Rehab Med. 2020;101:1447–61. https://doi.org/10.1016/j.apmr.2020.04.004.
Ferrer-Peña R, Cuenca-Martínez F, Romero-Palau M, et al. Effects of motor imagery on strength, range of motion, physical function, and pain intensity in patients with total knee arthroplasty: a systematic review and meta-analysis. Braz J Phys Ther. 2021;25(6):698–708. https://doi.org/10.1016/j.bjpt.2021.11.001.
Da YBW, Lim ECW. The effects of motor imagery on pain and range of motion in musculoskeletal disorders. Clin J Pain. 2019;35(1):87–99. https://doi.org/10.1097/AJP.0000000000000648.
Nicholson V, Watts N, Chani Y, Keogh JW. Motor imagery training improves balance and mobility outcomes in older adults: a systematic review. J Physiother. 2019;65(4):200–7. https://doi.org/10.1016/j.jphys.2019.08.007.
Acknowledgements
We thank Daan Marcellis for his assistance in designing the search strategy. We also thank Dr. Sidney Grosprêtre for providing additional information.
Funding
No financial grant was received for the current study.
Author information
Authors and Affiliations
Contributions
Luuk Siemes: Conceptualization, Methodology, Investigation, Writing – original draft, Visualization. Maarten van de Worp: Investigation, Writing – review and editing. Henk Nieuwenhuijzen: writing – review and editing. Niki Stolwijk: writing – review and editing. Thomas Pelgrim: Validation. Bart Staal: Conceptualization, Writing – review and editing, Supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Siemes, L.J., van der Worp, M.P., Nieuwenhuijzen, P.H.J. et al. The effect of movement representation techniques on ankle function and performance in persons with or without a lateral ankle sprain: a systematic review and meta-analysis. BMC Musculoskelet Disord 24, 786 (2023). https://doi.org/10.1186/s12891-023-06906-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-023-06906-9