Abstract
Background
Severe community-acquired pneumonia is one of the most lethal forms of CAP with high mortality. For rapid and accurate decisions, we developed a mortality prediction model specifically tailored for elderly SCAP patients.
Methods
The retrospective study included 2365 elderly patients. To construct and validate the nomogram, we randomly divided the patients into training and testing cohorts in a 70% versus 30% ratio. The primary outcome was in-hospital mortality. Univariate and multivariate logistic regression analyses were used in the training cohort to identify independent risk factors. The robustness of this model was assessed using the C index, ROC and AUC. DCA was employed to evaluate the predictive accuracy of the model.
Results
Six factors were used as independent risk factors for in-hospital mortality to construct the prediction model, including age, the use of vasopressor, chronic renal disease, neutrophil, platelet, and BUN. The C index was 0.743 (95% CI 0.719–0.768) in the training cohort and 0.731 (95% CI 0.694–0.768) in the testing cohort. The ROC curves and AUC for the training cohort and testing cohort (AUC = 0.742 vs. 0.728) indicated a robust discrimination. And the calibration plots showed a consistency between the prediction model probabilities and observed probabilities. Then, the DCA demonstrated great clinical practicality.
Conclusions
The nomogram incorporated six risk factors, including age, the use of vasopressor, chronic renal disease, neutrophil, platelet and BUN, which had great predictive accuracy and robustness, while also demonstrating clinical practicality at ICU admission.
Similar content being viewed by others
Introduction
Community-acquired pneumonia (CAP) is an acute infectious disease affecting the lung parenchyma and is acquired outside the hospital [1]. Although CAP is one of the leading causes of mortality in immunocompetent and immunocompromised patients, it is still easily neglected [2]. The Global Burden of Diseases, Injuries, and Risk Factors Study [3] revealed that in 2016, 336.5 million cases of lower respiratory tract infection were recorded, resulting in an incidence rate of 32.2 per 100,000 people worldwide. Advanced age, chronic lung disease, chronic heart disease, cardiovascular disease, diabetes mellitus, malnutrition, viral respiratory tract infections, immunocompromising conditions, and lifestyle factors such as smoking and excessive alcohol consumption were the factors that increased the risk of community-acquired pneumonia [4]. Severe community-acquired pneumonia (SCAP) is one of the most lethal forms of CAP with high mortality. Septic shock and respiratory failure are the most serious complications of SCAP, characterized by especially life-threatening. Intensive care unit (ICU) care is generally required [2, 5]. It has been reported that the one-year mortality of all CAP inpatients is approximately 30%, while it is around 50% in ICU CAP patients [4].
In elderly patients, impaired gag reflex, decreased mucociliary function, damaged immunity, impaired febrile response, and cardiopulmonary dysfunction contribute to an increased susceptibility to developing CAP [6]. Risk factors that predisposed the elderly to pneumonia included comorbid conditions, organ dysfunction, nutritional status, alcohol consumption and smoking [7]. The incidence of the disease was higher among elderly individuals, with a rate of 63.0/10,000 person-years in those aged 65–79 and increasing to 164.3/10,000 person-years after the age of 80 [8, 9]. SCAP occurs more frequently in those with comorbidities [10, 11]. All comorbidities were more frequent in the elderly group [12]. Compared to younger patients, elderly people might exhibit less prominent symptoms due to associated comorbidity or impaired immune systems. Consequently, pneumonia in elderly patients was characterized by increased mortality and morbidity compared to their younger counterparts [6].
There were rules used to determine the severity and prognosis prediction of CAP and to guide treatment. Both the pneumonia severity index (PSI) and CURB-65 were developed as prognostic models based on demographic characteristics and clinical data to predict 30-day mortality. Compared to the PSI, CURB-65 was rarely thought to be effective as clinical evidence in the site of care [2, 6]. Sepsis-3 is a new definition about the life-threatening organ dysfunction caused by a dysregulated host response to infection, in order to enable early recognition of critically ill patients and thereby improve their outcomes [13]. However, their predictability for elderly patients needs to be improved [14, 15].
As the population ages, the incidence is expected to rise. Therefore, it will become a severe problem for our society. To achieve a rapid and accurate decision for high-risk patients, our study aimed to develop a mortality prediction model specifically targeting elderly SCAP patients. This model could help clinicians rapidly recognize high-risk patients, so that they can receive adequate attention and treatment.
Methods
Study design and cohort
We performed a retrospective, observational, cohort study. The study was conducted in the medical ICU at West China Hospital in Chengdu, Sichuan Province from September, 2011 to September 2019 and was approved by the West China Hospital of Sichuan University Biomedical Research Ethics Committee (No. 2021 − 828).
The requirement for obtaining informed consent in this analysis was waived due to the retrospective noninterventional design. To construct and validate the nomogram, we randomly divided the patients in one database into two cohorts, the training and testing cohorts, in a 70% versus 30% ratio to ensure comparability between the two cohorts.
CAP was diagnosed if the onset occurred within 48 h after admission or before admission. SCAP was defined as meeting at least 1 major criterion: (1) septic shock with need for vasopressors; (2) respiratory failure requiring mechanical ventilation; or at least 3 minor criteria: (1) respiratory rate ≥ 30 breaths/min; (2) PaO2/FiO2 ratio ≤ 250; (3) multi-lobar infiltrates; (4) confusion/ disorientation; (5) blood urea nitrogen level ≥ 20 mg/dL; (6) white blood cell count < 100,000/µL; (7) core temperature < 36℃; and (8) hypertension requiring aggressive fluid resuscitation), according to the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) guidelines [16]. The elderly patients were at least 65 years old.
The exclusion criteria were as follows: (1) not elderly (< 65 years old), (2) severe immunosuppression: autoimmune diseases, human immunodeficiency virus infection, chemotherapy, or other immunosuppressive therapy, (3) residents of long-term care facilities and/or nursing homes, (4) repeated admission, (5) hospital acquired pneumonia, (6) discharged within 24 h of admission, and (7) incomplete data. All patients received standard care and therapy according to the CAP guidelines.
Study outcomes and measurements
Clinical data of patients were collected within 24 h after admission to the ICU from electronic medical records. The data encompassed demographic characteristics, comorbidities, vital signs, hematological indicators, biochemical parameters, inflammatory markers, coagulation indicators and other laboratory tests. In the case of repeated laboratory tests within the first 24 h of admission, we chose the initial values for analysis. Two trained respiratory clinicians reviewed the medical records with standardized data collection forms. Any controversy was resolved by team discussion. All patient data were anonymized. The follow-up ended at discharge. The primary outcome was in-hospital death, and the secondary outcomes were ICU mortality, 7-day, 14-day and 28-day mortality after the diagnosis of SCAP.
Statistical analysis
All statistical analyses and graphs were performed by SPSS for Windows (Version 25.0, Chicago, IL, USA) and R software (Version 4.1.1, https://www.R-project. org/). Continuous variables are described as medians [interquartile ranges (IQRs), 25–75%] or means ± standard deviations (SDs), while categorical variables are described as frequencies. ‘p ≤ 0.05’ was considered statistically significant. Demographic and clinical characteristics were compared between the survival group and dead group, as well as between the training group and testing group using Student’s t-test, Mann–Whitney U test, or chi-square test where appropriate. Univariate logistic regression analysis was initially conducted to find the potential variables related to in-hospital mortality within the training cohort (p < 0.05). The results are described as ORs with corresponding 95% confidence intervals (CIs). The variables above were enrolled in the next multivariate logistic regression analysis to identify the independent risk factors for hospital mortality, according to which the nomogram for the hospital mortality prediction model was constructed. Then, the robustness of this model was assessed using the concordance index (C index) and area under the receiver operating characteristic curve (ROC and AUC). Finally, calibration curve and decision curve analysis (DCA) were employed to evaluate the predictive accuracy of hospital mortality in elderly SCAP patients.
.
Results
Clinical characteristics of elderly SCAP patients
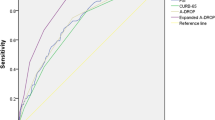
There were 3488 elderly SCAP patients enrolled in this study. People suffering from severe immunosuppression or hospital-acquired pneumonia, and people who were residents of long-term care facilities and/or nursing homes, had repeated admissions, were discharged within 24 h of admission, or had incomplete data were excluded (Fig. 1). Finally, a total of 2365 patients were enrolled in the subsequent analysis, which contained 1529 (64.7%) males and were 75.39 years old on average. Comorbidities including cancer, diabetes mellitus, chronic hepatic disease, chronic renal disease and chronic cardiac disease, were evaluated, as shown in Fig. 2A. The total in-hospital mortality was 36.7%. However, as shown in Fig. 2B, the Sepsis-3 (0.514; 95% CI 0.500, 0.528), as well as the two most widely used severity assessment tools in SCAP, PSI (0.550; 95% CI 0.526, 0.575) and CURB-65 (0.580; 95% CI 0.558, 0.602) scores, were poor predictors of in-hospital mortality in elderly SCAP patients in the present study.
The 2365 enrolled patients were divided into a training cohort (1655 patients) and a testing cohort (710 patients). Comparing the two cohorts, we found no significant differences in sex ratio (64.7% vs. 65.1% males, p = 0.780), mean age (75.30 vs. 75.31 years old, p = 0.971), prognosis, treatment, comorbidities, vital signs on admission, or most laboratory examinations (Table 1).
Construction of the nomogram
In the training cohort, 26 variables were found to be potentially significant difference in univariate logistic regression analysis. Next, these variables were reanalyse by multivariate logistic regression, and 6 factors were used as independent risk factors for in-hospital mortality to construct the prediction model, including age, the use of vasopressor, chronic renal disease, neutrophil, platelet, and blood urea nitrogen (BUN). Their ORs and 95% Cis were presented in Table 2.
The nomogram showed the prediction model for individual hospital morbidity illustrated by the 6 factors above in Fig. 3A. Each variable corresponded to a point on the top line. And the sum of these 6 points corresponded to the “total points” line vertically projected on the risk of in-hospital death on the bottom line.
Assessment of the nomogram
The C index was 0.743 (95% CI 0.719–0.768) in the training cohort and 0.731 (95% CI 0.694–0.768) in the testing cohort, which indicated that the prediction model had good predictive discrimination in elderly SCAP patients. The ROC curves and AUC for the training cohort and testing cohort (AUC = 0.742 vs. 0.728) were displayed in Fig. 3B and C, which indicated a robust discrimination of this prediction model. The calibration plots released a consistency between prediction model probabilities and observed probabilities in Fig. 4A and B. Then, the DCA demonstrated great net benefit across different threshold probabilities, as shown in Fig. 4C and D, possessing strong clinical practicality.
Discussion
CAP is a significant public health issue associated with substantial morbidity and mortality in all age groups globally [16, 17]. The incidence of CAP in the United States is a significant contributor to hospitalization and mortality, with an estimated annual report of approximately 6 million cases [4, 18]. A surveillance study (n = 2488 adults) reported that 21% of CAP patients had progressed to SCAP [19]. The mortality for SCAP patients ranged between 17% and 49% in some large multicenter cohort studies [20,21,22]. In elderly patients, CAP exhibits the absence of typical symptoms that are commonly observed in younger adults, owing to attenuated local and systemic inflammatory responses [23], making it difficult to notice in a timely manner. Moreover, it was reported that the incidence of SCAP increased significantly with age [19]. With an overall increase in the elderly population, the burden of caring for elderly SCAP patients would be further increased [23]. To determine the association between SCAP and in-hospital mortality in elderly patients and to make more accurate predictions, we analyzed data from a large, well-characterized retrospective study.
Our prediction model was employed to assess the mortality risk in elderly SCAP patients, showing excellent predictive discrimination ability and great robustness. Owing to the easily obtainable data in routine clinical practice, this model offered a reference about prognostic determination in elderly patients with SCAP, thereby assisting healthcare professionals. Song, Y., et al. [24] analyzed the data from 292 elderly patients with SCAP from 33 hospitals in China. They concluded that age (OR 1.138; 95% CI 1.037–1.253), Glasgow score (OR 0.908; 95% CI 0.838–0.985), blood platelet (OR 0.996; 95% CI 0.993–0.999), and BUN values (OR 1.061; 95% CI 1.023–1.102) were found to be significantly associated with 28-day mortality, which was similar to our results. However, it was regrettable that they did not perform internal validation or confirm their model. Pan, J., et al. [25] enrolled 455 patients with SCAP admitted to the ICU and discovered that lymphocytes, PaO2/FiO2, shock, and APACHE II score were independent risk factors for in-hospital mortality. They conducted an external validation for their prediction model, but the mean age in the development cohort and validation cohort was significantly different (p = 0.006). Wang, X., et al. [26] reported that serum creatinine, leukocyte, C reactive protein, GCS and serum HCO3− were carried out and that each index was an independent factor for hospital mortality in 37,348 SCAP patients in the ICU. They included a large population of SCAP patients and had a great predictability, but not aimed at elderly people, while more detailed elderly age group analysis was always ignored in most researches.
Age was thought to be an important factor that determined mortality. Previous studies showed that the mortality of CAP patients aged 65 or above was higher than that of those younger than 65 years old (10.3 versus 2.2%) [27]. In some studies that only enrolled elderly patients, the conclusion still held [24]. Age was also an independent risk factor in SCAP patients with heart disease [28] and type 2 diabetes [29]. Immunoreaction influences the prognosis. Qiu, Y., et al. [30] reported a higher neutrophil level in the death group in adult renal transplant recipients. Zhu, Y., et al. [31]found that the CD3+ CD4+ T cell count (OR 0.987; 95% CI 0.983–0.991) was an independent risk factor for mortality. Neutrophilia and lymphopenia were generally considered to be common immune responses during infection, which might be driven by emargination and delayed apoptosis of neutrophils as well as margination and accelerated apoptosis of lymphocytes [32]. Invasive mechanical ventilation was considered to be an important factor associated with high morbidity. Miquel Ferrer et al. [33] verified the hypothesis that invasive mechanical ventilation (OR 3.54; 95% CI 1.45–8.67) was an independent risk factor for death in SCAP patients. They did not limit the range of age, but the average age of the 644 enrolled patients was up to 65 years old. Impaired renal function also significantly affected the survival rate of elderly SCAP patients according to our model.
Fine and colleagues developed the PSI, a composite score consisting of 20 items that are aggregated to categorize patients into one of five risk groups [34]. Another widely employed prognostic score, the CURB-6, is commonly used due to its simplicity in assessment [35], which is widely used to evaluate the prognosis of patients with pneumonia [2]. However, the prognostic evaluation of PSI in the elderly population is not ideal [14, 15]. Naito, T., et al. [36] evaluated the PSI in patients aged 80 and older and found that the specificity was only 15% when defining PSI Class IV and V as a high-risk group. Baek, M.S., et al. [37] evaluated the PSI and CURB-65 in 160 patients aged 80 or older admitted to the medical ICU, but concluded that the performances of the CURB-65 and PSI were not excellent in very elderly patients. Chen, J.H., et al. [38] compared the PSI and CURB-65 categories across three age categories: younger adults (18–64 years), elderly adults (65–84 years) and very old adults (≥ 85 years). The AUCs for the PSI were 0.87, 0.85 and 0.69, respectively, and the AUCs for CURB-65 were 0.80, 0.73 and 0.60 in the three groups. The inappropriate weight given to the age variable was thought to be the reason for underperformance of the PSI in elderly patients. Sepsis-3 has been widely used in ICU patients, and also can be used in prognostic prediction in patients developing sepsis secondary to community-acquired pneumonia [13, 39]. Compared to the PSI, CURB-65 and Sepsis-3, our model focused specifically on elderly SCAP patients aged 65 years or older. The prognosis assessment is more predictive in elderly patients.
The advantage of our study was that we encompassed a large population in both the training and testing cohorts with complete information, thereby enhancing the robustness of our model. Disadvantages were also present. First, we excluded patients with severe immunosuppression, which indicated a significant burden of morbidity. This group of patients with an alternat immune system due to an underlying disease or medical treatment is at elevated risk of pneumonia by uncommon avirulent or opportunistic organisms [40], which may make the prediction model biased differently from the other group of patients. Therefore, we excluded patients with severe immunosuppression. Admittedly, this model is more applicable to non-immunodeficient populations. Then, we did not follow up long enough to record long-term mortality (> 3 months). However, further evaluation was needed to explore long-term mortality factors, incidence, prediction, and their implications for patient care in individuals with SCAP [13]. Several studies have reported that advanced age, sex, comorbidities, pneumonia type, and illness severity are associated with increased long-term mortality risk. Third, while internal verification was conducted, external validation was not achieved. The data collected from a single hospital limited the representativeness of the patient population. Fourth, despite a thorough analysis of potential risk factors, it is essential to know the possibility that some unadjusted confounders and untested variables may enhance the model.
The prognosis of elderly patients with SCAP is influenced by multiple factors. This model has a certain reference role in predicting mortality, and the indicators included in the model are easily available clinical examination results. We hope our model can offer a reference to clinicians in their daily work as a tool to convey prognostic information.
Conclusion
In conclusion, we constructed a prognosis prediction model for in-hospital mortality in elderly patients with SCAP. The nomogram incorporated six risk factors encompassing age, the use of vasopressor, chronic renal disease, neutrophil, platelet and BUN. It had great predictive accuracy and robustness, while also demonstrating clinical practicality at ICU admission. The assessment could facilitate the early identification of high-risk patients, thereby ensuring that high-risk individuals would receive adequate attention and timely interventions.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Community:
-
Acquired pneumonia (CAP)
- Severe community:
-
Acquired pneumonia (SCAP)
- ICU:
-
Intensive care unit
- PSI:
-
Pneumonia Severity Index
- IQR:
-
Interquartile range
- SD:
-
Standard deviation
- CIs:
-
Confidence intervals
- C index:
-
Concordance index
- ROC:
-
Receiver-operating characteristic
- AUC:
-
Area under curve
- DCA:
-
Decision curve analysis
- BUN:
-
Blood urea nitrogen
References
Nair GB, Niederman MS. Updates on community acquired pneumonia management in the ICU. Pharmacol Ther. 2021;217:107663.
Stefano Aliberti CSDC, Francesco Amati G, Sotgiu, Marcos I, Restrepo. Community-acquired pneumonia. Lancet Healthy Longev. 2021;2(9):e528.
Estimates of the. Global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210.
File TM, Ramirez JA. Community-Acquired Pneumonia. N Engl J Med. 2023;389(7):632–41.
Niederman MS, Torres A. Severe community-acquired pneumonia. Eur Respir Rev 2022, 31(166).
Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, Cooley LA, Dean NC, Fine MJ, Flanders SA, et al. Diagnosis and treatment of adults with community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67.
Fung HB, Monteagudo-Chu MO. Community-acquired pneumonia in the elderly. Am J Geriatr Pharmacother. 2010;8(1):47–62.
Lopardo GD, Fridman D, Raimondo E, Albornoz H, Lopardo A, Bagnulo H, Goleniuk D, Sanabria M, Stamboulian D. Incidence rate of community-acquired pneumonia in adults: a population-based prospective active surveillance study in three cities in South America. BMJ Open. 2018;8(4):e019439.
Ferreira-Coimbra J, Sarda C, Rello J. Burden of Community-Acquired Pneumonia and Unmet Clinical needs. Adv Ther. 2020;37(4):1302–18.
Elshamly M, Nour MO, Omar AMM. Clinical presentations and outcome of severe community-acquired pneumonia. Egypt J Chest Dis Tuberc. 2016;65(4):831–9.
Faverio P, Aliberti S, Bellelli G, Suigo G, Lonni S, Pesci A, Restrepo MI. The management of community-acquired pneumonia in the elderly. Eur J Intern Med. 2014;25(4):312–9.
Klapdor B, Ewig S, Pletz MW, Rohde G, Schutte H, Schaberg T, Welte T, Group CS. Community-acquired pneumonia in younger patients is an entity on its own. Eur Respir J. 2012;39(5):1156–61.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM et al. The Third International Consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA 2016, 315(8).
Frenzen FS, Kutschan U, Meiswinkel N, Schulte-Hubbert B, Ewig S, Kolditz M. Admission lactate predicts poor prognosis independently of the CRB/CURB-65 scores in community-acquired pneumonia. Clin Microbiol Infect. 2018;24(3):306. e301-306 e306.
Liu Y, Bai JS, Liu JX, Ren HQ, Fu AS, Ge YL. Nomogram Prediction Model of the severity of CAP patients based on blood indicators. Clin Lab 2023, 69(2).
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr., Musher DM, Niederman MS, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):27–72.
Wunderink RG. Guidelines to Manage Community-Acquired Pneumonia. Clin Chest Med. 2018;39(4):723–31.
Losier A, Dela Cruz CS. New testing guidelines for community-acquired pneumonia. Curr Opin Infect Dis. 2022;35(2):128–32.
Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, et al. Community-Acquired Pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–27.
Angus DC, Marrie TJ, Obrosky DS, Clermont G, Dremsizov TT, Coley C, Fine MJ, Singer DE, Kapoor WN. Severe community-acquired pneumonia: use of intensive care services and evaluation of American and British thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166(5):717–23.
Woodhead M, Welch CA, Harrison DA, Bellingan G, Ayres JG. Community-acquired pneumonia on the intensive care unit: secondary analysis of 17,869 cases in the ICNARC Case Mix Programme database. Crit Care. 2006;10(Suppl 2):1.
Walden AP, Clarke GM, McKechnie S, Hutton P, Gordon AC, Rello J, Chiche JD, Stueber F, Garrard CS, Hinds CJ. Patients with community acquired pneumonia admitted to European intensive care units: an epidemiological survey of the GenOSept cohort. Crit Care. 2014;18(2):R58.
Simonetti AF, Viasus D, Garcia-Vidal C, Carratala J. Management of community-acquired pneumonia in older adults. Ther Adv Infect Dis. 2014;2(1):3–16.
Song Y, Wang X, Lang K, Wei T, Luo J, Song Y, Yang D. Development and validation of a Nomogram for Predicting 28-Day mortality on Admission in Elderly patients with severe community-acquired pneumonia. J Inflamm Res. 2022;15:4149–58.
Pan J, Bu W, Guo T, Geng Z, Shao M. Development and validation of an in-hospital mortality risk prediction model for patients with severe community-acquired pneumonia in the intensive care unit. BMC Pulm Med. 2023;23(1):303.
Wang X, Jiao J, Wei R, Feng Y, Ma X, Li Y, Du Y. A new method to predict hospital mortality in severe community acquired pneumonia. Eur J Intern Med. 2017;40:56–63.
Kothe H, Bauer T, Marre R, Suttorp N, Welte T, Dalhoff K. Competence network for community-acquired pneumonia study g: outcome of community-acquired pneumonia: influence of age, residence status and antimicrobial treatment. Eur Respir J. 2008;32(1):139–46.
Gong L, He D, Huang D, Wu Z, Shi Y, Liang Z. Clinical profile analysis and nomogram for predicting in-hospital mortality among elderly severe community-acquired pneumonia patients with comorbid cardiovascular disease: a retrospective cohort study. BMC Pulm Med. 2022;22(1):312.
Ma CM, Wang N, Su QW, Yan Y, Yin FZ. Age, Pulse, Urea and Albumin (APUA) Model: A Tool for Predicting in-hospital mortality of Community-Acquired Pneumonia adapted for patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2020;13:3617–26.
Qiu Y, Su Y, Tu GW, Ju MJ, He HY, Gu ZY, Yang C, Luo Z. Neutrophil-to-lymphocyte ratio predicts mortality in adult renal transplant recipients with severe community-acquired pneumonia. Pathogens 2020, 9(11).
Zhu Y, Zheng X, Huang K, Tan C, Li Y, Zhu W, Liu N, Zhou Y, Chen H, Li P, et al. Mortality prediction using clinical and laboratory features in elderly patients with severe community-acquired pneumonia. Ann Palliat Med. 2021;10(10):10913–21.
Fantin B, Joly V, Elbim C, Golmard J-L, Pocidalo M-AG, Veni P, Carbon C. Lymphocyte subset counts during the Course of Community-Acquired Pneumonia: Evolution according to Age, Human Immunodeficiency Virus Status, and etiologic microorganisms. Clin Infect Dis. 1996;22(6):1096–8.
Ferrer M, Travierso C, Cilloniz C, Gabarrus A, Ranzani OT, Polverino E, Liapikou A, Blasi F, Torres A. Severe community-acquired pneumonia: characteristics and prognostic factors in ventilated and non-ventilated patients. PLoS ONE. 2018;13(1):e0191721.
M ICHAEL J. F INE MD, T HOMAS E. A UBLE PHD, D ONALD M. Y EALY MD, B ARBARA H. H ANUSA PHD, L ISA A. W EISSFELD PHD, D ANIEL E. S INGER MD, C HRISTOPHER M. C OLEY MD., T HOMAS J. M ARRIE MD, AND W ISHWA N. K APOOR MD, M.P.H.: A PREDICTION RULE TO IDENTIFY LOW-RISK PATIENTS WITH COMMUNITYACQUIRED PNEUMONIA. N Engl J Med 1997, 336.
Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, Lewis SA, Macfarlane JT. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–82.
Naito T, Suda T, Yasuda K, Yamada T, Todate A, Tsuchiya T, Sato J, Chida K, Nakamura H. A validation and potential modification of the pneumonia severity index in elderly patients with community-acquired pneumonia. J Am Geriatr Soc. 2006;54(8):1212–9.
Baek MS, Park S, Choi J-H, Kim C-H, Hyun IG. Mortality and prognostic prediction in very Elderly patients with severe pneumonia. J Intensive Care Med. 2020;35(12):1405–10.
Chen JH, Chang SS, Liu JJ, Chan RC, Wu JY, Wang WC, Lee SH, Lee CC. Comparison of clinical characteristics and performance of pneumonia severity score and CURB-65 among younger adults, elderly and very old subjects. Thorax. 2010;65(11):971–7.
Cillóniz C, Dominedò C, Ielpo A, Ferrer M, Gabarrús A, Battaglini D, Bermejo-Martin J, Meli A, García-Vidal C, Liapikou A et al. Risk and prognostic factors in very old patients with Sepsis secondary to Community-Acquired Pneumonia. J Clin Med 2019, 8(7).
Ramirez JA, Musher DM, Evans SE, Dela Cruz C, Crothers KA, Hage CA, Aliberti S, Anzueto A, Arancibia F, Arnold F, et al. Treatment of community-acquired pneumonia in immunocompromised adults: a Consensus Statement regarding initial strategies. Chest. 2020;158(5):1896–911.
Acknowledgements
None.
Funding
This work was supported by the Science and Technology Department of Sichuan Province (2022NSFSC1313, 2022NSFSC0693, 2023NSFSC1459, 2023NSFSC1464) and the Fundamental Research Funds for the Central Universities (2022SCU12055).
Author information
Authors and Affiliations
Contributions
CW, XYW, DXH and DH gave the study concept and design; all authors acquired, analyzed, and interpreted the data, and critically revised the manuscript for important intellectual content; CW,XYW and DH drafted the manuscript; LJG and DXH carried out the statistical analysis; ZAL and LJG supervised the study; All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was performed in accordance with the declaration of Helsinki and was approved by ethic committee of the West China Hospital of Sichuan University (No. 2021 − 828). Written informed consent was waived approved by ethic committee of the West China Hospital of Sichuan University due to the retrospective noninterventional design.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wei, C., Wang, X., He, D. et al. Clinical profile analysis and nomogram for predicting in-hospital mortality among elderly severe community-acquired pneumonia patients: a retrospective cohort study. BMC Pulm Med 24, 38 (2024). https://doi.org/10.1186/s12890-024-02852-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-02852-x