Abstract
Purpose
Cervical laminoplasty (CLP) is a developed surgical procedure for the treatment of cervical spondylotic myelopathy (CSM), but only a few of those studies focus on preoperative dynamic cervical sagittal alignment and the study of different degrees of loss of cervical lordosis (LCL) is lacking. This study aimed to analyze patients who underwent CLP to investigate the effect of cervical extension and flexion function on different degrees of LCL.
Methods
In this retrospective case–control study, we analyzed 79 patients who underwent CLP for CSM between January 2019 and December 2020. We measured the cervical sagittal alignment parameters on lateral radiographs (neutral, flexion, and extension positions) and used Japanese Orthopedic Association (JOA) score to assess clinical outcomes. We defined the extension ratio (EXR) as 100 × Ext ROM (cervical range of extension)/ROM (cervical range of motion). We observed the relationships between collected variables (demographic and radiological variables) and LCL. Patients were classified into the following three groups according to the LCL: stability group: (LCL ≤ 5°); mild loss group (5° < LCL ≤ 10°); and severe loss group (LCL > 10°). We compared the differences of collected variables (demographic, surgical and radiological variables) among the three groups.
Results
Seventy-nine patients were enrolled (mean age 62.92 years; 51 men, 28 women) in the study. Among the three groups, cervical Ext ROM was the best in the stability group (p < 0.01). Compared with the stability group, range of flexion (Flex ROM) was significantly higher (p < 0.05) and EXR was significantly lower (p < 0.01) in the severe loss group. Compared with the severe loss group, JOA recovery rates were better (p < 0.01) in the stability group. Receiver-operating characteristic curve (ROC) analysis to predict LCL > 10° (area under the curve = 0.808, p < 0.001). The cutoff value for EXR was 16.80%, with sensitivity and specificity of 72.5% and 82.4%, respectively.
Conclusion
CLP should be carefully considered for patients with a preoperative low Ext ROM and high Flex ROM, as a significant kyphotic change is likely to develop after surgery. EXR is a useful and simple index to predict significant kyphotic changes.
Similar content being viewed by others
Introduction
Cervical spondylotic myelopathy (CSM) is one of the most prevalent neurological disorders in older population which is characterized by neck pain, and sensory, motor, and/or reflex deficits [1]. Cervical laminoplasty (CLP) is a developed surgical procedure for the treatment of CSM [2,3,4,5]. CLP can preserve the motion of the operated levels and is more suitable for patients undergoing a multi-segmental cervical spinal cord surgery. However, cervical laminoplasty, as posterior non-fusion decompression surgery, can lead to some potential complications, such as loss of cervical lordosis (LCL), decreased neck motion range, axial neck pain, C5 nerve root palsy, and lamina closure. Among them, LCL is a significant issue.
The alignment of the cervical spine needs to maintain adequate lordosis to provide enough space for the shifting of the spinal cord. Therefore, prevention of LCL and investigation of its risk factors after CLP are necessary. Several studies have reported many preoperative predictors of LCL after laminoplasty [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. and the relationship between static cervical sagittal alignment and kyphotic changes has been well described [6,7,8,9, 13, 15, 17, 18, 20,21,22]. However, only a few of those studies focus on preoperative dynamic cervical sagittal alignment [11, 14, 16] and the study of different degrees of LCL is lacking.
We aimed to retrospectively analyze patients who underwent laminoplasty to investigate the effect of preoperative static and dynamic cervical sagittal alignment on LCL after laminoplasty. We hypothesized preoperative cervical extension function and flexion function were associated with different degrees of LCL and neurological recovery and found out potential risk factors for serious post-laminoplasty LCL.
Materials and methods
Patient enrollment
This study was approved by the IRB of our affiliated institution (IRB number: 2018-086). This was a retrospective case–control study of the patients who underwent cervical laminoplasty at our institution between January 2019 and December 2020. Patients were eligible for our study if they met the following inclusion criteria: 1) aged 18 years or older without previous cervical surgery; 2) a lesion involving more than three levels of CSM 3) clinical signs of myelopathy (difficulty with manual dexterity, upper extremity numbness, gait disturbance); and 4) at least twelve months of follow-up. Patients with fractures, infections, tumors, combinations with fusion surgery, decompression levels including C2 or thoracic spine levels, and invisible T1 upper endplates were excluded. Finally, seventy-nine patients were enrolled.
Surgical procedures
After the induction of general endotracheal anesthesia, patients were positioned prone on the operating table. The surgeons performed an incision in the back of the neck, and detached the paravertebral muscle from the spinous process and lamina, preserving the facet capsule. All patients underwent open-door laminoplasty with a mini titanium plate system for decompression. One side of the lamina was opened, and the other side served as the hinge.
Radiological parameters
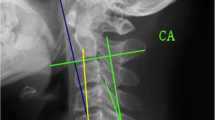
The cervical sagittal alignment parameters were measured on lateral radiographs (Fig. 1): cervical lordosis (CL) was the angle between the C2 lower endplate and the C7 lower endplate; T1 slope (T1S) was the angle between a horizontal plane and a line parallel to the superior T1 endplate; cervical sagittal vertical axis (cSVA) was defined as the horizontal offset from a plumbline dropped from the C2 vertebral body to the posterosuperior corner of the C7 vertebra; CL in flexion (Flex CL) and extension (Ext CL) were measured on radiographs in the flexion and extension positions.
The cervical spine range of motion (ROM) was calculated as Ext CL—Flex CL. Cervical spine range of flexion (Flex ROM) was calculated as CL—Flex CL and cervical spine range of extension (Ext ROM) was calculated as Ext CL—CL. The extension ratio (EXR) was defined as 100 × Ext ROM/ROM. LCL was defined as preoperative CL—postoperative CL. Patients were classified into the following three groups according to the LCL [11, 21]: stability group: (LCL ≤ 5°); mild loss group (5° < LCL ≤ 10°); and severe loss group (LCL > 10°). The flowchart of study is displayed in Fig. 2.
Clinical parameters
The Japanese Orthopedic Association (JOA) score [23], before surgery and at the 1-year follow-up visit, was used to evaluate clinical outcomes. The recovery rate was calculated as follows: JOA recovery rate = 100 × (postoperative JOA—preoperative JOA) / (17—preoperative JOA).
Statistical analysis
All the data were analyzed using SPSS version 22.0 software (SPSS, Inc, Chicago, IL, USA). Variables were described as mean ± standard deviation and interclass correlation coefficient was used to indicate the measurement consistency between two observers. Pearson correlation analysis was used to analyze the correlation; multiple linear regression model was used to explore the risk factors for LCL. Chi-square test was used to compare categorical data among the groups; T-tests, ANOVA, and Kruskal–Wallis tests were used to assess the differences of radiographic parameters among the groups. A receiver-operating characteristic curve (ROC) analysis was used to determine the optimal cutoff value. P value < 0.05 was considered as evidence of statistical significance.
Results
Seventy-nine patients were enrolled (mean age 62.92 years; 51 men, 28 women) in the study. CL decreased significantly after CLP (pre-, 17.34 ± 10.44 vs. post 12.37 ± 11.42, p < 0.01). The overall demographic, surgery segments, and proximal level are summarized in Table 1.
Correlations between LCL and preoperative parameters
In the correlation analysis, LCL was positively correlated with cervical flexion capacity (r = 0.278, p < 0.05) and negatively correlated with cervical extension capacity (r = -0.456, p < 0.01). No significant correlations were observed between the other evaluated parameters (Table 2).
Risk factors for LCL
Multiple linear regression analysis was conducted by using variables that were found to be significantly correlated with the LCL. The results suggested that LCL decreased by 0.421° (p < 0.001) for each extension CL, and increased by 0.208° (p = 0.042) for flexion CL. LCL could be predicted by using the following regression equation: LCL = 5.507—0.421 * Ext ROM + 0.208 * Flex ROM (Table 3).
Comparison of evaluated parameter variables according to postoperative LCL
Compared with the stability group (LCL ≤ 5°), the preoperative CL was significantly higher while postoperative CL was significantly lower in the severe loss group (LCL > 10°). Among the three groups, cervical extension Ext ROM was the best in the stability group. Compared with the stability group, Flex ROM was significantly higher and the extension ratio (EXR) was significantly lower in the severe loss group. As for clinical symptoms, pre- and postoperative JOA did not significantly differ among the three groups. Compared with the severe loss group, JOA recovery rates were better in the stability group (Table 4).
Effectiveness of EXR to predict severe lordosis loss
The ROC curve in Fig. 3 shows good discriminative power of EXR to predict severe lordosis loss after CLP (area under the curve = 0.808, p < 0.001; cutoff value, 16.80%; sensitivity: 72.5%; specificity: 82.4%; positive predictive value: 60.0%; negative predictive value: 96.3%). In the severe loss group, 15/17 patients were subclassified as low EXR (EXR ≤ 16.80%); in the mild loss group, 6/15 patients were subclassified as the low EXR group. In the stability group, only 4/47 patients were subclassified into the low EXR group.
Discussion
Having sufficient postoperative cervical lordosis is a prerequisite for CLP to obtain the indirect anterior decompression effect. LCL has been reported to be associated with poor outcomes after laminoplasty in many studies [7,8,9, 17, 24, 25]. Kim et al. [17] and Miyazaki et al. [19] reported that preoperative higher T1S was a risk factor for LCL. T1S-CL [8, 17, 22] and CL/T1S [26] have also been considered as predictors for LCL after CLP. However, there are some studies showing different results regarding the correlation between T1S and LCL [16, 27,28,29]. Michael et al. [12] and Seo et al. [8] emphasize the importance of cephalad vertebral level and cervical foraminal stenosis in LCL after laminoplasty. Some studies also report other factors for LCL, such as cSVA [15, 17, 22], C7-SVA [20, 21], CGH-C7 SVA [7, 9] and age [6]. In the present study, we evaluated regional static parameters to identify possible risk factors for postoperative kyphotic alignment change. However, no significant correlations were observed between the static parameters and LCL in our study. These static parameters have their own limitations in accounting for LCL after surgery. According to our knowledge, there are no theories with consensus that explain why these static parameters can affect LCL after surgery. Many researchers think that the posterior neck muscular-ligament complex may play an important role in these processes [6, 12, 16,17,18, 22, 30, 31].
Recently, some studies have shown the relationship between preoperative dynamic cervical sagittal alignment and LCL after CLP. Lee et al. [16] reported the extension function of the cervical spine as an indicator to predict kyphotic change after CLP, and showed that significant kyphotic change occurred in patients whose Ext ROM was < 14°. Moreover, some studies have shown that higher Flex ROM results in greater LCL after CLP [11, 18, 30,31,32]. The present study showed similar results to those studies: preoperative Ext ROM (β = -0.421) and Flex ROM (β = 0.208) were predictors for postoperative LCL. Our study reported a high negative correlation between Ext ROM and LCL, which implies that enough Ext ROM is a highly reliable factor in preventing LCL after CLP. Similar to the results of previous studies [11, 18, 30,31,32], our study shows the positive correlation between Flex ROM and LCL. Cervical flexion mobility is blocked by degenerative structures, such as bone, ligaments, or muscles. Fujishiro et al. [30] speculated that increased motion in the flexional direction indicates that such structural forces restricting motion toward the kyphotic position are weak. Because of the surgical injury, the equilibrium necessary to maintain cervical sagittal alignment is disrupted and results in a higher prevalence of LCL.
In our study, we discovered that different degrees of postoperative LCL implied different degrees of neurological recovery. Worse JOA recovery rate was reported in patients in the severe loss group compared with the stability group. Similar tendencies were also shown between the stability group and the mild loss group; however, there was no evidence of statistical significance (p > 0.05). Postoperative mild LCL occurred in patients with a low level of Ext ROM and the influences of LCL on postoperative neurological recovery were limited. Preoperative high levels of Flex ROM aggravate postoperative LCL for patients with low Ext ROM, and severe LCL implies poor clinical outcomes (Table 4, Figs. 4 and 5).
Representative cases. Sagittal preoperative radiographs (a-1, 2, 3) showing increased Ext ROM (20.3°) in a 65-year-old man who underwent laminoplasty of C4–7; no significant LCL was observed 18 months after surgery (a-2, a-4: pre CL 20.1° vs. post CL 24.9°). Sagittal preoperative radiographs (b-1, 2, 3) showing low Ext ROM (8.2°) and Flex ROM (29°) in a 48-year-old man who underwent laminoplasty of C3–7; mild LCL was observed 16 months after surgery (b-2, b-4: pre CL 16.2° vs. post CL 8.6°). Sagittal preoperative radiographs (c-1, 2, 3) showing low Ext ROM (3.1°) and increased Flex ROM (38.3°) in a 55-year-old man who underwent laminoplasty of C4–6; severe LCL was observed 24 months after surgery (c-2, c-4: pre CL 15.4° vs. post CL -7.6°). CL, cervical lordosis; LCL, loss of cervical lordosis; Flex ROM, cervical spine range of flexion; Ext ROM, cervical spine range of extension
Ranges of Ext ROM and Flex ROM (25% quartile-75% quartile). The ranges of Ext ROM in the three groups were 7–15°; 4–11.5°; and 2–8°, respectively. The ranges of Flex ROM in three groups were 23–37°; 23–41°; and 27–46°, respectively. Flex ROM, cervical spine range of flexion; Ext ROM, cervical spine range of extension
Some researchers have speculated that the degree of cervical extension mobility indicates the cervical constriction reservoir [16] and cervical flexion mobility indicates the forces inhibiting cervical kyphosis [30]. Both Flex ROM and Ext ROM were important factors for LCL after CLP. Ono et al. [11] proposed CL ratio (100 × Flex ROM / total ROM) as a novel predictor for the loss of cervical lordosis after laminoplasty and reported the cut off value of CL ratio for predicting postoperative LCL. Compared with Flex ROM, Ext ROM had a greater influence on postoperative LCL in our study. Therefore, we reported EXR (100 × Ext ROM / total ROM) as a predictor, and EXR showed better prediction in severe lordosis loss than Ext ROM or Flex ROM alone. The optimal cutoff value of EXR to discriminate between severe LCL and not severe LCL was 16.8% (Fig. 3). For patients with a preoperative EXR less than 16.8%, more cervical exercises should be encouraged after surgery due to the high prevalence of severe postoperative LCL. Multilevel posterior cervical fusion or anterior cervical fusion surgery can also be considered, if necessary.
This study has several limitations. First, because our study was retrospective, a selection bias may exist. Second, the number of patients was low. Only 17 cases were assigned to the severe loss group. Third, the follow-up period was 1 year. Choi et al. [33] reported that changes in cervical sagittal alignment generally reach a plateau at 6 months after CLP. Thus, the follow-up period was enough to investigate the risks for LCL after CLP. Finally, only the JOA score was used to evaluate clinical outcomes in the present study.
Conclusion
Preoperative dynamic cervical sagittal alignment is a highly useful indicator to predict the LCL after CLP. CLP should be carefully considered for patients with a preoperative low Ext ROM and high Flex ROM, as a significant kyphotic change is likely to develop after surgery. EXR is a useful and simple index to predict significant kyphotic changes.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due [This study is part of a series of studies that have not been completely completed] but are available from the corresponding author on reasonable request.
Abbreviations
- CLP:
-
Cervical laminoplasty
- CSM:
-
Cervical spondylotic myelopathy
- LCL:
-
Loss of cervical lordosis
- CL:
-
Cervical lordosis
- T1S:
-
T1 slope
- cSVA:
-
Cervical sagittal vertical axis
- Flex CL:
-
Cervical lordosis in flexion
- Ext CL:
-
Cervical lordosis in extension
- ROM:
-
Cervical spine range of motion
- Flex ROM:
-
Cervical spine range of flexion
- Ext ROM:
-
Cervical spine range of extension
- EXR:
-
Extension ratio
- JOA:
-
Japanese Orthopedic Association
- ROC:
-
Receiver-operating characteristic curve
References
Bono CM, Ghiselli G, Gilbert TJ, et al. An evidence-based clinical guideline for the diagnosis and treatment of cervical radiculopathy from degenerative disorders. Spine J. 2011;11:64–72.
Oshima Y, Miyoshi K, Mikami Y, et al. Long-term outcomes of cervical laminoplasty in the elderly. Biomed Res Int. 2015;2015:713952.
Kimura A, Seichi A, Inoue H, et al. Long-term results of double-door laminoplasty using hydroxyapatite spacers in patients with compressive cervical myelopathy. Eur Spine J. 2011;20:1560–6.
Choi SH, Kang CN. Degenerative cervical myelopathy: pathophysiology and current treatment strategies. Asian Spine J. 2020;14:710–20.
Chiba K, Ogawa Y, Ishii K, et al. Long-term results of expansive open-door laminoplasty for cervical myelopathy–average 14-year follow-up study. Spine (Phila Pa 1976). 2006;31:2998–3005.
Zhang JT, Li JQ, Niu RJ, et al. Predictors of cervical lordosis loss after laminoplasty in patients with cervical spondylotic myelopathy. Eur Spine J. 2017;26:1205–10.
Xu C, Zhang Y, Dong M, et al. The relationship between preoperative cervical sagittal balance and clinical outcome of laminoplasty treated cervical ossification of the posterior longitudinal ligament patients. Spine J. 2020;20:1422–9.
Seo J, Suk K, Kwon J, et al. Cervical foraminal stenosis as a risk factor for cervical kyphosis following cervical laminoplasty. Spine J. 2022;22:1271–80.
Sakai K, Yoshii T, Hirai T, et al. Cervical sagittal imbalance is a predictor of kyphotic deformity after laminoplasty in cervical spondylotic myelopathy patients without preoperative kyphotic alignment. Spine. 2016;41:299–305.
Pan Y, Ma X, Feng H, et al. Effect of posterior cervical expansive open-door laminoplasty on cervical sagittal balance. Eur Spine J. 2020;29:2831–7.
Ono K, Murata S, Matsushita M, et al. Cervical lordosis ratio as a novel predictor for the loss of cervical lordosis after laminoplasty. Neurospine. 2021;18:311–8.
Michael KW, Neustein TM, Rhee JM. Where should a laminoplasty start? The effect of the proximal level on post-laminoplasty loss of lordosis. The Spine Journal. 2016;16:737–41.
Matsuoka Y, Suzuki H, Endo K, et al. Small sagittal vertical axis accompanied with lumbar hyperlordosis as a risk factor for developing postoperative cervical kyphosis after expansive open-door laminoplasty. J Neurosurg Spine. 2018;29:176–81.
Machino M, Ando K, Kobayashi K, et al. Postoperative Kyphosis in Cervical Spondylotic Myelopathy. Spine. 2020;45:641–8.
Lin B, Hong K, Lin C, et al. Impact of global spine balance and cervical regional alignment on determination of postoperative cervical alignment after laminoplasty. Medicine. 2018;97:e13111.
Lee SH, Son DW, Lee JS, et al. Does extension dysfunction affect postoperative loss of cervical lordosis in patients who undergo laminoplasty? Spine. 2019;44:E456–64.
Kim K, Lee C, Park J, et al. Preoperative parameters for predicting the loss of lordosis after cervical laminoplasty. Spine. 2020;45:1476–84.
Kim B, Cho S, Hur JW, et al. Kinematics after cervical laminoplasty: risk factors for cervical kyphotic deformity after laminoplasty. The Spine Journal. 2021;21:1822–9.
Miyazaki M, Ishihara T, Notani N, et al. Relationship of T1 slope with loss of lordosis and surgical outcomes after laminoplasty for cervical ossification of the posterior longitudinal ligament. Clin Neurol Neurosur. 2018;164:19–24.
Oshima Y, Takeshita K, Taniguchi Y, et al. Effect of preoperative sagittal balance on cervical laminoplasty outcomes. Spine. 2016;41:E1265–70.
Li XY, Wang Y, Zhu WG, et al. Impact of cervical and global spine sagittal alignment on cervical curvature changes after posterior cervical laminoplasty. J Orthop Surg Res. 2022;17:521.
Chen HY, Yang MH, Lin YP, et al. Impact of cervical sagittal parameters and spinal cord morphology in cervical spondylotic myelopathy status post spinous process-splitting laminoplasty. Eur Spine J. 2020;29:1052–60.
Fukui M, Chiba K, Kawakami M, et al. Japanese Orthopaedic Association Back Pain Evaluation Questionnaire. Part 2. Verification of its reliability : The Subcommittee on Low Back Pain and Cervical Myelopathy Evaluation of the Clinical Outcome Committee of the Japanese Orthopaedic Association. J Orthop Sci. 2007;12:526–32.
Cao J, Zhang J, Yang D, et al. Multivariate analysis of factors associated with kyphotic deformity after laminoplasty in cervical spondylotic myelopathy patients without preoperative kyphotic alignment. Sci Rep. 2017;7:43443.
Kato M, Namikawa T, Matsumura A, et al. Effect of cervical sagittal balance on laminoplasty in patients with cervical myelopathy. Global Spine J. 2017;7:154–61.
Kong C, Li XY, Sun XY, et al. The ratio of C2–C7 Cobb angle to T1 slope is an effective parameter for the selection of posterior surgical approach for patients with multisegmental cervical spondylotic myelopathy. J Orthop Sci. 2020;25:953–9.
Cho JH, Ha JK, Kim DG, et al. Does preoperative T1 slope affect radiological and functional outcomes after cervical laminoplasty? Spine (Phila Pa 1976). 2014;39:E1575-81.
Lee SH, Son DW, Lee JS, et al. Differences in cervical sagittal alignment changes in patients undergoing laminoplasty and anterior cervical discectomy and fusion. Neurospine. 2018;15:91–100.
Sakamoto R, Nakamoto H, Yoshida Y, et al. Does T1 slope minus cervical lordosis mismatch affect surgical outcomes of cervical laminoplasty in the absence of preoperative severe kyphosis? BMC Musculoskelet Disord. 2022;23:810.
Fujishiro T, Nakano A, Yano T, et al. Significance of flexion range of motion as a risk factor for kyphotic change after cervical laminoplasty. J Clin Neurosci. 2020;76:100–6.
Fujishiro T, Hayama S, Obo T, et al. Gap between flexion and extension ranges of motion: a novel indicator to predict the loss of cervical lordosis after laminoplasty in patients with cervical spondylotic myelopathy. J Neurosurg Spine. 2021;35:8–17.
Obo T, Fujishiro T, Mizutani M, et al. Segmental cervical instability does not drive the loss of cervical lordosis after laminoplasty in patients with cervical spondylotic myelopathy. Spine J. 2022;22:1837–47.
Choi I, Roh SW, Rhim SC, et al. The time course of cervical alignment after cervical expansive laminoplasty: determining optimal cut-off preoperative angle for predicting postoperative kyphosis. Medicine (Baltimore). 2018;97:e13335.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Chengxin Liu: Writing, Reviewing, Editing, Methodology and Data Curation. Bin Shi: Writing, Reviewing, Data Curation and Supervision. Wei Wang: Editing, Data Curation and Supervision. Xiangyu Li: Editing and Supervision. Shibao Lu: Supervision. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethical committee of Beijing Xuanwu Hospital (clinical research NO. [2018]086). Informed consent was obtained from all subjects and/or their legal guardian(s). All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, C., Shi, B., Wang, W. et al. Effect of preoperative dynamic cervical sagittal alignment on the loss of cervical lordosis after laminoplasty. BMC Musculoskelet Disord 24, 233 (2023). https://doi.org/10.1186/s12891-023-06335-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-023-06335-8