Abstract
Introduction
Dysphagia occurs in multiple respiratory pathophysiologies, increasing the risk of pulmonary complications secondary to aspiration. Reflux associated aspiration and a dysregulated lung microbiome is implicated in Idiopathic Pulmonary Fibrosis (IPF), but swallowing dysfunction has not been described. We aimed to explore oropharyngeal swallowing in IPF patients, without known swallowing dysfunction.
Methods
Fourteen consecutive outpatients with a secure diagnosis of IPF were recruited and the 10-item Eating Assessment Tool (Eat 10) used to assess patient perception of swallowing difficulty. Oropharyngeal swallowing was assessed in ten patients using Videofluoroscopy Swallow Studies (VFSS). The studies were rated using validated scales: Penetration-Aspiration Scale (PAS); standardised Modified Barium Swallow Impairment Profile (MBSImP).
Results
EAT-10 scores indicated frank swallowing difficulty in 4/14 patients. Videofluoroscopy Studies showed that 3/10 patients had airway penetration, and one aspirated liquid without a cough response. Median MBSImp for oral impairment was 5, range [3–7] and pharyngeal impairment 4, range [1–14] indicating, overall mild alteration to swallowing physiology.
Conclusion
We conclude that people with IPF can show a range of swallowing dysfunction, including aspiration into an unprotected airway. To our knowledge, this is the first report on swallowing physiology and safety in IPF. We believe a proportion of this group may be at risk of aspiration. Further work is indicated to fully explore swallowing in this vulnerable group.
Similar content being viewed by others
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic life-threatening lung disease, with poor prognosis, characterised anatomically by progressive scarring of the lung and symptomatically by exertional dyspnoea, with irreversible loss of pulmonary function [1].
While the aetiology is unknown, emerging data suggest an important association with aspiration [2] linked with gastric reflux. The term aspiration refers either to the direct inhalation of secretions or ingested materials from the oesophagus or from the oropharynx into the airways. Aspiration by either route can lead to the presence of damaging agents into the airways and the lungs, which is thought to drive the onset and/or progression of a broad range of pathological diseases that affect the airways and lungs [3]. To date, investigations of IPF and aspiration have been limited to gastro-oesophageal reflux disease in which gastric content refluxes up into the oesophagus, with the potential to pass into the airway via the pharynx (microaspiration) [4]. Concerns about the potential role of gastro oesophageal reflux-associated aspiration in IPF pathophysiology have been sufficient to prompt an influential multi-centre pilot of fundoplication [5]. A dysregulated lung microbiome has also been implicated in high profile studies [6].
The pharynx is a shared tube for breathing and swallowing. Because of this, normal swallowing is a complex process, coordinated with breathing to protect the airway. Patients with chronic respiratory diseases are at risk of oropharyngeal dysphagia and swallowing difficulties may go unreported [7, 8]. Early evidence suggests 20% of COPD patients have a swallowing impairment, and subclinical aspiration, identified on Videofluoroscopy Swallow Studies (VFSS) [9]. Prior study found 44% of COPD patients reported swallowing difficulties symptoms on a patient-reported outcome measure [7]. The parenchymal lung scarring, and hypoxaemia seen in IPF patients may act to disrupt respiratory-swallow coordination leading to dysfunction. Surprisingly we are unaware of studies investigating swallowing dysfunction in people with IPF. The purposes of this study were to explore the perception of swallowing difficulty and oropharyngeal swallowing physiology in people with IPF. This was carried out in outpatients without known or suspected swallowing problems.
Material and methods
Participants
Consecutive outpatients with a secure diagnosis of IPF according to European Respiratory Society/American Thoracic Society were recruited from the regional Interstitial Lung Disease (ILD) clinic at the Royal Victoria Infirmary, Newcastle upon Tyne between March 2014 and February 2015. Outpatients with IPF are reviewed on a regular basis at the ILD clinic at the Royal Victoria Infirmary. Recruits were drawn from within this clinic population. Written informed consent was obtained using a printed form prior to the study assessment. Inclusion criteria included competent adults (over the age of 18) with a secure diagnosis of IPF according to the American Thoracic Society (ATS), European Respiratory Society (ERS), Japanese Respiratory Society (JRS) and Latin American Thoracic Society (LATS) clinical practice guidelines [10]. Accurate diagnosis is also supported by discussions among the Newcastle Hospitals Multi-Disciplinary Team (MDT). Exclusion criteria: pregnancy; neurological disease; dementia; gastro-intestinal disease, excepting controlled reflux symptoms; head & neck pathology excepting tonsillectomy/adenoidectomy; previous thoracic surgery; stroke. The patients were screened and excluded if they had a neurological diagnosis that may be associated with dysphagia. The study was described in details, with full written consent taken at the outset, before study activities commenced. All methods were performed in accordance with the approval guidelines and regulations.
Swallowing questionnaire
Eating Assessment tool (EAT-10) is a quick, self-administered and widely used validated questionnaire, which can be used to assess dysphagia symptoms [11]. It has been used previously with patients with chronic respiratory disease such as COPD [7, 12]. It consists of 10 questions regarding swallowing difficulty. Each question is scored on a 5 point Likert scale from 0 (no problem) to 4 (severe problem). The total EAT-10 score is calculated by adding up the scores across the 10 statements (highest score = 40). A total score of 3 or more indicates swallowing difficulty [13].
Swallowing assessment
Swallowing was assessed by a Videofluoroscopy Swallow Study (VFSS). VFSS is a dynamic radiographic examination using fluoroscopy to capture and record real-time bolus flow throughout all stages of swallowing. A Speech and Language Therapist and a radiologist, performed the VFSS examinations. The examination typically includes testing different bolus volumes and constituents [14]. The participants were seated in an upright position. Test boluses were thin liquid (5 and 20 mL); 5 mL paste (custard) and solid (1/4 biscuit) mixed with radiopaque barium sulphate (E-Z-PAQUE), conducted in the lateral plane and one 10 mL liquid bolus with an anterior–posterior view. Liquids were administered first to avoid confounding the results due to remaining residue in the pharynx after ingesting solid consistencies. A penny was taped to the subject’s chin during the swallowing study. The circular shape of the penny minimizes the impact of head rotation and the known diameter of the coin allows for calibration of pixels per cm and thus calculation of areas and displacement on VF.
VFSSs analysis
Studies were rated using two validated scales:
-
1.
The standardised Modified Barium Swallow Impairment Profile (MBSImP), a tool used to evaluate swallowing efficiency that measures 17 physiological components of adult swallowing mechanism using ordinal scaling. Ratings of 0 to 2, 3, or 4 points per component, with each score representing a unique observation of either structural movement, bolus flow or both from the VFSS [14, 15]. It covers 3 functional domains of the swallow; oral (0–22), pharyngeal (0–29) and oesophageal (0–4). The Overall impression (OI) score is the worst and the most impaired score for all bolus amounts and consistencies. The Oral impairment score and Pharyngeal impairment score were calculated by summing up the OI scores [14]. Scores were interpreted according to a clinically validated classification system [16].
-
2.
The Penetration Aspiration Scale (PAS) is a tool used to evaluate swallowing safety, ranging in value from 1 to 8, recording the presence of laryngeal penetration/sub-glottic aspiration (1 = no airway invasion, 2–5 = penetration, 6–8 = aspiration) [17]. Penetration is defined as the passage of food or fluid into the airway just above the level of the vocal cords. Whereas, aspiration is defined as the passage of food or fluid below the level of the vocal cord [18].
Higher scores for both tools indicate poorer swallowing.
Results
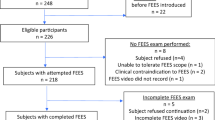
We recruited 14 participants with IPF: 10 males, 4 females, median: 68 years, range 51–82 years. Ten IPF patients: 7 males, 3 females, median: 63 years, range 51–77 years underwent the VFSS (Fig. 1: Consort flow diagram for study).
EAT-10: self-reported swallowing symptoms
A total of 14 participants with IPF completed the EAT-10 questionnaires. All data are presented in Fig. 2. The total median EAT-10 score was 0, range 0–25. Scores were raised in four patients, with values of 25, 15, 14 and 13. These exceeded the normal cut off < 3, indicating swallowing difficulty [13].
Swallowing physiology and safety
The clinical characteristics
The clinical characteristics for the IPF patients underwent VFSS are presented in Table 1. Overall, there were 10 IPF participants who completed the VFSS, the median age of participants was 63 years [range: 51–77]. Most participants were males (7/10, 70%) and ex-smokers (7/10, 70%). The median Body Mass Index (BMI) was 26.2 kg/m2 [range: 20.3–45.6]. The median modified Medical Research Council dyspnoea scale (mMRC) was 2 [range: 1–4]. The median FEV1/FVC% was 80.5% [range: 74–93] and the median FVC % of predicted was 72% [range: 51–92] indicating a restrictive lung pattern [19]. The median TLCO % of predicted was 60% [range: 27–78], showing impaired lung gas transfer.
The Videofluoroscopy Swallow Study (VFSS)
All data are presented in Table 2. Median MBSImp for oral impairment was 5 [range: 3–7] and pharyngeal impairment 4 [range 1–14] indicating, overall mild alteration to swallowing physiology. Patient 1 scored 14 in the pharyngeal impairment indicating mild/moderate impairment [16].
Swallow physiology: the MBS impairment profile
The MBSImP scores suggested none-to-mild swallowing impairment during oral and pharyngeal stages [16].
Lip closure, bolus preparation and transport, soft palate elevation were uniformly normal. However, all participants had evidence of oral residue, six had a reduction in tongue control and eight participants had a late initiation of pharyngeal swallow.
For the pharyngeal stage of swallowing five participants had evidence of incomplete laryngeal closure and one participant had a bilateral bulging of both pharyngeal walls. Eight participants had a reduction in tongue base retraction. Nine participants had evidence of post-swallow pharyngeal residue.
Swallow safety: the penetration/aspiration scale
On PAS, 3/10 patients (Patients 2, 4 and 8) had airway penetration. Only patient 1 aspirated liquid without a cough response; this patient had reduced laryngeal elevation and incomplete laryngeal vestibular closure, resulting in the residue laying below the true vocal chords without a response to eject the aspirated liquid (Fig. 3).
Discussion
To our knowledge, dysphagia has not been described in people with IPF. The purpose of this study was to describe oropharyngeal swallowing physiology and safety in unselected patients with a secure, mixed-disciplinary, diagnosis of IPF, without previous evidence for swallowing difficulty.
The widely used patient- reported symptoms of swallowing impairment, EAT-10 tool was used to assess patients’ perception of swallowing difficulty. In our study the median EAT-10 score was 0, range 0 -25. Four out of 14 patients (29%), had markedly raised total EAT-10 scores, with values of 25, 15, 14 and 13.
We are unaware of previous EAT-10 data in people with IPF. A recent study in 30 patients with Acute Exacerbations of COPD showed that 67% of patients had a raised EAT-10 score, compared with 23% of patients with cardiac disease [12].
In our study, subjective dysphagia symptoms reported by the EAT-10 tool were not consistently related to the nature and severity of the oropharyngeal swallow impairment observed during VFSS. Patient 1 who demonstrated aspiration on videofluorocopy had the highest EAT-10 score of 25, but patients who scored 13 and 14 in the EAT-10 tool had relatively normal swallow physiology detected during VFSS (Table: 2). Our exploratory findings in a limited number of patients are consistent with previous studies in COPD which have previously shown a weak association of EAT-10 with objective measurements of dysphagia [7, 12]. Further studies are therefore indicated in people with IPF, within which it is important to identify patients who may have difficulty swallowing regardless of whether aspiration is present. The EAT-10 tool helps to extend understanding about broad aspects of swallowing, which includes patient centred social and emotional information, not captured by objective instrumental tests.
Videofluoroscopy studies demonstrated a range of physiology in the ten patients studied. The swallow from laryngeal elevation onwards was consistently and highly disrupted in patient 1 and 2; these two patients had an abnormal physiology according to the MBSImP classification system [16]. However, patient 2 had no airway invasion, despite objectively the worst physiology of all, suggesting that a high score on MBSImP may not correspond to an unsafe swallow. Higher scores on some components of the MBSImP may be regarded as ‘normal’; for example, the bolus may enter the pharynx before swallow is initiated even in healthy individuals and some features may be part of a healthy ageing profile [20]. On PAS patients 2, 4 and 8 had airway penetration, we also noted that Patient 4 and 8 had relatively normal physiology by MBSImP. In the scarce literature, normal older swallowers sometimes have scores of 2 and 3 in PAS and our findings may therefore represent the combined effects of both normal aging and IPF pathophysiology and require further study [17]. There is no available evidence in the literature of which we are aware comparing the prevalence of swallowing impairments in IPF patients compared to the general population of similar age, with no known neurological disorders.
Parenchymal lung scarring and hypoxaemia may disrupt the complex coordination of normal swallowing and breathing function and in principle dysphagia may contribute to a complex dysregulated aerodigestive homeostasis in people with IPF. The true incidence of dysphagia in IPF is unknown but oral dysbiosis has been linked with a range of lung diseases including, pneumonia, COPD, and lung cancer [21]. The oral cavity has been shown to be a source of diverse bacteria and it is of interest that this can include non-gastric reservoirs of Helicobacter pylori [22], which has been associated with a more severe disease phenotype, higher mortality and lung function decline in people with IPF [23].
As fibrotic changes progress and lung function declines in IPF, the affected lung may be expected to become more susceptible to external challenges such as aspiration. Non-sterile aspiration, related to dysphagia and unprotected by cough, therefore represents a candidate source of complex lung injury and microbiome dysregulation. Aspiration is noted to be a trigger of threatening acute exacerbations [24]. Acute exacerbations in IPF are of high concern as they represent the most common cause of death in IPF. Just under a half of deaths in IPF are preceded by an acute exacerbation and the median survival after an acute exacerbation is approximately 3 to 4 months [25].
This prospective consecutive case series is descriptive in a limited number of patients. Further exploration is needed to establish the association between dysphagia and IPF, and the clinical significance of such a link. It would be of interest for further studies to assess if the prevalence of dysphagia in IPF is above that expected in a population of a similar age and to compare our IPF findings to other patient groups with comorbidities that could increase the risk of swallowing problems/aspiration (e.g. frailty). Our experience indicated that such studies are possible in people with IPF but also underline that these are challenging. Of 18 patients approached in our study five died, and three deaths occurred in fourteen consented patients, before videofluoroscopy could be performed. In other settings, simple bedside tests of dysfunction are clinically informative in swallowing pathophysiology [26] and together with selected patient reported outcome measures, [27] such approaches may be useful in frail patients. Safe approaches to augmented personalised therapy in selected patients, including speech and language intervention, could be rapidly implemented given the established model of mixed disciplinary care in IPF, if dysphagia is confirmed in further studies.
Availability of data and materials
The anonymised patient-level data used for this study cannot be shared for reasons of information governance. Data may be available to affiliated researchers given the North West- Preston Research Ethics committee, REC reference 14/NW/1056 ethical approval, and are available from the corresponding author on reasonable request.
References
Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–68.
Brownlee I, Aseeri A, Ward C, et al. From gastric aspiration to airway inflammation. Monaldi Arch Chest Dis. 2010;73(2):54.
Nishino T. Swallowing as a protective reflex for the upper respiratory tract. Anesthesiol. 1993;79:588–601.
Wang Z, Bonella F, Li W, et al. Gastroesophageal reflux disease in idiopathic pulmonary fibrosis: uncertainties and controversies. Respir. 2018;96(6):571–87.
Raghu G, Freudenberger T, Yang S, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27(1):136–42.
Spagnolo P, Molyneaux PL, Bernardinello N, et al. The role of the lung’s microbiome in the pathogenesis and progression of idiopathic pulmonary fibrosis. Int J Mol Sci. 2019;20(22):5618.
Garand KL, Strange C, Paoletti L, et al. Oropharyngeal swallow physiology and swallowing-related quality of life in underweight patients with concomitant advanced chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2018;13:2663.
Lin T-F, Shune S. Chronic obstructive pulmonary disease and dysphagia: a synergistic review. Geriatrics. 2020;5(3):45.
Mokhlesi B, Logemann JA, Rademaker AW, Stangl CA. oropharyngeal dysfunction in COPD patient. Chest. 2002;121(2):361–9.
Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824.
Belafsky PC, Mouadeb DA, Rees CJ, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol, Rhinol Laryngol. 2008;117(12):919–24.
Gonzalez Lindh M, Janson C, Blom Johansson MB, et al. Swallowing dysfunction in patients hospitalised due to a COPD exacerbation. ERJ Open Res. 2021;7:00173-2021.
Cheney DM, Siddiqui MT, Litts JK, et al. The ability of the 10-item eating assessment tool (EAT-10) to predict aspiration risk in persons with dysphagia. Ann Otol, Rhinol Laryngol. 2015;124(5):351–4.
Martin-Harris B, Brodsky MB, Michel Y, et al. MBS measurement tool for swallow impairment—MBSImp: establishing a standard. Dysphagia. 2008;23(4):392–405.
Martin-Harris B, Humphries K, Garand KL. The modified barium swallow impairment profile (MBSImP™©)–innovation, dissemination and implementation. Perspect ASHA Spec Interest Groups. 2017;2(13):129–38.
Beall J, Hill EG, Armeson K, et al. Classification of physiologic swallowing impairment severity: a latent class analysis of modified barium swallow impairment profile scores. Am J Speech Lang Pathol. 2020;29(2S):1001–11.
Robbins J, Coyle J, Rosenbek J, et al. Differentiation of normal and abnormal airway protection during swallowing using the penetration–aspiration scale. Dysphagia. 1999;14(4):228–32.
Allen JE, White CJ, Leonard RJ, Belafsky PC. Prevalence of penetration and aspiration on videofluoroscopy in normal individuals without dysphagia. Otolaryngol Head Neck Surg. 2010;142(2):208–13.
Aaron SD, Dales RE, Cardinal P. How accurate is spirometry at predicting restrictive pulmonary impairment? Chest. 1999;115(3):869–73.
Dua KS, Ren J, Bardan E, et al. Coordination of deglutitive glottal function and pharyngeal bolus transit during normal eating. Gastroenterol. 1997;112(1):73–83.
Pathak JL, Yan Y, Zhang Q, et al. The role of oral microbiome in respiratory health and diseases. Respir Med. 2021;185:106475.
Kashyap D, Baral B, Verma TP, et al. Oral rinses in growth inhibition and treatment of pylori infection. BMC Microbiol. 2020;20(1):1–18.
Bennett D, Bargagli E, Refini RM, et al. Helicobacter pylori seroprevalence in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2014;43(2):635–8.
Kamiya H, Panlaqui OM. A systematic review of the incidence, risk factors and prognosis of acute exacerbation of systemic autoimmune disease-associated interstitial lung disease. BMC Pulm Med. 2021;21:150.
Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis An international working group report. Am J Respir Crit Care Med. 2016;194(3):265–75.
Patterson JM, McColl E, Carding PN, et al. Swallowing performance in patients with head and neck cancer: a simple clinical test. Oral Oncol. 2009;45(10):904–7.
Regan J, Lawson S, De Aguiar V. The eating assessment Tool-10 predicts aspiration in adults with stable chronic obstructive pulmonary disease. Dysphagia. 2017;32(5):714–20.
Acknowledgements
We thank the patient volunteers and their carers and are grateful for extensive discussions with Mr Ian Foote and the IPF Support Group Northern Region.
Funding
This work was supported.
KTP 008,821, ‘an aero digestive approach for lung fibrosis’ from Innovate UK, (Rhys Jones, John Simpson, Mike Griffin, Ian Forrest and Chris Ward).
British Lung Foundation (grant IPFPSG12-7 (John Simpson, Ian Forrest and Chris Ward).
The Oesophagoose medical charity (Rhys Jones).
The Newcastle Hospitals NHS Charity. An observational study of swallow competence in idiopathic pulmonary fibrosis.
PhD Project funding from Imam Abdulrahman Bin Faisal University, Kingdom of Saudi Arabia (Amal Alamer), Al-Baha University, Albaha, Saudi Arabia (Abdullah Althuwaybi) and Saudi Arabia Cultural Bureau in London (Amal Alamer and Abdullah Althuwaybi).
John Simpson is an NIHR Research Professor.
Author information
Authors and Affiliations
Contributions
Mrs Amal Alamer: analysed the study and wrote all drafts of the manuscript. Mr Rhys Jones: designed and recruited for the study and contributed to analysis and writing. Dr. Michael Drinnan: analysed and co-wrote the study. Prof. A. John Simpson: study design and patient recruitment. Prof. Mike Griffin: study design and protocol development. Prof. Joanne M Patterson: study design, protocol development and writing the study. Mr Abdullah Althuwaybi study discussion and literature review. Prof. Chris Ward designed, analysed and wrote the study. Dr. Ian Forrest initiated, designed, recruited, analysed and was clinical lead for the study. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were performed in accordance with the approval guidelines and regulations.
The study was approved by the Health Research Authority (HRA), North West- Preston Research Ethics committee, REC reference 14/NW/1056 and carried out in accordance with approval guidelines. All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
No competing interests or conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alamer, A., Jones, R., Drinnan, M. et al. Oropharyngeal swallowing physiology and safety in patients with Idiopathic Pulmonary Fibrosis: a consecutive descriptive case series. BMC Pulm Med 22, 422 (2022). https://doi.org/10.1186/s12890-022-02232-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02232-3