Abstract
Background
This study assessed the diagnosis, staging and treatment guidance of lung cancer (LC) based on seven tumor-associated autoantibodies (TAAbs) —p53, PGP9.5, SOX2, GBU4-5, MAGE A1, CAGE, and GAGE7.
Methods
ELISA was used to determine the TAAb serum levels in 433 patients diagnosed with LC (161 surgical patients) and 76 patients with benign lung disease (16 surgical patients). The statistical characteristic of the TAAbs was compared among patients with different clinicopathological features. Pre- to postoperative changes in TAAb levels were analyzed to determine their value of LC.
Results
Among all patients, the positive rate of the seven TAAbs was 23.4%, sensitivity was 26.3%, accuracy was 36.3%, specificity was 93.4%, positive predictive value was 95.8%, and negative predictive value was 18.2%; the positive rate for the LC group (26.3%) was significantly higher than that for the benign group (6.6%; P < 0.001). Significant differences in the positive rate of the seven autoantibodies according to age (P < 0.001), smoking history (P = 0.009) and clinical LC stage (P < 0.001) were found. Smoking was positively associated with the positive of TAAbs (Τ = 0.118, P = 0.008). The positive rates of the seven TAAbs for squamous carcinoma (54.5%), other pathological types (44.4%) and poorly differentiated LC (57.1%) were significantly higher than those for the other types. The positive rate of GBU4-5 was highest among all TAAbs, and the SOX2 level in stage III-IV patients was much higher than that in other stages. For patients undergoing surgery, compared with the preoperative levels, the postoperative levels of the 7 markers, particularly p53 (P = 0.027), PGP9.5 (P = 0.007), GAGE7 (P = 0.014), and GBU4-5 (P = 0.002), were significantly different in the malignant group, especially in stage I-II patients, while no clear pre- to postoperative difference was observed in the benign group.
Conclusions
When the seven TAAbs was positive, it was very helpful for the diagnosis of LC. The 7 TAAbs was valuable for staging and guiding treatment of LC in surgical patients.
Similar content being viewed by others
Background
Lung cancer (LC) is the most frequently occurring cancer and the leading cause of cancer death in men and the third most commonly diagnosed cancer and the leading cause of cancer death in women [1]. LC is classified broadly into non-small cell LC (NSCLC) (85% of total diagnoses) or small cell LC (SCLC) (15% of total diagnoses); among NSCLC classifications, adenocarcinomas are the most common subtype, followed by squamous-cell carcinomas [2]. The current mortality rate of LC remains relatively high, and the 5-year survival rate is unsatisfactory [3].
Early detection and treatment of lung cancer are a promising task to decrease lung mortality [4,5,6]. In contrast to computed tomography (CT)-guided lung biopsy (an invasive operation with numerous risks), low-dose CT scans (excessive false-positive results making subsequent medical procedures costlier, and repeated CT scanning raises the concern of an increased risk of developing radiation-related cancer) and molecular biology techniques (such as gene sequencing, which possess a low application rate due to their high costs) [4, 7], the assessment of blood tumor biomarkers has the potential for the early diagnosis of LC, as it has advantages including noninvasiveness and convenience of accessibility [8, 9].
Tumor-associated autoantibodies (TAAbs) are produced in the early stage of cancers by the humoral immune response, triggered by abnormal expression of tumor-associated antigens (TAAs). In comparison with other types of biomarkers, serum TAAbs appear earlier and are more stable [10]. They are promising biomarkers that could be applied for the early diagnosis of cancers [11].
A previous study found that a panel consisting of 4 autoantibodies (NOLC1, HMMR, MALAT1 and SMOX) was associated with early stage lung cancer in Chinese patients, and TAAb panels have shown better diagnostic performance than single TAAbs [12]. Given the heterogeneity of human lung cancers, researchers have tried to include more autoantibodies (AABs) to achieve higher sensitivity with a study that used 7 AABs (p53, c-myc, HER2, NY-ESO-1, CAGE, MUC1, and GBU4-5) in European patients with lung cancer (n D 104), a sensitivity of 76% and specificity of 92% were observed [13]; and an audit study of EarlyCDT(R)-Lung (6-AABs or 7-AABs) in 1600 patients also showed high specificity of 83% or 91% [14]. Since there are noticeable differences in the genetic makeup of European and Asian lung cancer patients, this panel of AABs may not be ideal for the Chinese population, and a similar study needs to be performed in Chinese patients to confirm these results, then 7 antigens (p53, PGP9.5, SOX2, GAGE7, GBU4-5, MAGE A1 and CAGE) were identified from 43 cancer-related antigens in a large clinical multicenter study and almost all of the AABs demonstrated good discriminative ability between lung cancer and healthy controls; researchers also compared the sensitivity values for traditional tumor markers, the 7-TAAbs panel showed a higher sensitivity in the early stages of lung cancer [4].
Specifically, p53 is a tumor suppressor gene involved in regulating the cell cycle [15, 16]. PGP9.5 is a ubiquitinase expressed in neural tissue and various malignant tumors, including LC cells [17, 18]. MAGE A1 belongs to the human melanoma antigen family and is a special tumor antigen that is thought to be involved in the occurrence of various tumors [19]. SOX2 is a transcription factor belonging to the SOX family that is involved in the proliferation and development of various cancers, demonstrating increased abundance [20]. CAGE is a cancer-associated gene that is expressed in a variety of cancers but not in normal tissues except the testis [21]. GBU4-5 is another protein described as inducing autoantibodies in LC [22]. Finally, GAGE7 is one of ten members of the GAGE family that have been identified; GAGE2-8 differ from each other mainly by single nucleotide substitutions resulting in amino acid substitutions. GAGE proteins share no homology with any protein of known function, and their functions remain unknown [23].
However, as clinical biomarkers, single functionality is clearly not enough, and more functions need to be explored. Hence, the aim of this study was to verify the diagnostic value of these seven TAAbs and explore their other value of LC.
Methods
Sample information
We collected data for 480 patients with pulmonary nodules and 29 patients with nonneoplastic disease from The First Affiliated Hospital of Ningbo University from July 2022 to December 2022; 433 patients were diagnosed with LC (161 surgical patients), and 76 patients had benign lung disease (16 surgical patients). Among all patients, 227 males and 282 females were included, ranging in age from 23 to 91 years, with a median age of 60 years. A total of 433 patients were in the LC group, namely, 192 males and 241 females, aged 23–91 years old, with a median age of 60 years; according to histopathological staging, 344 patients had adenocarcinoma, 44 had squamous cell carcinoma, 11 had small-cell carcinoma, 9 had other types of LC, and 7 had poorly differentiated carcinoma. In terms of TNM staging, 277 patients had stage I cancer, 13 had stage II, 53 had stage III, and 29 had stage IV cancer. There were 76 patients with benign lung disease, namely, 35 males and 41 females, aged 23–89, with a median age of 58.5 years.
This study was reviewed and approved by the ethics committee of The First Affiliated Hospital of Ningbo University. Informed consent was obtained from all participants. The ethical approval number: 2024-089RS.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) pulmonary nodules diagnosed as LC or benign lung disease by pathology examination and (2) other benign diseases with no evidence of malignancy. The exclusion criteria were as follows: (1) unclear LC staging, (2) evidence of active malignancy other than LC within six months and (3) autoimmune disease.
Serum sample collection and processing
Serum from 5 mL of fasting blood was separated by centrifugation at 3500 r/min (2410 g) for 5 min, completed within 8 h if the specimen could not be detected in time, and stored at 2–8 °C.
Reagents and equipment
An ELISA was used in the test according to the 7-TAAbs assay kit (Hangzhou Cancer probe Biotech Company). The OD value of each sample was measured with a microplate reader (ST360, Shanghai Kehua Biotechnology Co., Ltd.).
Enzyme-linked immunosorbent assay (ELISA)
The ELISA kit was used according to the manufacturer’s instructions. The positive reference values of the seven TAAbs were as follows: p53 ≥ 13.1 U/ml, PGP9.5 ≥ 11.1 U/ml, SOX2 ≥ 10.3 U/ml, GAGE7 ≥ 14.4 U/ml, GBU4-5 ≥ 7.0 U/ml, MAGE A1 ≥ 11.9 U/ml, and CAGE ≥ 7.2 U/ml. If one of the seven autoantibodies was positive, the patient was said to be positive for the 7 TAAbs; if all seven autoantibodies were negative, the patient was considered negative. The optimal cutoff values for the 7 AABs were defined as an optical density (OD) value greater than either the mean plus 2 standard deviations (SDs) or the mean plus 3 SDs of the normal cohort in the training set and the cutoff values were optimized using a Monte Carlo direct search method to find a set of antigen-specific cutoffs yielding the maximum sensitivity for a fixed specificity of 90%; The more stringent cut-off point (3 SDs) was applied to the PGP9.5, SOX2, p53, GAGE7 and CAGE autoantibody assays, incorporating, on average, 99% of the distribution of the data. An OD value greater than the mean plus 2 SDs of the normal population was applied to the GBU4-5 and MAGEA1 autoantibody assays [4].
Statistical analysis
SPSS version 26.0 software was used for data analysis. Chi-square analysis was used to compare the positive rate of the 7 TAAbs between groups. Kendall correlation analysis was used to find the correlation between smoking and the seven TAAbs. The paired sample T test was used to compare the changes in the levels of TAAbs preoperatively and postoperatively. A P value < 0.05 was considered to indicate statistical significance. GraphPad Prism 10.0.2 software was used for image processing.
Results
Positive rate of the seven autoantibodies for different characteristics and different groups
In terms of age, there was an obvious difference in the positive rate (χ2 = 19.463, P < 0.001) of the seven autoantibodies: 31.3% in the older group (≥ 60 years) and 14.8% in the younger group (< 60 years). There was no significant difference in the positive rate (χ2 = 0.381, P = 0.599) of the seven TAAbs between the sexes (24.7% male, 22.3% female). The positive rate of the 7 TAAbs among smokers (32.7%) was significantly higher than that in nonsmokers (20.7%) (χ2 = 7.11, P = 0.009), and smoking was positively associated with the positive of TAAbs (Τ = 0.118, P = 0.008). Regarding clinical stage, the positive rate of the seven TAAbs for stage III-IV cancer (54.7% in stage III, 51.7% in stage IV) was significantly higher than that for stages I (18.8%) and II (23.1%; χ2 = 39.599, P < 0.001). Regarding the different types of LC, the positive rates of the seven TAAbs for squamous carcinoma (54.5%), other pathological types (44.4%) and poorly differentiated LC (57.1%) were significantly higher than those for other types (Table 1).
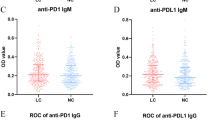
Among all patients, the positive rate of the seven autoantibodies was 23.4%, sensitivity was 26.3%, accuracy was 36.3%, specificity was 93.4%, positive predictive value was 95.8%, and negative predictive value was 18.2%.
In the LC group, the positive rate of the seven autoantibodies (26.3%) was significantly higher than that in the benign group (6.6%; χ2 = 14.077, P < 0.001) (Table 1). Notably, in the benign group, 60% of all positive patients had a history of chronic obstructive pulmonary disease (COPD).
Diagnostic efficacy of the different markers for different types and clinical stages of lung cancer
For precancer, adenocarcinoma and other pathological types, the positive rate of GBU4-5 was highest among all markers (11.1% for precancer, 8.1% for adenocarcinoma, 22.2% for other pathological types). SOX2, GBU4-5 and MAGE A1 accounted for the highest proportion (18.2%) of squamous carcinomas. For SCLC and poorly differentiated cancer, SOX2 was most commonly observed (18.2% and 28.6%, respectively). In stage I-IV cancer, the positive rates of GBU4-5 (8.3%), MAGE A1 (15.4%), GBU4-5 and SOX2 (both 18.9%), and SOX2 (27.6%) were the highest, respectively (Table 2).
Role of the levels of the seven TAAbs in surgical patients
When comparing preoperative and postoperative values in patients undergoing surgery, there was no clear difference in any of the seven markers in the benign group (P > 0.05). In contrast, a significant difference in the levels of the markers was found in the malignant group, including p53 (P = 0.027), PGP9.5 (P = 0.007), GAGE7 (P = 0.014), and GBU4-5 (P = 0.002). In a subgroup analysis of the malignant group, we divided the patients by clinical stage and whether they were positive for the 7 TAAbs. For precancerous and preinvasive lesions, there was no clear difference in any of the seven markers (P > 0.05). For stage I-II cancer, obvious differences in 3 markers, namely, PGP9.5 (P = 0.034), GAGE7 (P = 0.03), and GBU4-5 (P = 0.011), were found. For stage III-IV cancer, a significant difference in GBU4-5 (P = 0.049) was found. Among patients positive for the 7 TAAbs, a clear pre- to postoperative difference in PGP9.5 (P = 0.014) and GBU4-5 (P = 0.019) was found. For the negative group, obvious differences in SOX2 (P = 0.007), GAGE7 (P = 0.046), GBU4-5 (P = 0.003) and MAGE A1 (P = 0.04) were found (Table 3) (Fig. 1).
Comparison of the preoperative and postoperative levels of each marker
A: Pre- and postoperative levels of each marker in benign patients. B: Pre- and postoperative levels of each marker in malignant patients. C: Pre- and postoperative levels of each marker in patients with precancerous and preinvasive lesions. D: Pre- and postoperative levels of each marker in stage I-II lung cancer (LC) patients. E: Pre- and postoperative levels of each marker in stage III-IV LC patients. F: Pre- and postoperative levels of each marker in LC patients positive for the 7 TAAbs. G: Pre- and postoperative levels of each marker in LC patients negative for the 7 TAAbs
Discussion
LC is the most common tumor worldwide; yet more methods need to be developed to diagnose the disease early, staging and guiding treatment. Although early detection of the 7 TAAbs in hematological tests is necessary, it is insufficient for improving the survival rate of patients. In recent years, studies on the efficacy of TAAbs have shown varied but analogous results with increasingly abundant clinical evidence.
In a systematic review [24], studies of various TAAbs in different countries and ethnic groups were recorded. Twelve articles reported on autoantibodies against p53 and found sensitivities ranging from 12.6 to 40.3% and specificities ranging from 94.9 to 100%. TAAb panels supplied relatively high sensitivities, and some panels even yielded promising specificities (both > 90%) [25, 26]. A study by Boyle et al. reported a sensitivity of 37.0% for the antigens in the panel of six TAAbs they used, including p53, CAGE, GBU4-5, and SOX2 [27].
In a trial of more than 15,000 people [28], researchers found that the 7-TAAb panel demonstrated its potential as a powerful diagnostic tool for LC detection in a real-world cohort, particularly when combined with LDCT, and it showed a greater sensitivity for detecting ground-glass nodules. They highlight the clinical utility of the 7-TAAb panel in facilitating early detection of LC and that it may have significant implications for improving patient outcomes in this population.
In our study, the positive rate of the 7 TAAbs in elderly patients was obviously higher than that in younger patients, so for the early diagnosis of LC, patients older than 60 seem to be more suitable for the application of a 7-TAAb panel. Therefore, it is more suitable for LC screening in elderly individuals.
The positive rate of the seven TAAbs in patients with a smoking history was much higher than that in nonsmokers and smoking was positively associated with the positive of TAAbs, which may be closely related to smoking as a risk factor for LC. Thus, the 7-TAAb panel may be more suitable for LC screening in smokers.
Regarding the early diagnosis of LC by the seven TAAbs, the positive rate in the malignant group was significantly higher than that in the benign group, consistent with the findings of many studies [29,30,31,32,33], which showed that higher positive rates were observed in the later stage of LC. Combined with promising specificity and positive predictive value, the above results indicate that when the seven TAAbs was positive for patients suspected of lung cancer, it may have good prompt effect in the diagnosis.
In a systematic review and meta-analysis of 11 studies, researchers found that both COPD and emphysema seemed to increase the risk of developing LC [34]. In the benign group, we found that 60% of all positive patients had a COPD diagnosis, consistent with the findings of another study [35]. It seems that such patients are at higher risk of developing LC, but clinical screening with larger samples is needed to evaluate this hypothesis.
Among all 7 TAAbs, the positive rate of GBU4-5 was much higher than that of the other TAAbs in early-stage cancer, suggesting that GBU4-5 is the earliest diagnostic marker to demonstrate substantial changes in level in LC. On the other hand, the positive rate of SOX2 was significantly higher than that of the other markers in patients with stage III-IV disease, similar to previously reported results [20]. SOX2 may thus be a marker of poor prognosis.
Among the different types of LC, especially adenocarcinoma and squamous cell carcinoma, which are the most common types, there are clear differences in the positive rates of the seven autoantibodies. It may indicate that squamous carcinoma cells had faster and stronger humoral immune response to the seven TAAbs than adenocarcinomas, especially in SOX2, GBU4-5 and MAGE A1. The seven autoantibodies have a higher diagnostic value for squamous cell carcinoma. Based on the above data, for patients with high probability of squamous cell carcinoma indicated by clinical history and imaging in the future, the seven TAAbs may be of good benefit to the diagnosis and the change of its value may be of great value for the development of squamous cell carcinoma, the guidance of subsequent treatment and the evaluation of efficacy.
Among surgical patients, our results demonstrated the benefit of comparing the pre- and postoperative levels of LC autoantibodies, regardless of whether the patients were positive or negative for the 7 TAAbs. The possible effect of surgical resection on TAAbs serum concentrations has already excluded when 93 patients’ serum samples collected before and after surgery and the results showed that the serum concentrations of each TAAbs did not change significantly after surgery [4]. This is the most innovative part of this study since, to date, no other study has investigated this topic. The pre- to postoperative change in the levels of the 7 TAAbs may have guiding significance in staging and postoperative treatment of LC. The reason may be that immediately after the complete surgical removal of the lesions in the early stage of LC, a significant decline in the levels of the 7 TAAbs can be detected. Among all markers, changes in the levels of p53, PGP9.5, GAGE7 and GBU4-5 are worthy of attention.
In this research, we used ELISA to detect the pre- and postoperative levels of 7 TAAbs in patients with different features, and we summarized important characteristics on the basis of data differences. The results of the study were used to screen for the specific value of the seven tumor-associated autoantibodies in distinguishing different features of LC and in determining the value of these autoantibodies as biomarkers, enriching the tools available to clinicians for diagnosing, staging and guiding the postoperative treatment of LC.
However, our study only preliminarily suggested the relationship between autoantibodies and lung cancer as well as identified valuable markers. More in-depth studies with larger sample sizes are needed to prove their universality.
Conclusions
When the seven TAAbs was positive, it was very helpful for the diagnosis of LC. The 7 TAAbs was valuable for staging and guiding treatment of LC in surgical patients.
Data availability
Data is provided within supplementary information files.
Abbreviations
- LC:
-
lung cancer
- TAAbs:
-
tumor-associated autoantibodies
- NSCLC:
-
non-small cell lung cancer
- SCLC:
-
small cell lung cancer
- CT:
-
computed tomography
- TAAs:
-
tumor-associated antigens
- AABs:
-
autoantibodies
- ELISA:
-
enzyme-linked immunosorbent assay
- OD:
-
optical density
- SDs:
-
standard deviations
- COPD:
-
chronic obstructive pulmonary disease
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021;398(10299):535–54.
Nasim F, Sabath BF, Eapen GA. Lung cancer. Med Clin North Am. 2019;103(3):463–73.
Ren S, Zhang S, Jiang T, He Y, Ma Z, Cai H, Xu X, Li Y, Cai W, Zhou J, Liu X, Hu X, Zhang J, Yu H, Zhou C, Hirsch FR. Early detection of lung cancer by using an autoantibody panel in Chinese population. Oncoimmunology. 2017;7(2):e1384108.
Sullivan FM, Farmer E, Mair FS, Treweek S, Kendrick D, Jackson C, Robertson C, Briggs A, McCowan C, Bedford L, Young B, Vedhara K, Gallant S, Littleford R, Robertson J, Sewell H, Dorward A, Sarvesvaran J, Schembri S. Detection in blood of autoantibodies to tumour antigens as a case-finding method in lung cancer using the early CDT®-lung test (ECLS): study protocol for a randomized controlled trial. BMC Cancer. 2017;17(1):187.
Huang H, Luo W, Ni Y, Sun S, Wang C, Zhang L. The diagnostic efficiency of seven autoantibodies in lung cancer. Eur J Cancer Prev. 2020;29(4):315–20.
Nooreldeen R, Bach H. Current and future development in lung cancer diagnosis. Int J Mol Sci. 2021;22(16):8661.
Djureinovic D, Dodig-Crnković T, Hellström C, Holgersson G, Bergqvist M, Mattsson JSM, Pontén F, Ståhle E, Schwenk JM, Micke P. Detection of autoantibodies against cancer-testis antigens in non-small cell lung cancer. Lung Cancer. 2018;125:157–63.
Mordente A, Meucci E, Martorana GE, Silvestrini A. Cancer biomarkers Discovery and Validation: state of the art, problems and future perspectives. Adv Exp Med Biol. 2015;867:9–26.
Yang G, Xiao Z, Tang C, Deng Y, Huang H, He Z. Recent advances in biosensor for detection of lung cancer biomarkers. Biosens Bioelectron. 2019;141:111416.
Wang T, Liu H, Pei L, Wang K, Song C, Wang P, Ye H, Zhang J, Ji Z, Ouyang S, Dai L. Screening of tumor-associated antigens based on Oncomine database and evaluation of diagnostic value of autoantibodies in lung cancer. Clin Immunol. 2020;210:108262.
Yao Y, Fan Y, Wu J, Wan H, Wang J, Lam S, Lam WL, Girard L, Gazdar AF, Wu Z, Zhou Q. Potential application of non-small cell lung cancer-associated autoantibodies to early cancer diagnosis. Biochem Biophys Res Commun. 2012;423(3):613–9.
Chapman CJ, Murray A, McElveen JE, Sahin U, Luxemburger U, Türeci O, Wiewrodt R, Barnes AC, Robertson JF. Autoantibodies in lung cancer: possibilities for early detection and subsequent cure. Thorax. 2008;63(3):228–33.
Jett JR, Peek LJ, Fredericks L, Jewell W, Pingleton WW, Robertson JF. Audit of the autoantibody test, EarlyCDT®-lung, in 1600 patients: an evaluation of its performance in routine clinical practice. Lung Cancer. 2014;83(1):51–5.
Berke TP, Slight SH, Hyder SM. Role of reactivating mutant p53 protein in suppressing growth and metastasis of Triple-negative breast Cancer. Onco Targets Ther. 2022;15:23–30.
Futamura M, Tokumaru Y, Takabe K, Arakawa H, Asano Y, Mori R, Mase J, Nakakami A, Yoshida K. MIEAP, a p53-downstream gene, is associated with suppression of breast cancer cell proliferation and better survival. Am J Cancer Res. 2021;11(12):6060–73.
Fang Y, Shen X. Ubiquitin carboxyl-terminal hydrolases: involvement in cancer progression and clinical implications. Cancer Metastasis Rev. 2017;36(4):669–82.
Mandelker DL, Yamashita K, Tokumaru Y, Mimori K, Howard DL, Tanaka Y, Carvalho AL, Jiang WW, Park HL, Kim MS, Osada M, Mori M, Sidransky D. PGP9.5 promoter methylation is an independent prognostic factor for esophageal squamous cell carcinoma. Cancer Res. 2005;65(11):4963–8.
Yi E, Chang JE, Leem C, Jeon CH, Jheon S. Association of MAGE A1-6 expression with lung cancer progression. J Cancer. 2017;8(8):1324–9.
Grimm D, Bauer J, Wise P, Krüger M, Simonsen U, Wehland M, Infanger M, Corydon TJ. The role of SOX family members in solid tumours and metastasis. Semin Cancer Biol. 2020;67(Pt 1):122–53.
Krause P, Türeci O, Micke P, Buhl R, Huber C, Sahin U. SeroGRID: an improved method for the rapid selection of antigens with disease related immunogenicity. J Immunol Methods. 2003;283(1–2):261–7.
Brichory FM, Misek DE, Yim AM, Krause MC, Giordano TJ, Beer DG, Hanash SM. An immune response manifested by the common occurrence of annexins I and II autoantibodies and high circulating levels of IL-6 in lung cancer. Proc Natl Acad Sci U S A. 2001;98(17):9824–9.
Cilensek ZM, Yehiely F, Kular RK, Deiss LP. A member of the GAGE family of tumor antigens is an anti-apoptotic gene that confers resistance to Fas/CD95/APO-1, Interferon-gamma, taxol and gamma-irradiation. Cancer Biol Ther. 2002 Jul-Aug;1(4):380–7.
Yang B, Li X, Ren T, Yin Y. Autoantibodies as diagnostic biomarkers for lung cancer: a systematic review. Cell Death Discov. 2019;5:126.
Wu L, Chang W, Zhao J, Yu Y, Tan X, Su T, Zhao L, Huang S, Liu S, Cao G. Development of autoantibody signatures as novel diagnostic biomarkers of non-small cell lung cancer. Clin Cancer Res. 2010;16(14):3760–8.
Doseeva V, Colpitts T, Gao G, Woodcock J, Knezevic V. Performance of a multiplexed dual analyte immunoassay for the early detection of non-small cell lung cancer. J Transl Med. 2015;13:55.
Gnjatic S, Nishikawa H, Jungbluth AA, Güre AO, Ritter G, Jäger E, Knuth A, Chen YT, Old LJ. NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30.
Liu Z, Zhang F, Jiang J, Zhao C, Zhu L, Liu C, Li N, Qiu L, Shen C, Sheng D, Zeng Q. Early detection of lung cancer in a real-world cohort via tumor-associated immune autoantibody and imaging combination. Front Oncol. 2023;13:1166894.
Dai L, Qu Y, Li J, Wang X, Wang K, Wang P, Jiang BH, Zhang J. Serological proteome analysis approach-based identification of ENO1 as a tumor-associated antigen and its autoantibody could enhance the sensitivity of CEA and CYFRA 21 – 1 in the detection of non-small cell lung cancer. Oncotarget. 2017;8(22):36664–73.
Tang ZM, Ling ZG, Wang CM, Wu YB, Kong JL. Serum tumor-associated autoantibodies as diagnostic biomarkers for lung cancer: a systematic review and meta-analysis. PLoS ONE. 2017;12(7):e0182117.
Healey GF, Lam S, Boyle P, Hamilton-Fairley G, Peek LJ, Robertson JF. Signal stratification of autoantibody levels in serum samples and its application to the early detection of lung cancer. J Thorac Dis. 2013;5(5):618–25.
Qin J, Zeng N, Yang T, Wan C, Chen L, Shen Y, Wen F. Diagnostic value of autoantibodies in lung cancer: a systematic review and meta-analysis. Cell Physiol Biochem. 2018;51(6):2631–46.
Zhao H, Zhang X, Han Z, Wang Z, Wang Y. Plasma anti-BIRC5 IgG may be a useful marker for evaluating the prognosis of nonsmall cell lung cancer. FEBS Open Bio. 2018;8(5):829–35.
Mouronte-Roibás C, Leiro-Fernández V, Fernández-Villar A, Botana-Rial M, Ramos-Hernández C, Ruano-Ravina A. COPD, emphysema and the onset of lung cancer. A systematic review. Cancer Lett. 2016;382(2):240–4.
Tarro G, Perna A, Esposito C. Early diagnosis of lung cancer by detection of tumor liberated protein. J Cell Physiol. 2005;203(1):1–5.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
HM conceived and designed the study. HM collected and analyzed the clinical data. HM was the major contributor to writing the manuscript. TTW and QPZ provided proofreading of the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the ethics committee of The First Affiliated Hospital of Ningbo University. Informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

















































Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, H., Wu, T., Zhang, Q. et al. The role of seven tumor-associated autoantibodies in the diagnosis, staging and treatment guidance of lung cancer. BMC Pulm Med 24, 250 (2024). https://doi.org/10.1186/s12890-024-03060-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-03060-3