Abstract
Objective
The present study aimed to determine the effect and mechanical mechanism of spontaneous breathing during mechanical ventilation on oxygenation and lung injury using Beagles dogs mild or moderate acute respiratory distress syndrome (ARDS) model.
Methods
After inducing mild or moderate ARDS by infusion of oleic acid, Eighteen Beagles dogs were randomly split into Spontaneous breathing group (BIPAPSB, n = 6), and Complete muscle paralysis group (BIPAPPC, n = 6),Six Beagles without ventilator support comprised the control group. Both groups were ventilated for 8 h under BIPAP mode. High-pressure was titrated TV to 6 ml/kg. A multi-pair esophageal balloon electrode catheter was used to measure respiratory mechanics and electromyogram. End-expiratory lung volume (EELV), gas exchange and respiratory variables were recorded in the process of mechanical ventilation. The contents of Interleukin (IL)-6 and IL-8 in lung tissue were measure using qRT-PCR. Besides, lung injury score was calculated in the end of mechanical ventilation.
Results
Based on the comparable setting of ventilator, BIPAPSB group exhibited higher safety peak transpulmonary pressure, abdominal pressure, EELV and P/F(PaO2/FiO2) than BIPAPPC group, whereas mean transpulmonary pressure, the mRNA levels of the IL-6 and IL-8 in the lung tissues and lung injury score in BIPAPSB group were lower than those in BIPAPPC group.
Conclusion
In mild to moderate ARDS animal models, during mechanical ventilation, SB may improve respiratory function and reduce ventilator-induced lung injury. The mechanism may be that spontaneous inspiration up-regulates peak transpulmonary pressure and EELV; Spontaneous expiration decreases mean transpulmonary pressure by up-regulating intra-abdominal pressure, thereby reducing stress and strain.
Similar content being viewed by others
Introduction
Acute respiratory distress syndrome (ARDS) is common in critically ill patients admitted to intensive care units. Besides, the major supportive therapy for this syndrome is mechanical ventilation [1]. However, mechanical ventilation has side-effects, and it is likely to induce P-SILI (patient self-inflicted lung injury) and ventilator-induced lung injury (VILI) [2]. Despite the wide use of lung protective ventilation strategies [3], the overall intensive care units and hospital mortality of ARDS patients remain above 40% [4, 5].
Spontaneous breathing(SB) and Complete muscle paralysis(PC) are two auxiliary treatments for ARDS.Among them, SB is divided into assisted SB and unassisted SB according to the presence or absence of mechanical assistance. Completely controlled ventilation is primarily with the use of neuromuscular blocking agents [6]. During mechanical ventilation in patients with ARDS, however, the role of SB is contradictory [7]. Several studies reported that spontaneous breathing followed by a strong SB effort can induce lower pleural pressure, high transpulmonary pressure and rapid respiratory rate (RR), thereby up-regulating intrathoracic blood volume, worsening pulmonary edema, and then increasing lung damage, may induce patient self-inflicted lung injury(P-SILI)and ventilator-induced lung injury (VILI) [8]. However, numerous experimental and clinical studies also reported that SB with activity of the inspiratory muscles can induce greater pleural pressures and transpulmonary pressure, thus facilitating the homogenous distribution of ventilation, diminishing atelectasis [9, 10], and further reducing lung mechanical stress and strain [11, 12]. Recently, Marcelo et al. found that in animal with severe ARDS, SB could aggravate lung injury; in animal with mild or moderate ARDS, SB might be more protective for injured lung, whereas the precise mechanism is unclear [13]. Our previous studies have also confirmed that SB aggravates lung injury in severe ARDS animal models, and the mechanism may be related to abdominal muscle activity [8], however, the role and mechanism of spontaneous breathing in mild to moderate ARDS has not been fully elucidated. In mild to moderate ARDS, the role of abdominal muscle activity in mechanical ventilation is not clear. In this study, we explored the effect of spontaneous breathing on lung tissue and its mechanism in oleic acid-induced mild to moderate ARDS animal model, in order to provide theoretical basis for the treatment of mild to moderate ARDS.
Method and material
This study was approved by the ethics committee of Guizhou medical university. The care, and handling of the animals were in compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals standard.
Preparation of animal samples
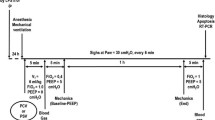
A total of 18 male beagle dogs were taken in this study. The weights ranged from 9.5 to 12.8 kg. The animals were pre-medicated with ketamine hydrochloride at a dose of 100 mg and fentanyl citrate at a dose of 3 μg/kg intravenously. General anesthetic was used by continuous infusion pentobarbital (5 to 6 mg/kg/h) or combination of propofol (75 to 150 mg/kg/h) in supine position [14], and was adjusted upward as tolerance developed. Paralysis was achieved with Pancuronium (bolus = 0.16 mg /kg, followed by 0.08 mg /kg/ h) [15]. Orotracheal intubation was performed using a cuff tube of 8.0mmID, and lungs were ventilated using EVITA4 ventilator ( Dräger Medical AG,Germany). A/C-V mode was first adopted. The tidal volume (VT) was set to 10 ml/kg. PEEP was 5 cmH2O, I:E ratio 1:1, and FiO2 1.0, RR (respiratory rate) was regulated, thus keeping PaCO2 between 35 and 45 mmHg. The femoral artery and the jugular vein on the right were catheterized to the PiCCO system (Pulsion Medical Systems, Munich, Germany) to measure the average arterial pressure and the core temperature. A catheter combined with multiple pairs of esophageal balloon electrodes (Guangzhou Yinghui Medical Technology Co. Ltd, China) was inserted into esophagus. The appropriate position was checked using airway occlusion technique [16]. Gastric pressure (Pgas)、airway pressure (Paw)、 esophageal pressure (Peso) 、electromyography of diaphragmatic esophagus(EMGdi)and Electromyography of abdominal muscle (EMGab) were recorded by PowerLab 16/30SP and Chart7.2 software (ADInstruments,Ltd,Australia). A respiratory flow head (MLT300L) was adopted to measure the airflow. Body temperature was kept constant at 37 ℃ throughout the experiment using an electric warming pad. Lactated Ringer’s intravenous fluid was injected at a rate of 6 ml/kg/h to keep the average arterial pressure as 70 mmHg. PiCCO calibrated after 8 h by transpulmonary thermodilution.
Experiment protocol
Respiratory mechanics data of beagle dogs were measured after 30 min., and then,a total of 0.2 ml/kg purified Oleic Acid (OA)was injected to induce lung injury, if needed, additional infusion oleic acid (0.1 ml each time) would be given. Until PaO2/FiO2 were consistently between 100 to 300 mmHg for 30 min, a stable model of mild or moderate ARDS was considered to be established successfully [17,18,19]. After lung injury, the ventilator mode was switched to the BIPAP mode. Beagles were split into (1) SB group (BIPAPSB group) and (2) Complete muscle paralysis group (BIPAPPC group). In the BIPAPPC group, the Phigh was regulated, thus keeping the VT around 6 ml/kg. Plow was pre-set to 10 cm H2O, FiO2 at 1.0, and I:E at 1:1. RR were regulated to keep the level of PaCO2 between 45 and 60 mmHg. In BIPAPSB group, the infusion of pancuronium bromide was ceased, and the dosage of pentobarbital and propofol decreased gradually to recover SB, and other ventilator settings were identical to those of BIPAPPC group. The control group was only induced by OA. After 8 h ventilation, all the animals were euthanized through venous infusion of potassium chloride. Lung tissue samples were collected from the upper lobes, the latera lobes, the dorsal and ventral parts of the lower lobe of the right lung, respectively, and then placed in 10% buffered formalin for the subsequent histological analysis. Tis experiment was carried out by observing the “Guidelines for the Care and Use of Laboratory Animals” (NIH Publication No. 85–23, 2011) published by the National Institutes of Health. All of the animal procedures are approved by the Animal Experimental Ethical Inspection Form of Guizhou Medical University (approve number: 1603175) and carried out in compliance with the ARRIVE guidelines.
Measurements of respiratory mechanics, EELV and VD/VT
All the variables were constantly recorded by PowerLab. For BIPAP mode, the following equation could be adopted to calculate the mean airway pressure (mean Paw) [20]: (Phigh × Thigh + Plow × Tlow) / (Thigh + Tlow), where Thigh denotes the length of time for Phigh, and Tlow that for Plow. If Thigh: Tlow was set to 1:1, the mean value of Paw could be remained constant even though the RR changes. By regulating the ventilator using the method mentioned above, a comparable mean Paw level could be kept in this study. The transpulmonary pressure (PL) was calculated by the equation: PL = Paw–Peso. The peak airway pressure (Ppeak) was recorded, and the total RR was calculated by the swings of Pes. End-expiratory volume (EELV) was ascertained using a simplified closed-circuit helium dilution method [21]. The alveolar dead space-to-tidal volume ratio (VD/VT) was calculated by: VD/VT = PaCO2- ETCO2/ PaCO2 [22].
Inflammatory mediators
The lower lobes on the left were lavaged with 40 ml sterilized normal saline and recycled after 5 s. Plasma was gathered before the induction of ARDS, during the injury as well as in the end of the experiment. These plasma samples and Bronchoalveolar lavage fluid (BALF) were centrifuged at a rate of 3,000–4,000 rpm at once for 15 min. The protein levels of IL-6 and IL-8 were measured with enzyme-linked immunosorbent assay kit specially made for dogs (Genequick, Guangzhou, China). The expression levels of the mRNA of IL-6 and IL-8 were ascertained through the quantitative real-time reverse-transcription polymerase chain reaction. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were adopted as the internal control for the normalization of RNA template. The senses and anti-senses of the taken primers (5’-3’) for IL-6 and -8 were presented as follows:
-
IL-6 F: TGACCACTCCTGACCCAACC, R: TCCAGACTCCGCAGGATGAG;
-
IL-8 F: ACTTCCAAGCTGGCTGTTGC, R: CTGGCATCGAAGTTCTGAACTG.
Histopathological examination
The animals were sacrificed by 100 mg/kg of intravenous 10% potassium chloride [23]. Biopsies were collected on the middle lobes, upper lobes, and lateral, ventral and dorsal parts of the right lower lobe, respectively. Subsequently, they were placed into 10% buffered formaldehyde and then stained with HE (hematoxylin–eosin). A pathologist was appointed to examine all the collected biopsies with the lung injury histopathology scoring system. The scoring system included: 1, none; 2, mild; 3, moderate; 4, severe. The following criteria for each level: alveolar and interstitial edema, granulocytes, lymphocytes and erythrocytes infiltrate, fibrinous exudates and micro thrombi. The total score was obtained by up-regulating all the sub-scores [24].
Statistical analysis
All the dates are expressed as the means ± SDs. The data of hemodynamics and respiratory mechanics between the two experimental groups were compared by unpaired t-test, and paired t-test was used to evaluate the difference before and after modeling in the same group. The differences of EELV, VD/VT, Pao2/FiO2 and inflammatory factors between groups were analyzed by one-way ANOVA and post-test by LSD-t or Dunn’s procedure. The changes of hemodynamics and respiratory mechanics parameters affected by intergroup and time were measured by double analysis of variance (ANOVA). Differences were considered statistically significant if P value was below 0.05. GraphPad Prism 8. 3 and SPSS21.0 software were used to drawing and perform statistical analyses. The power analysis was calculated by analyzing the final pathological injury score of lung tissue, inflammatory factors and oxygenation index of two groups of beagle dogs.GPower3.1 were used to calculate power analysis.
Results
There was no significant difference in the basic data (weight, length and age, or the dosage of OA injection) in the experimental groups. Hypoxia and acidosis appeared, and respiratory system static compliance decreased significantly after the infusion of OA.
Hemodynamics and gas exchanges
Hemodynamics
Table 1 suggests that there was no difference in the hemodynamic parameters at the beginning and the end of the experiment.
Respiratory mechanics
Figure 1 and Table 1 suggest that there was also a comparable mean Paw between the two experimental groups throughout the experiments. The pressure–time curve showed that spontaneous breathing appeared in BIPAPSB group, and spontaneous breathing mainly occurred in low pressure. In BIPAPPC group, there was no spontaneous breathing, which was a typical curve of pressure controlled ventilation. Compared with BIPAPPC, the EMGdi of BIPAPSB group was visible. Due to the diaphragm activity, BIPAPSB group exhibited higher peak PL, Pes, and Pgas and lower mean transpulmonary pressure compared with BIPAPPC. BIPAPPC group had neither diaphragmatic activity nor abdominal muscle activity. As a result, its Peso experienced a positive variation in the inspiratory phase. In addition, After 6 h of modeling, the respiratory rate in BIPAPPC was significantly higher than that in BIPAPSB, but there was no significant difference in tidal volume between the two groups.
Representative respiratory tracings of airway pressure (Paw), esophageal pressure (Pes), intragastric pressure (Pgas), transpumonary pressure (PL), Airflow, abdominal muscles surface electromyography (EMGab) and diaphragmatic esophageal surface electromyography (EMGdi) in BIPAP, BIPAP group in representative animals. BIPAPSB = biphasic positive airway pressure with spontaneous breathing, SB efforts were regained; BIPAPPC = biphasic positive airway pressure with muscles paralysis, Animals’ SB efforts were fully depressed. Therefore, BIPAP was equal to pressure-controlled ventilation
EELV
Figure 2A suggests that the EELV decreased after the induction of lung injury, and no difference was detected in these groups at the beginning of the ventilation. After the planned MV strategy was adopted, the experimental groups showed an overt difference in EELV after 8 h of ventilation (P < 0.001). The EELV of BIPAPSB group was higher than that of BIPAPPC group (P < 0.05).
A Time course of the end- expiratory lung volume (EELV). B Time course of the dead space volume to tidal volume (VD/VT) ratio. C Time course of the oxygenation index.BIPAPSB = Biphasic positive airway pressure with SB; BIPAPPC = Biphasic positive airway pressure with muscles paralysis; SB = Sspontaneous Breathing; *P < 0.05, vs. other groups
VD/VT
Figure 2B shows no significant difference in VD/VT before and after the induction of lung injury. After the planned MV strategy was adopted, the experimental groups showed a significantly difference in VD/VT after 8 h of ventilation (P < 0.05). BIPAPSB group (50.6 ± 6.7%) led to a lower VD/VT than BIPAPPC group after 2 h of ventilation, but a significant difference was also found after 6 h of ventilation (P < 0.05).
Gas exchanges
As shown in Fig. 2C, there was no significant difference in P/F before and after lung injury. The P/F of all the beagle dogs decreased between 100 to 200 mmHg after injection of OA. After the planned MV strategy was adopted, BIPAPSB group showed a higher P/F than that of the BIPAPPC group after 6 h of ventilation (P < 0.05).
Lung and systemic inflammatory mediators
As displayed in Fig. 3, the IL-6 and IL-8 levels in the plasma were comparable among groups after the induction of lung injury. Nevertheless, BIPAPSB group yielded lower IL-8 levels compared with BIPAPPC (P < 0.05); Moreover, BIPAPSB group exhibited lower mRNA expression levels of IL-6 and IL-8 compared with BIPAPPC (P < 0.05), whereas all were higher than control group.
The Levels of interleukin (IL)-6 and IL-8 in plasma after 8 h mechanical ventilation(*P < 0.05 vs. other groups).Control = Control group;BIPAPSB = Biphasic positive airway pressure with SB; BIPAPPC = Biphasic positive airway pressure with muscles paralysis SB = Spontaneous Breathing; NS = No Significantly Difference
Lung histopathology
Table 2 and Fig. 4 suggest that the overall cumulative histopathological lung injury score of BIPAPSB group was lower than that of BIPAPPC group, but all were higher than that of control group. BIPAPSB group presented less lung congestion, pulmonary edema, alveolar neutrophils infiltration and interstitial lymphocyte infiltration. In the meantime, BIPAPPC group took on more alveolar rupture, inflammatory cell infiltration, and alveolar congestion, as well as thicker alveolar wall and greater interstitial edema accompanied with the formation of hyaline membrane.
Representative appearances and photomicrographs of hematoxylineosin–stained lung sections (magnification × 200) from in Control (A), BIPAPSB (B), BIPAPPC (C) group in representative animals. BIPAPSB = Biphasic positive airway pressure with SB; BIPAPPC = Biphasic positive airway pressure with muscles paralysis. The BIPAP SB group had minimal alveolar congestion, and inflammatory cell infiltration. The BIPAPPC group showed mild thickening of the alveolar walls, alveolar congestion, and hemorrhage
Discussion
The mechanical ventilation is required to support gas exchange in the ARDS, whereas it may aggravate lung damage, a phenomenon known as ventilator-induced lung injury (VILI). Spontaneous breathing and complete muscle paralysis are two treatment methods of ARDS mechanical ventilation. but it is currently in dispute with the role of spontaneous breathing in ARDS ventilation. It is now widely considered that maintaining spontaneous breathing may have different physiological effects in mild, moderate and severe ARDS,In severe ARDS, SB could aggravate lung injury, whereas in animal with mild or moderate ARDS, SB might be more protective for injured lung,For patients with severe ARDS, excessive spontaneous breathing will lead to increased transpulmonary pressure, lung gas swing, pulmonary edema and man–machine asynchrony, which will lead to the aggravation of lung injury and increase the mortality of patients;For patients with mild to moderate ARDS, spontaneous breathing may improve alveolar ventilation and oxygenation in gravity-dependent areas by increasing diaphragm activity [7, 13]. However, few research has clarified the mechanical mechanism of SB on oxygenation and lung injury in mild or moderate ARDS. The present study is the first to demonstrate the mechanism of SB improved respiratory function and mitigated VILI in mild or moderate ARDS.
Based on an OA-induced ARDS model in beagles, we found SB improved respiratory function and mitigated lung injury, which is consistent with the previous animal and clinical experiments [11, 25,26,27]. In our study, we found that BIPAPSB group presented a higher EELV than BIPAPpc group, as well as a lower VD/VT than BIPAPpc group in the same mean airway pressure. Those two research results could explain mechanism why preserving SB improved respiratory function. Spontaneous inspiratory preserving diaphragm muscle contraction result in a higher and safety Peak PL, which would promote the dorsal-caudal distribution of ventilation, thereby increasing EELV. The factors contribute to more aeration in dependent lung regions, so as to improve gas exchange. Douglas et al. [28] have also proven that the increase in EELV was parallel to oxygenation. Furthermore, preserving SB presents lower VD/VT, which means a more appropriate ventilation-perfusion matching, as well as an improved respiratory function [8].
In this study, it was also found that the lung mRNA expressions of IL-6 and IL-8, VD/VT, and lung histopathological score were lower in BIPAPSB group than those in BIPAPPC group, which was consistent with other study results [29], whereas few studies have elucidated the mechanism. Several mechanical mechanisms observed in our study can explain these phenomena. Firstly, In SB group, inspiratory and expiratory muscle were all retained. As soon as spontaneous inspiratory induced the ventilation, inspiratory activity would be reflex suppressed due to mechanical inflation. Ventilator could activate the expiratory muscles, especially abdominal muscles, as can be seen from the esophagus pressures and intragastric, representing the intrathoracic pressures and intra-abdominal, respectively. Our study found that the mean transpulmonary pressure in SB group was significantly lower than that in BIPAPPC group, while intra-abdominal pressure was significantly higher than that in BIPAPPC group. This was because spontaneous expiration unopposed increased the intra-abdominal pressure and reduced the mean transpulmonary pressure. The major factors of mechanical damage were peak transpulmonary pressure and mean transpulmonary pressure. Accordingly, by increasing intra-abdominal pressure and decreasing the mean pulmonary pressure, spontaneous abdominal muscles activity would cause less VILI in mild or moderate ARDS. On the other hand,SB up-regulates peak transpulmonary pressure led to more aeration for dorsal lung tissue, recruits dependent lung tissues that were less aerated, and decreased the repeat opening and closing cycle of lung tissue, thereby mitigating VILI.Second,strain( tidal volume/end-expiratory volume) is one of the major determinants of VILI,In this experiment, compared with completely controlled ventilation, the average VT of spontaneous breathing group decreased, while EELV increased, so the strain value decreased relatively [30]. Third, compared with BIPAPPC, tidal volume in SB group was variable. Studies have shown that variable tidal volume has a protective effect on VILI [31]. Last,SB may help to alleviate Ventilator induced diaphragm dysfunction(VIDD). Our study shows that Complete muscle paralysis is more relaxed than diaphragm. Previous studies have confirmed that significant diaphragm fiber atrophy occurs at 18–69 h after controlled ventilation, which leads to VIDD;VIDD is related to weaning failure and prolonged hospitalization in patients with mechanical ventilation, while keeping spontaneous breathing during mechanical ventilation can prevent diaphragmatic atrophy and effectively avoid VIDD [32]. In addition, compared with keeping SB, PC has a higher demand for sedation, analgesia and NMBA, while the use of high-dose sedation, analgesia and NMBA is related to the difficulty of weaning [33]. Therefore, proper retention of SB may help to shorten the time of mechanical ventilation and promote early activity by reducing the dose of sedation, analgesia and NMBA.
There are several major limitations in this study. Firstly, we used BIPAP ventilated mode in this study. Therefore, we are not sure whether these results can be extended to other modes. Secondly, the ARDS model induced by oleic acid cannot be extrapolated to other ARDS models. Third, because long-term ventilation time may affect the accuracy of the experiment, such as hypercapnia, drug overuse, this study used 8h ventilation observation. In fact, longer study time may lead to significant physiological and morphological differences between the experimental groups. Finally,We used the pathological injury score of lung tissue and inflammatory factors in three groups to evaluate the statistical power. There were 6 beagle dogs in each group, and the statistical power was 0.97.However, when comparing the oxygenation index of the two groups alone, our power is low, and each group may need at least 9 beagles. The number of beagles is still one of the defects of our article.
Conclusions
To sum up, in mild to moderate ARDS animal models, SB improved respiratory function and reduced VILI. The mechanism may be that spontaneous inspiration up-regulates peak transpulmonary pressure and EELV, thus reducing strain, and spontaneous expiration decrease mean transpulmonary pressure by up-regulating intra-abdominal pressure, thereby reducing stress.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Hraiech S, Yoshida T, Annane D, et al. Myorelaxants in ARDS patients. Intensive Care Med. 2020;46(12):2357–72.
Marini JJ, Rocco P. Which component of mechanical power is most important in causing VILI. Crit Care. 2020;24(1):39.
Yoshida T, Roldan R, Beraldo MA, et al. Spontaneous Effort During Mechanical Ventilation: Maximal Injury With Less Positive End-Expiratory Pressure. Crit Care Med. 2016;44(8):e678–88.
Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021;398:622–37.
Zhang X, Du J, Wu W, Zhu Y, Jiang Y, Chen R. An experimental study on the impacts of inspiratory and expiratory muscles activities during mechanical ventilation in ARDS animal model. Sci Rep. 2017;7:42785.
Hurford WE. Neuromuscular Blockade Applicability in Early Acute Respiratory Distress Syndrome. Anesthesiology. 2020;132(6):1577–84.
Yoshida T, Fujino Y, Amato MB, et al. Fifty Years of Research in ARDS. Spontaneous Breathing during Mechanical Ventilation. Risks, Mechanisms, and Management. Am J Respir Crit Care Med. 2017;195(8):985–92.
Zhang X, Wu W, Zhu Y, Jiang Y, Du J, Chen R. Abdominal Muscle Activity during Mechanical Ventilation Increases Lung Injury in Severe Acute Respiratory Distress Syndrome. PLoS ONE. 2016;11(1):e0145694.
Wrigge H, Zinserling J, Neumann P, et al. Spontaneous breathing improves lung aeration in oleic acid-induced lung injury. Anesthesiology. 2003;99(2):376–84.
Forel JM, Voillet F, Pulina D, et al. Ventilator-associated pneumonia and ICU mortality in severe ARDS patients ventilated according to a lung-protective strategy. Crit Care. 2012;16(2):65.
Putensen C, Mutz NJ, Putensen-Himmer G, Zinserling J. Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1241–8.
Carvalho NC, Guldner A, Beda A, et al. Higher levels of spontaneous breathing reduce lung injury in experimental moderate acute respiratory distress syndrome. Crit Care Med. 2014;42(11):e702–15.
de Abreu MG, Guldner A, Pelosi P. Spontaneous breathing activity in acute lung injury and acute respiratory distress syndrome. Curr Opin Anaesthesiol. 2012;25(2):148–55.
Yu L, Wang Y, Zhou X, et al. Leptin injection into the left stellate ganglion augments ischemia-related ventricular arrhythmias via sympathetic nerve activation. Heart Rhythm. 2018;15(4):597–606.
Mitaka C, Hirata Y, Habuka K, et al. Atrial natriuretic peptide improves pulmonary gas exchange by reducing extravascular lung water in canine model with oleic acid-induced pulmonary edema. Crit Care Med. 2002;30(7):1570–5.
Miao MY, Chen W, Zhou YM, et al. Validation of the flow index to detect low inspiratory effort during pressure support ventilation. Ann Intensive Care. 2022;12(1):89.
Wei F, Wen S, Wu H, et al. Partial liquid ventilation-induced mild hypothermia improves the lung function and alleviates the inflammatory response during acute respiratory distress syndrome in canines. Biomed Pharmacother. 2019;118:109344.
Schweiger JW, Downs JB, Smith RA. Chest wall disruption with and without acute lung injury: effects of continuous positive airway pressure therapy on ventilation and perfusion relationships. Crit Care Med. 2003;31(9):2364–70.
Guizhi Du, Wang S, Li Z, et al. Sevoflurane Posttreatment Attenuates Lung Injury Induced by Oleic Acid in Dogs. Anesth Analg. 2017;124(5):1555–63.
Xia J, Sun B, He H, Zhang H, Wang C, Zhan Q. Effect of spontaneous breathing on ventilator-induced lung injury in mechanically ventilated healthy rabbits: a randomized, controlled, experimental study. Crit Care. 2011;15(5):R244.
Chiumello D, Chidini G, Calderini E, et al. Respiratory mechanics and lung stress/strain in children with acute respiratory distress syndrome. Ann Intensive Care. 2016;6(1):11.
Hardman JG, Aitkenhead AR. Estimating alveolar dead space from the arterial to end-tidal CO(2) gradient: a modeling analysis. Anesth Analg. 2003;97(6):1846–51.
Spieth PM, Knels L, Kasper M, et al. Effects of vaporized perfluorohexane and partial liquid ventilation on regional distribution of alveolar damage in experimental lung injury. Intensive Care Med. 2007;33(2):308–14.
Dembinski R, Hochhausen N, Terbeck S, et al. Pumpless extracorporeal lung assist for protective mechanical ventilation in experimental lung injury. Crit Care Med. 2007;35(10):2359–66.
de Abreu MG, Cuevas M, Spieth PM, et al. Regional lung aeration and ventilation during pressure support and biphasic positive airway pressure ventilation in experimental lung injury. Crit Care. 2010;14(2):R34.
Wrigge H, Zinserling J, Neumann P, et al. Spontaneous breathing with airway pressure release ventilation favors ventilation in dependent lung regions and counters cyclic alveolar collapse in oleic-acid-induced lung injury: a randomized controlled computed tomography trial. Crit Care. 2005;9(6):R780–9.
Yoshida T, Rinka H, Kaji A, et al. The impact of spontaneous ventilation on distribution of lung aeration in patients with acute respiratory distress syndrome: airway pressure release ventilation versus pressure support ventilation. Anesth Analg. 2009;109(6):1892–900.
Douglas WW, Rehder K, Beynen FM, Sessler AD, Marsh HM. Improved oxygenation in patients with acute respiratory failure: the prone position. Am Rev Respir Dis. 1977;115(4):559–66.
Xia J, Zhang H, Sun B, Yang R, He H, Zhan Q. Spontaneous Breathing with Biphasic Positive Airway Pressure Attenuates Lung Injury in Hydrochloric Acid-induced Acute Respiratory Distress Syndrome. Anesthesiology. 2014;120(6):1441–9.
Chiumello D, Carlesso E, Cadringher P, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med. 2008;178(4):346–55.
Perinel-Ragey S, Baboi L, Guérin C. Variability of Tidal Volume in Patient-Triggered Mechanical Ventilation in ARDS. Respir Care. 2017;62(11):1437–46.
Marin-Corral J, Dot I, Boguña M, et al. Structural differences in the diaphragm of patients following controlled vs assisted and spontaneous mechanical ventilation. Intensive Care Med. 2019;45(4):488–500.
Stephens RJ, Dettmer MR, Roberts BW, et al. Practice patterns and outcomes associated with early sedation depth in mechanically ventilated patients: a systematic review and metaanalysis. Crit Care Med. 2018;46(3):471–9.
Acknowledgements
The authors thank Department of Science and Technology of Guizhou Province、the Guizhou Health Committee、Guiyang Science and Technology Bureau and Guizhou Medical University,Thank you for the financial support of the above units to this project.
Funding
This work was funded by Technology Support Program of Science and Technology Department of Guizhou Province (NO: [2021–061]; Science and Technology Fund Project of Guizhou Health Committee, China(NO: gzwkj2021-090);the Cultivate project 2021 for National Natural Science Foundation of China, Guizhou Medical University. (20NSP038); Basic Research Project of Guizhou Provincial Department of Science and Technology (NO:Qian Ke HeJi Chu[2022]Yi Ban 450);Science and Technology Planning Project of Guiyang, China (NO:2018–1-27).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: ZX,YR, ZL. Performed the experiments: ZX, YR, ZL, CZ, HS,LS,SY. Analyzed the data: ZX, YR, ZL. Wrote the paper: ZX, YR, ZL. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study approved by the Institutional Animal Care and Use Committee of Guizhou Medical University (no. 1603175).
Consent for publication
Not applicable (animal study).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, R., Zhou, L., Chen, Z. et al. Effect and mechanical mechanism of spontaneous breathing on oxygenation and lung injury in mild or moderate animal ARDS. BMC Pulm Med 23, 428 (2023). https://doi.org/10.1186/s12890-023-02730-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02730-y