Abstract
Background
Heterotopic ossification (HO) is noted most frequently in periarticular muscles and has not yet been reported in the cruciate ligaments of the knee. We present a rare case of symptomatic ossification of the posterior cruciate ligament (PCL).
Case presentation
A 59-year-old woman had a 2-year history of knee pain that was getting worse during knee motion and had restricted knee motion for 1 year. X-rays could not show the lesion clearly. Multi-planar computed tomography demonstrated ossification within the PCL with mild osteoarthritic changes and excluded any other intra-articular pathology. The patient underwent arthroscopic debridement and then experienced immediate relief of pain and complete recovery of range of motion.
Conclusion
This is the first report of HO in the PCL as a possible cause of knee pain and restricted knee motion. On the basis of literature review, this case elaborates the difference between HO and calcification in the ligaments, the related factors inducing HO and the undefined pathogenesis, and favorable prognosis after adequate treatment.
Similar content being viewed by others
Background
Heterotopic ossification (HO) refers to the pathologic formation of new bone in non-ossified tissues. Different from metastatic calcification and dystrophic calcification, HO commonly occurs after injuries and surgery and forms mature lamellar bone with limited capacity for growth [1]. HO commonly occurs in the skeletal muscle, subcutaneous tissue, skin, and fibrous tissue adjacent to joints and is noted most frequently in periarticular muscles, with decreasing frequencies in the hip, elbow, knee, and shoulder [2, 3]. The prevalence of HO is as high as 90% after hip arthroplasty and acetabular fracture, 40% after elbow fracture, and 25% after knee dislocation [3, 4]. However, this pathologic process has not yet been reported in the cruciate ligaments of the knee. Here, a case report of a patient with knee pain and restricted knee motion is presented, which was diagnosed as HO of the posterior cruciate ligament.
Case presentation
A 59-year-old female presented to our outpatient clinic complaining of pain in the left knee, with decreased range of motion (ROM) for 1 year. When she visited our inpatient department on the next day, her ROM of the left knee was restricted from 0° to 15°. She had a history of left knee pain in the past 2 years with the absence of trauma. The pain was non-specific and significantly exacerbated by knee activity. She had no prior consultation for her symptoms but occasionally took diclofenac sodium capsules for pain relief. The pain became so severe that she could no longer walk. Her past history of illness revealed that she had atrial fibrillation and a 1-week history of swelling and pain in both legs 3 years ago. Therefore, she underwent ultrasound examination and was diagnosed with multiple thrombosis in the bilateral lower limb arteries, which was more serious in the right lower limb with complete thrombus occlusion occurring in the right popliteal artery. She underwent interventional right iliac artery thrombectomy with stent implantation and balloon dilatation of the right anterior tibial artery in our hospital 3 years ago. Since then, she has been taking anticoagulants (warfarin) for atrial fibrillation until she presented at our department. The patient had no history of endocrine and metabolic disorders such as diabetes, hyperthyroidism, and gout. During physical examination, obvious and unbearable pain upon movement was noted without any knee swelling and tenderness. Signs related to meniscus pathology or joint laxity were not found.
Investigations
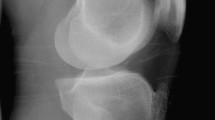
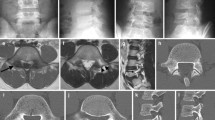
Blood tests were performed while the patient was hospitalized. The results revealed normal range of calcium and glucose at 2.35 and 4.99 mmol/L, respectively; however, the patient’s blood uric acid was high (480 μmol/L). The patient had normal hemoglobin of approximately 130 g/L. Three days after arthroscopic debridement, the patient developed mild leukocytosis, which was believed to be a response to surgical stress. The results of biochemical and hematologic tests were within the normal range. Anteroposterior and lateral radiographs of the left knee demonstrated a high-density shadow within the intercondylar notch with mild osteoarthritic changes (Fig. 1). The high-density mass on plain radiographs was so inconspicuous that our radiologist did not notice or report it. Multi-planar computed tomography (CT) positioned the lesion precisely and excluded any other intra-articular pathology that could cause limitation of joint movement. The mass appeared as a non-structural lesion in the intercondylar area by coronal CT (Fig. 2a). The sagittal CT image revealed the mass inside the PCL (Fig. 2b). CT showed the mass with heterogeneous density. In both X-ray and CT images, the mass was shown as high density, which made it difficult to determine whether it was an ossified or a calcified lesion. Although magnetic resonance imaging (MRI) is meaningful and necessary for a full evaluation of the ligaments in our patient, it was not recommended because of the presence of a stent of unknown material in the body.
Treatment
Because we speculated that the patient’s discomfort resulted from mechanical locking and entrapment by the dense mass, we recommended surgery and performed arthroscopy accordingly. Intraoperatively, a hump of the PCL was revealed by arthroscopic examination, while other pathological changes were not found. The fiber fascicles of the PCL appeared after debridement of the synovial membrane covering the pathological site of the PCL. We slit open the fascicles and exposed the mass, which appeared grayish-white and seemed to be hard based on the macroscopic appearance (Fig. 3). The mass was so closely adhered to the fibrous bundles of the ligament that debridement of the lesion was carefully performed. The pathological tissue obtained from surgery was sent for histopathology.
Pathologically, the tissues were calcified mature lamellar bone with completely fibrotic bone marrow, which was infiltrated by a few chronic inflammatory cells (Fig. 4). The pathological tissue obtained from surgery was stained with hematoxylin–eosin. The finding of osteogenic components or lamellar bone and chondrogenic components or cartilage led to the diagnosis of HO. The hallmark of osteogenic components, which were stained clearly red, is lamellar characteristics. The chondrogenic components were stained light blue with a few chondroblasts.
Outcome and follow-up
Following the surgery, the patient verbalized complete relief of her symptoms. Moreover, she was allowed full ROM and weight bearing with no assistance. No complications were observed during the postoperative course. The patient was discharged after wound healing 2 weeks later. At 1-year follow-up, clinical examination demonstrated no signs of recurrence, and the patient gained full ROM with no pain or instability.
Discussion and conclusions
To our knowledge, this is the first case of isolated PCL ossification reported in the literature. Two studies reported the occurrence of an ossicle within the substance of the anterior cruciate ligament (ACL); unfortunately, both of them considered that it was a variation of the ACL [5, 6]. Jung described posterolateral capsular heterotopic bone formation with decreased ROM of the knee, which needed surgical excision after PCL reconstruction [7]. In this study, only radiographs were used to diagnose HO without pathological findings, which were deficient or inadequate in terms of the methodology used to diagnose HO. Unlike cases of calcification, cases involving isolated symptomatic ossification of a ligament within the knee have been rarely reported [8,9,10,11,12]. Although their clinical manifestations and treatment methods are largely similar, the pathologies are completely different. Typical clinical findings such as restricted ROM and knee pain with or without a history of major trauma are common in ossification and calcification of a ligament [8,9,10,11,12].
Plain radiographs are primarily used to detect HO, but they can lead to misdiagnosis such as loose bodies [13]. CT is often used for the early evaluation of the extremity and demonstrates calcium within the ligament with high Hounsfield units (> 200). However, the density of ligament calcification is more uniform than that of ligament ossification, which is often accompanied by a shadow with slightly lower density in the center of the lesion. Although MRI was not performed in our case, some studies showed that MRI has advantages in displaying the specific location and identifying the nature of the mass [13,14,15]. MRI not only assesses the relation with lesion and peripheral structures, but also shows the isointensity to the normal bone marrow with a hypointense rim [14], which is helpful to differentiate it from calcified lesions. In addition, MRI can display abnormalities of other intra-articular structures, such as ligamentous and meniscal tears.
The difference between ligament ossification and calcification can be elaborated on the basis of the pathological findings. Pathologically, ligament calcification, which resembles calcific tendinitis of the rotator cuff, appears as a simple deposition of calcium salts, and inflammatory cells occasionally infiltrate the ligament [8,9,10]. However, the pathological material of our case consists of a mature lamellar bone with a central bone marrow resembling that acquired from HO [16].
HO has been increasingly recognized as a complication following major orthopedic surgeries, spinal cord injury, traumatic brain injury, blast injury, elbow and acetabular fractures, and thermal injury [2, 3]. Chantraine proposed that repeated microtrauma at the site of the joints and vascular disorders such as venous stasis and arteriovenous shunting, which cause metabolic changes that lead to cell differentiation, might play a major role in HO metaplasia [1]. Our case had no obvious history of trauma and relevant family history. We speculated that her medical history of artery thrombosis or repeated minor injuries that went unnoticed contributed to her HO. Nevertheless, the related factors inducing the disease cannot be verified.
Although current research on ligament ossification seeks to better understand the underlying cellular, biochemical, and mechanical processes, its pathogenesis remains unclear. One predominant theory has emerged to explain its occurrence [16,17,18]. Chalmers proposed a pathogenesis on the basis of three essential elements: an inducing agent, progenitor cells, and an environment conducive to bone production [17]. This concept is currently widely accepted. On the basis of Chalmers’ research, Kaplan suggested that progenitor cells are ubiquitous to various tissues, including ligament, muscle, and vessels. As a result of injury or other causes, the local inflammatory environment may result in the differentiation of these cells into osteoprogenitor cells capable of forming HO and is affected by oxygen tension, pH, availability of micronutrients, and mechanical stimuli [18]. To characterize the properties of cells derived from tissue containing pre- and mature ectopic bone, Benjamin studied several mouse models of HO, including two models of trauma-induced HO [19]. They noted that cells isolated from trauma sites in two distinct models exhibited increased proliferation and osteogenic differentiation compared with cells isolated from uninjured controls [19]. That is, trauma as an inducer enhances the proliferation and osteogenic differentiation properties of cells at the injured site. Although our case had no history of injury, the metabolic changes resulting from vascular disorders or unnoticed minor injuries, which led to local necrosis or degeneration, probably created an inflammatory environment that induced the occurrence of HO.
In previous studies, conservative treatments such as oral nonsteroidal anti-inflammatory drugs, diphosphonates, and low-dose local radiotherapy have been used to treat HO [18, 20, 21]. However, we could not find any literature report about ossification within the PCL. Several studies have reported the use of arthroscopic debridement operation in the treatment of calcification lesions within the cruciate ligament [8,9,10]. Likewise, the patient in the present case was treated with arthroscopic debridement of the lesion within the PCL. Although the ossified tissue could not be completely removed due to the close adhesion between HO and ligament fibers, we removed the lesion as much as possible to maintain the integrity of the PCL intraoperatively. The patient’s pain and restricted ROM subsided immediately after the surgery.
Arthroscopic debridement of the lesion can directly eliminate the symptoms of entrapment and mechanical locking, which cannot be achieved by conservative treatments. Our findings suggest that the arthroscopic removal of the lesion is an effective and necessary procedure for treating ossification of the PCL, especially when the symptoms are considered to be primarily caused by entrapment and mechanical locking.
In conclusion, HO in the PCL is rare. This is the first report of HO in the PCL as a possible cause of knee pain and restricted knee motion. HO is often easily misdiagnosed as calcification of the ligament. CT scan and MRI are important in differentiating between ossification and calcification; however, histopathological examination can confirm the bone nature of the lesion, which would make a definitive diagnosis. Arthroscopic debridement of the lesion is an effective and necessary procedure for treating ossification of the PCL.
Availability of data and materials
All the data needed to achieve the conclusion are presented in the pater.
Abbreviations
- HO:
-
Heterotopic ossification
- PCL:
-
Posterior cruciate ligament
- CT:
-
Computerized tomography
- MRI:
-
Magnetic resonance imaging
- ROM:
-
Range of motion
- ACL:
-
Anterior cruciate ligament
References
Chantraine A, Minaire P. Para-osteo-arthropathies: a new theory and mode of treatment. Scand J Rehabil Med. 1981;13(1):31–7.
Stannard JP, Wilson TC, Sheils TM, Mcgwin JG, Volgas DA, Alonso JE. Heterotopic ossification associated with knee dislocation. Arthroscopy. 2002;18(8):835–9. https://doi.org/10.1053/jars.2002.32842.
Nauth A, Giles E, Potter BK, Nesti LJ. Heterotopic ossification in orthopaedic trauma. J Orthop Trauma. 2012;26(12):684–8. https://doi.org/10.1097/BOT.0b013e3182724624.
Ranganathan K, Loder S, Agarwal S. Heterotopic ossification: basic-science principles and clinical correlates. J Bone Joint Surg Am. 2015;97(13):1101–11. https://doi.org/10.2106/JBJS.N.01056.
Devgan A, Mukhopadhyay R, Singh A, Gogna P, Singla R, Magu NK. Ossicle in anterior cruciate ligament: a rare occurrence. Case Rep Orthop. 2014;2014:1–3. https://doi.org/10.1155/2014/616715.
Sarsilmaz A, Gelal F. A new variation: an anterior cruciate ligament attached to the accessory ossicle. Clin Anat. 2011;24(8):991–3. https://doi.org/10.1002/ca.21113.
Jung YB, Jung LHJ. Heterotopic bone formation after posterior cruciate ligament reconstruction using inlay method and posterolateral corner sling with tibia tunnel: report of one case. Knee Surg Sports Traumatol Arthrosc. 2007;15(6):729.
Tsujii A, Tanaka Y, Yonetani Y, Iuchi R, Shiozaki Y, Horibe S. Symptomatic calcification of the anterior cruciate ligament: a case report. Knee. 2012;19(3):223–5. https://doi.org/10.1016/j.knee.2011.05.008.
Nikolaos EK, Stergios GP. Isolated posterior cruciate ligament calcification. BMJ Case Rep. 2011;2011:bcr1020114916.
Pellegrini A. Traumatic calcification of the collateral tibial ligament of the knee joint. Clin Med. 1905;11:433–9.
Muschol M, Müller I, Petersen W, Hassenpflug J. Symptomatic calcification of the medial collateral ligament of the knee joint: a report about five cases. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):598–602. https://doi.org/10.1007/s00167-004-0598-1.
Hayashi H, Fischer H. Incidental anterior cruciate ligament calcification: case report. J Radiol Case Rep. 2016;10(3):20.
Forsberg JA, Pepek JM, Wagner S, Wilson K, Flint J, Andersen RC, et al. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009;91(5):1084–91. https://doi.org/10.2106/JBJS.H.00792.
Yu JS, Resnick D. Meniscal ossicle: MR imaging appearance in three patients. Skelet Radiol. 1994;23(8):637–9. https://doi.org/10.1007/BF02580385.
Yao J, Yao L. Magnetic resonance imaging of a symptomatic meniscal ossicle. Clin Orthop Relat Res. 1993;64(293):225–8.
Kaplan FS, Glaser DL, Hebela N, Shore EM. Heterotopic ossification. Acta Orthop Scand. 1998;69(2):103–6.
Chalmers J, Gray DH, Rush J. Observations on the induction of bone in soft tissues. J Bone Joint Surg Br. 1975;57(1):36.
Schuetz P, Mueller B, Christ CM, Dick W, Haas H. Amino-bisphosphonates in heterotopic ossification: first experience in five consecutive cases. Spinal Cord. 2005;43(10):604–10. https://doi.org/10.1038/sj.sc.3101761.
Agarwal S, Drake J, Qureshi AT, Benjamin L. Characterization of Cells Isolated from Genetic and Trauma-Induced Heterotopic Ossification. Plos One. 2016;11(8):e0156253.
Chao ST, Lee SY, Borden LS. External beam radiation helps prevent heterotopic bone formation in patients with a history of heterotopic ossification. J Arthroplasty. 2006;21:731–6.
Winkler S, Wagner F, Weber M. Current therapeutic strategies of heterotopic ossification – a survey amongst orthopaedic and trauma departments in Germany. BMC Musculoskelet Disords. 2015;16(1):313. https://doi.org/10.1186/s12891-015-0764-2.
Acknowledgements
Not applicable.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
All listed authors have made substantial contribution to the following aspects of the manuscript: CL, ZGH, HMJ participated in diagnosing and treating the patient, acquisition of data. CL wrote the case report including performing the literature review. CDL provided guidance for the literature search, the writing of the paper and guaranteed the integrity of this work. Anil KC and QHW revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors declare that all investigations were conducted in conformity with ethical standards.
Consent for publication
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, C., Huang, Z., Anil, K.C. et al. Heterotopic ossification in the post cruciate ligament of the knee: a case report and literature review. BMC Musculoskelet Disord 22, 304 (2021). https://doi.org/10.1186/s12891-021-04176-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-021-04176-x