Abstract
Background
The disease course of idiopathic pulmonary fibrosis (IPF) is progressive and occasionally, other types of interstitial lung disease (ILD) may progress similarly to IPF. This study aimed to evaluate risk factors for disease progression within 24 months in patients with various ILDs.
Methods
This prospective study obtained 97 patients with a suspected ILD who underwent a transbronchial lung cryobiopsy. The extent of several high-resolution computed tomography (HRCT) patterns was assessed. Due to the inclusion criteria the study population presented a low extent of honeycombing and definite usual interstitial pneumonia (UIP) pattern on HRCT suggesting an early stage of ILD. Disease progression within 24 months despite treatment was defined as a relative decline of ≥ 10% in forced vital capacity (FVC), or a relative decline in FVC of ≥ 5% and one of the three additional criteria: (1) a decline in diffusion capacity to carbon monoxide (DLCO) ≥ 15%; (2) increased fibrosis on HRCT; (3) progressive symptoms, or progressive symptoms and increased fibrosis on HRCT. The same definition was utilized in patients with IPF and other ILDs. Risk factors for disease progression were evaluated in a multivariable logistic regression model.
Results
Disease progression was revealed in 52% of the patients with ILD, 51% of the patients with IPF, and 53% of the patients with other types of ILD. A high extent of reticulation on HRCT (Odds ratio [OR] 3.11, 95% Confidence interval [CI] 1.21–7.98, P = 0.019) and never smoking (OR 3.11, CI 1.12–8.63, P = 0.029) were associated with disease progression whereas platelet count (OR 2.06 per 100 units increase, CI 0.96–4.45, P = 0.065) did not quite reach statistical significance.
Conclusion
Higher extent of reticulation on HRCT and never smoking appeared to associate with the risk of disease progression within 24 months in ILD patients without honeycombing. Approximately half of the patients with ILD revealed disease progression, and similar proportions were observed in patients with IPF and in other types of ILD.
Similar content being viewed by others
Background
Idiopathic pulmonary fibrosis (IPF) is usually a progressive disease with dismal prognosis [1], although the disease course may vary and is difficult to predict individually [2, 3]. IPF has been thought as the architype of progressive fibrosis even though recent studies have shown that a significant number of patients survived for over a decade before antifibrotic treatment was available [2, 4]. Occasionally, other types of interstitial lung disease (ILD), such as rheumatoid arthritis associated ILD (RA-ILD), systemic scleroderma associated ILD (SSc-ILD), fibrotic hypersensitivity pneumonia (fHP), and acute fibrinous and organizing pneumonia, may progress similarly to IPF [5]. It has been suggested that rather than a specific ILD diagnosis, the disease course should determine the management of ILDs [6] since patients with a progressive disease course have revealed similar survival rates than patients with IPF [7, 8]. Moreover, an antifibrotic drug nintedanib has been found to be effective also in non-IPF ILD patients with disease progression [9].
Disease progression in ILD has been studied in several studies that have excluded patients with IPF [8, 10,11,12]. Two recent studies included also patients with IPF within the group of progressive disease course [13, 14]. In the results of the above-mentioned studies, the patients with disease progression were older and had lower baseline pulmonary function tests than the patients with a stable disease [13]. Furthermore, disease progression was independently associated with mortality in the whole cohort and when patients with IPF and other ILDs were analyzed separately [14]. Recent studies revealed that more deterioration on high-resolution computed tomography (HRCT) was observed in the progressive patients treated with pirfenidone than in the stable patients with IPF after the initial forced vital capacity (FVC) decline [15]. Furthermore, the stable IPF patients survived longer than those with a rapid disease progression [16]. In an IPF cluster analysis, progressive patients were divided into three different clusters based on three unique expression patterns of proteins, which were pondered to refer different progressive profiles [17]. In addition, among patients with non-IPF ILDs the survival was shorter in patients with disease progression than in patients with a stable disease course [8, 10].
In the recent years, the research on progressive fibrosis has increased. Still, there is little knowledge of the risk factors for disease progression in patients with ILD. Since the progressive disease course is currently an indicator to consider antifibrotic treatment, we created a multivariable model evaluating the risk factors associated with disease progression in patients with ILD. The aim of this study was to evaluate the proportion of progressive ILD cases and to determine the risk factors associated with disease progression within 24 months. Disease progression was defined similarly in the whole ILD cohort also including patients with IPF.
Methods
Patient selection and data collection
Consecutive patients with a suspected ILD and a necessity of a histological investigation were prospectively recruited from Kuopio University Hospital (KUH) and Tampere University Hospital (TAUH) pulmonology clinics between January 2015 and December 2019 to this observational cohort study. A written informed consent was obtained from all participants. Inclusion and exclusion criteria are described in the Additional file 1. Due to inclusion criteria the extent of honeycombing and definite usual interstitial pneumonia (UIP) pattern on HRCT were low suggesting the patients were at an early stage of their ILD. Patient data were collected from the electronic medical records of the hospitals. The diagnoses were concluded in a multidisciplinary meeting according to the contemporary international guidelines [18,19,20]. The study protocol was approved by the Research Ethics Committee of the Northern Savo Hospital District (statement 80/2014) and Tampere University Hospital (R15149), and the study was conducted in compliance with the Declaration of Helsinki (as revised in 2013). The follow-up of the patients was conducted by the routine clinical practices of each hospital.
Histological and radiological investigations and questionnaires
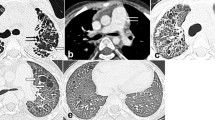
The 100 study subjects underwent transbronchial lung cryobiopsy and bronchoalveolar lavage (BAL) (see Additional file 1). Before the TBLC, digital volume HRCT scans were available from 84 out of 100 patients. Eleven patients had sequenced HRCT scans. Other comparable CT examinations, such as CT pulmonary angiography, were available from 5 patients. The radiologists evaluated 97% of the images as good quality and 3% as suboptimal quality. The extent of several specific HRCT patterns was assessed separately in three zones of each lung as described previously [21]. The extents of reticulation, honeycombing and ground-glass opacity (GGO) were semi-quantitatively graded on a scale of 0 to 4 (0 = finding absent, 1 = minor peripheral scattered changes, 2 = uniform peripheral or minor central scattered changes, 3 = substantial peripheral changes that penetrated deeply into the lung parenchyma, 4 = very abundant peripheral and central changes) the maximum score being 24. The mean score of the two radiologists for each pattern was used in the analysis. The different reticulation scores and progression of findings on HRCT during follow-up are illustrated in Fig. 1. A more detailed illustration of the scoring has been published previously [21, 22]. Furthermore, the two radiologists agreed on a consensus of the HRCT scans according to the 2018 international statement as a definite UIP, probable UIP, indeterminate with UIP, and alternative diagnosis [19]. The study subjects filled in The University of California, San Diego Shortness of Breath Questionnaire (SOBQ), Leicester Cough Questionnaire (LCQ) and The St George’s Respiratory Questionnaire (SGRQ) at the beginning of the study and in every six months. [23,24,25].
High-resolution computed tomography (HRCT) scans illustrating different reticulation scores. A HRCT coronal scan of the whole lung. A patient with reticulation score of the whole lung 6.0, B another patient with reticulation score of the whole lung 11.0. C HRCT obtained below the level of carina. A third patient with reticulation score of the whole lung 5.0 at the beginning of the study and D the same patient with reticulation score of the whole lung 9.0 at 24 months follow-up. This is an original figure created for this manuscript
The definition of disease progression
Disease progression was defined as a relative decline of ≥ 10% in FVC, or a relative decline in FVC of ≥ 5% and one of the three additional criteria: (1) a decline in diffusion capacity to carbon monoxide (DLCO) ≥ 15%; (2) increased fibrosis on HRCT; (3) progressive symptoms, or progressive symptoms and increased fibrosis on HRCT over 24 months despite treatment [26]. The progressive symptoms were defined as increased (SGRQ, SOBQ) or decreased (LCQ) total scores of a minimum clinically significant difference of the questionnaires [27,28,29]. The information of progression on HRCT was collected from all the HRCTs available during follow-up. All together 97 patients had sufficient data available at 24 months follow-up.
Statistical analysis
Data were expressed as medians and interquartile range (IQR) or frequencies with percentages. Independent-samples T-test was used for normally distributed continuous parameters and Mann-Whitney U tests for not normally distributed continuous variables, and Chi-square test was used for distribution counts when appropriate. The reticulation score was divided into tertiles which revealed a cut off value of ≥ 9.0 (Fig. 2). Independent variables with a plausible association with the risk of disease progression were selected in the multivariable model using expert evaluation and previous literature of the risk factors for disease progression [30]. Since there were more current or former smokers among the male than the female and higher emphysema score among current or former smokers than the never smokers, gender and emphysema were excluded from the multivariable model. Of the independent variables with strong interrelationships, such as FVC% and DLCO%, SGRQ and SOBQ, and white blood cell count and platelet count, the variable with closer association with disease progression in the bivariable analyses was chosen for the multivariable analysis. A backward stepwise logistic regression model was build using the selected variables (age, smoking status, body mass index [BMI], FVC%, reticulation score, traction bronchiectasis score, platelet count, SOBQ, UIP histology, antifibrotic treatment, and immunosuppressive therapy). The Kaplan-Meier estimator was used to evaluate survival. Inter-observer agreements of the specific HRCT patterns were presented as a kappa (κ) value: good agreement κ = 0.61–0.80, moderate agreement κ = 0.41–0.60 and fair agreement κ = 0.21–0.40. Missing data was excluded. P values < 0.05 were considered statistically significant. IBM statistics SPSS software, version 27.0, was used in the statistical analysis.
A bar diagram representing the tertiles of reticulation score and the distribution of patients with progressive ILD among these tertiles (Tertile 1 = reticulation score ≤ 6.0, Tertile 2 = reticulation score 6.1–8.9, Tertile 3 = reticulation score ≥ 9.0). ILD = interstitial lung disease. This is an original figure created for this manuscript
Results
Patient characteristics
Characteristics of the study population are presented in Table 1. Disease progression was revealed in 50 out of 97 patients (52%). There were more former smokers compared to the never smokers in the stable group than in the progressive group. The extent of reticulation on HRCT was higher in the progressive group than in the stable group. The number of immunosuppressive therapies did not differ between the progressive and stable groups (Table 1). Immunosuppressive therapies are presented in detail in the supplementary material (Additional file 1: Table S1). Acute exacerbations were more common in the progressive group than in the stable group (12 vs. 4, P = 0.044). Causes of death are presented in the supplementary material (Additional file 1: Table S2). In the subgroup of patients with IPF, 32 out of 63 patients (51%) revealed disease progression within 24 months, and among ILDs other than IPF, 18 out of 34 patients (53%) presented disease progression.
Risk factors for disease progression and survival
The predictor candidates included in the logistic regression model were age, smoking status, BMI, FVC%, reticulation score, traction bronchiectasis score, SOBQ total score, platelet count, UIP histopathology, antifibrotic treatment, and immunosuppressive therapy. The reticulation score ≥ 9.0 on HRCT and never smoking were associated with the risk of disease progression in ILD whereas platelet count did not quite reach statistical significance (Table 2). The median follow-up time of patients with a stable disease course was 53 months (IQR 41.0–65.0). Patients with higher extent of reticulation on HRCT had shorter median survival (66 months, 95% CI 56.9–75.1) than patients with lower extent of reticulation (72 months, 95% CI 64.6–79.4) (Fig. 3). Never smokers had shorter median survival (65 months, 95% CI 61.6–68.4) than current or former smokers (75 months, 95% CI 65.2–84.8) (Fig. 4). Patients with two risk factors (reticulation score ≥ 9.0 and never smoking) had shorter median survival (63 months, 95% CI 55.5–70.5) than patients with one (71 months, 95% CI 61.3–80.7) or no risk factors (79 months, 95% CI not applicable) (Fig. 5).
An unadjusted Kaplan-Meier survival curve in ILD patients with higher and lower extent of reticulation on HRCT (Log rank 4.65, P = 0.031). Survival curve and P-value represent the time to death or lung transplantation. HRCT = high-resolution computed tomography, ILD = interstitial lung disease. This is an original figure created for this manuscript
An unadjusted Kaplan-Meier survival curve in patients with risk factors for progressive ILD (Log Rank 9.38, P = 0.009). Survival curve and P value represent the time to death or lung transplantation. One risk factor = reticulation score ≥ 9.0 on HRCT or never smoking, two risk factors = reticulation score ≥ 9.0 on HRCT and never smoking. HRCT = high-resolution computed tomography, ILD = interstitial lung disease. This is an original figure created for this manuscript
The interobserver agreement
The inter-observer agreement between the two radiologists was moderate to good regarding the different HRCT patterns (ground-glass opacity κ = 0.481, honeycombing κ = 0.655, emphysema κ = 0.697, and traction bronchiectasis κ = 0.440).
Discussion
In this real-life study we demonstrated that higher extent of reticulation on HRCT and never smoking were associated with the risk of disease progression within 24 months in patients with ILD whereas high platelet count did not quite reach statistical significance. The proportion of patients with disease progression was 52% of the patients with ILD. Furthermore, we observed somewhat unexpectedly that only 51% of patients with IPF presented disease progression regardless of antifibrotic treatment. In addition, we observed that the patients with higher extent of reticulation on HRCT had shorter median survival than patients with lower extent of reticulation as well as never smokers had shorter median survival than current or former smokers. Furthermore, patients with two risk factors for disease progression (reticulation score ≥ 9.0 and never smoking) had shorter median survival than patients with one or no risk factors.
Our study subjects consisted of patients whose HRCT scans did not present a definite UIP pattern, and the extent of honeycombing was minimal, making a histological investigation of lung tissue samples necessary. We observed that higher extent of reticulation in the absence of honeycombing on HRCT was associated with the risk of disease progression in patients with ILD. To the best of our knowledge, this kind of association has not been observed previously, although in several studies, reticulation pattern has been associated with mortality in patients with fibrotic idiopathic interstitial pneumonia (IIP), IPF, and ILD [16, 32,33,34]. In patients with IPF, visually scored reticulation and higher interstitial HRCT score have shown to be associated with death [16, 33]. In addition, a study of 255 patients with ILD showed that an automated CT analysis of global reticulation volume was associated with mortality [34]. Likewise, a fibrosis score defined as a combination of reticulation and honeycombing has been associated with mortality in patients with ILD other than IPF [35, 36]. An increase of 10% in the extent of fibrosis on HRCT was associated with disease progression in 6 months among IPF patients treated with pirfenidone [15]. In our study, the extent of honeycombing was minimal due to the study protocol which included patients who needed a transbronchial lung cryobiopsy to establish the diagnosis. In a recent study focusing on peripheral blood proteins, patients with progressive ILD other than IPF and a high-risk proteomic signature showed a larger 1-year change in FVC than patients with a low-risk proteomic signature [37]. The difference in FVC change was similar in a subgroup of patients with definite or probable UIP pattern on HRCT [37].
Platelets are associated with the progression, control, and resolution of inflammation but the mechanisms of these processes are poorly understood [38]. Platelet hyperactivity has been observed in patients with IPF compared to controls [39] and a detectable platelet activation was found in the lungs of patients with SSc-ILD but not in patients with SSc without ILD [40]. A recent animal study has demonstrated that in mice with bleomycin-induced pulmonary fibrosis, platelets accumulated into the lung tissue, the platelet number in BAL correlated with fibrotic markers, and that platelet depletion reduced fibrosis and modestly inhibited pulmonary function changes [41]. Thus, our observation of a higher platelet count associating with the risk of disease progression in ILD is in line with the previous studies.
Smoking has been shown to associate with the risk of IPF [42, 43]. However, in several studies current smoking has been associated with a longer survival in patients with IPF [44,45,46]. Another study of IPF patients showed that current or former smokers had longer survival than never smokers [47]. In patients with autoimmune disease associated ILD, current or former smoking was associated with disease progression and mortality [48] whereas, in a study of patients with RA-ILD, the survival was similar between current or former and never smokers [49]. In our study, never smoking was associated with the risk of disease progression in ILD and never smokers had shorter median survival than current or former smokers. Instead, in recent studies, smoking was not associated with mortality in patients with progressive ILD [14] and an increase in pack years did not associate with the risk of disease progression in patients with ILD in a multivariable model [13]. Thus, the association between smoking and disease progression in ILD merits further investigations.
The proportion of patients with disease progression was similar in our study compared to other recently published studies [13, 14]. Disease progression was presented in 50% of ILD patients in the Canadian study and in 42% in the Japanese study compared to the 51% in our study [13, 14]. The Japanese study used the same definition of disease progression as we did, but they did not include HRCT analysis since they estimated that the proportion of patients who would be defined as progressive due to HRCT changes would be small [14]. The Canadian study had different definition for disease progression but most of the patients (49%) in that study presented FVC decline ≥ 10% as the sign of disease progression [13]. Similarly, in our study most of the patients (68%) presented FVC decline ≥ 10% as the sign of disease progression within 24 months. We observed only 51% of the patients with IPF to have a progressive disease course which could not be explained by the antifibrotic treatment. Similarly, the Canadian and Japanese studies observed that 59% and 59.4% of the patients with IPF revealed disease progression, respectively [13, 14]. The results of the above-mentioned studies and our study showed that not all patients with IPF fulfill the definition of disease progression within 24 months. The proportion of non-IPF ILD patients with disease progression was similar in our study compared to the previous studies despite the variable definitions used to describe disease progression [10, 11].
Previous studies have demonstrated that non-IPF ILD patients with disease progression have worse survival than patients with stable ILD [8, 10]. In addition, it has been demonstrated that non-IPF ILD patients with disease progression have similar survival than patients with IPF [7, 8]. However, Torrisi et al. did not find differences in survival between the progressive ILD and stable ILD in patients with non-IPF ILDs, whereas the survival was longer in patients with disease progression than in patients with IPF [11]. Another study demonstrated a greater three-year survival among patients with IPF (72%) than non-IPF ILD patients with disease progression (64%) [12]. Likewise, in a recent study, the median survival was longer in patients with stable IPF (84.8 months) than in patients with progressive non-IPF (52.3 months) whereas the median survival was shortest in patients with progressive IPF (31.8 months) [14].
We demonstrated a shorter median survival in patients with risk factors for disease progression observed in the present study than in patients with no risk factors. Different categorization, different amounts of specific ILD types, and a different proportion of UIP pattern on HRCT may explain the differences in the survival results. However, a similar survival has been demonstrated between the different disease progression definitions used in non-IPF patients [10]. The majority of our study population constituted of patients with IPF and none of the patients had a definite UIP pattern on HRCT. Furthermore, the number of patients with connective tissue associated ILD (CTD-ILD) (n = 1) in our study was minimal since the role of lung biopsy is small in the diagnosis of CTD-ILDs. Kwon et al. and Chen et al. included mostly patients with autoimmune disease associated ILD or CTD-ILD (67.7% and 38.6% respectively) when the UIP pattern was observed in 45.7% and 38.6% of patients, respectively [8, 10]. Whereas most of the patients in the study of Torrisi et al. were diagnosed with fHP (35.5%) and the proportion of UIP pattern on HRCT was not described [11].
The small number of patients can be regarded as a limitation of this study. The requirement of a histological investigation and an exclusion of patients with severe pulmonary function impairment could have caused selection bias in the study population. In addition, the multivariable model was not validated. For this reason, the results should be considered as observational rather than representing causality. Furthermore, nearly 80% of the study population constituted of patients with IIP, which should be taken into consideration when assessing the generalizability of the results. However, this study population included well characterized patients with ILD who underwent histopathological investigation and whose HRCTs were reanalyzed, and HRCT patterns were quantified. Moreover, the symptoms were evaluated systematically by questionnaires, and furthermore, laboratory tests and lung function tests were available.
Conclusion
Reticulation on HRCT was associated with the risk of disease progression within 24 months in patients with ILD not revealing a definite UIP pattern on HRCT. Approximately half of the patients presented a progressive disease course. Similarly, in a subgroup of patients with IPF 51% presented disease progression within 24 months. The results reinforce the role of HRCT in the evaluation of disease progression among patients with ILD. Furthermore, the results support the evidence that the disease progression of IPF is not as uniform as it has been assumed.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due the gathering of the data is continuing but are available from the corresponding author on reasonable request.
Abbreviations
- BAL:
-
Bronchoalveolar lavage
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CTD-ILD:
-
Connective tissue disease associated interstitial lung disease
- DLCO:
-
Diffusion capacity to carbon monoxide
- fHP:
-
Fibrotic hypersensitivity pneumonia
- FVC:
-
Forced vital capacity
- GGO:
-
Ground-glass opacity
- HRCT:
-
High-resolution computed tomography
- IIP:
-
Idiopathic interstitial pneumonia
- ILD:
-
Interstitial lung disease
- IPF:
-
Idiopathic pulmonary fibrosis
- IQR:
-
Interquartile range
- KUH:
-
Kuopio University Hospital
- LCQ:
-
Leicester Cough Questionnaire
- OR:
-
Odds ratio
- RA-ILD:
-
Rheumatoid arthritis associated interstitial lung disease
- SGRQ:
-
St George Respiratory Questionnaire
- SOBQ:
-
University of California, San Diego Shortness of Breath Questionnaire
- SSc-ILD:
-
Systemic scleroderma associated interstitial lung disease
- TAUH:
-
Tampere University Hospital
- TBLC:
-
Transbronchial lung cryobiopsy
- UIP:
-
Usual interstitial pneumonia
References
Caminati A, Madotto F, Conti S, Cesana G, Mantovani L, Harari S. The natural history of idiopathic pulmonary fibrosis in a large European population: the role of age, sex and comorbidities. Intern Emerg Med. 2021;16(7):1793–802. https://doi.org/10.1007/s11739-021-02651-w.
Nathan SD, Shlobin OA, Weir N, Ahmad S, Kaldjob JM, Battle E, et al. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest. 2011;140(1):221–9. https://doi.org/10.1378/chest.10-2572.
Kärkkäinen M, Kettunen H, Nurmi H, Selander T, Purokivi M, Kaarteenaho R. Comparison of disease progression subgroups in idiopathic pulmonary fibrosis. BMC Pulm Med. 2019;19(1):228. https://doi.org/10.1186/s12890-019-0996-2.
Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, et al. A Multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684–91. https://doi.org/10.7326/0003-4819-156-10-201205150-00004.
Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18-47. https://doi.org/10.1164/rccm.202202-0399ST.
Wells AU, Brown KK, Cottin V. The progressive fibrotic phenotype in current clinical practice. Curr Opin Pulm Med. 2021;27(5).
Brown KK, Martinez FJ, Walsh SLF, Thannickal VJ, Prasse A, Schlenker-Herceg R, et al. The natural history of progressive fibrosing interstitial lung diseases. Eur Respir J. 2020;55(6):2000085. https://doi.org/10.1183/13993003.00085-2020.
Chen X, Guo J, Yu D, Jie B, Zhou Y. Predictors of mortality in progressive fibrosing interstitial lung diseases. Front Pharmacol. 2021;12:754851. https://doi.org/10.3389/fphar.2021.754851.
Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–27. https://doi.org/10.1056/NEJMoa1908681.
Kwon BS, Choe J, Chae EJ, Hwang HS, Kim Y, Song JW. Progressive fibrosing interstitial lung disease: prevalence and clinical outcome. Respir Res. 2021;22(1):282. https://doi.org/10.1186/s12931-021-01879-6.
Torrisi SE, Kahn N, Wälscher J, Polke M, Lee JS, Molyneaux PL, et al. Outcomes and incidence of PF-ILD according to different definitions in a real-world setting. Front Pharmacol. 2021;12:790204. https://doi.org/10.3389/fphar.2021.790204.
Gagliardi M, Berg DV, Heylen C, Koenig S, Hoton D, Tamirou F, et al. Real-life prevalence of progressive fibrosing interstitial lung diseases. Sci Rep. 2021;11(1):23988. https://doi.org/10.1038/s41598-021-03481-8.
Hambly N, Farooqi MM, Dvorkin-Gheva A, Donohoe K, Garlick K, Scallan C, et al. Prevalence and characteristics of progressive fibrosing interstitial lung disease in a prospective registry. Eur Respir J. 2022;. 2102571 [pii].
Takei R, Brown KK, Yamano Y, Kataoka K, Yokoyama T, Matsuda T, et al. Prevalence and prognosis of chronic fibrosing interstitial lung diseases with a progressive phenotype. Respirology. 2022;. https://doi.org/10.1111/resp.14245.
Higo H, Miyahara N, Taniguchi A, Senoo S, Itano J, Watanabe H, et al. Deterioration of high-resolution computed tomography findings predicts disease progression after initial decline in forced vital capacity in idiopathic pulmonary fibrosis patients treated with pirfenidone. Respir Investig. 2020;58(3):185–9. https://doi.org/10.1016/j.resinv.2019.12.007.
Doubková M, Švancara J, Svoboda M, Šterclová M, Bartoš V, Plačková M, et al. EMPIRE Registry, Czech Part: Impact of demographics, pulmonary function and HRCT on survival and clinical course in idiopathic pulmonary fibrosis. Clin Respir J. 2018;12(4):1526–35. https://doi.org/10.1111/crj.12700.
Norman KC, O’Dwyer D,N., Salisbury ML, DiLillo KM, Lama VN, Xia M, et al. Identification of a unique temporal signature in blood and BAL associated with IPF progression. Sci Rep. 2020;10(1):12049. https://doi.org/10.1038/s41598-020-67956-w.
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. https://doi.org/10.1164/rccm.2009-040GL.
Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44-68. https://doi.org/10.1164/rccm.201807-1255ST.
Travis WD, Costabel U, Hansell DM, King J, Talmadge E, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–48. https://doi.org/10.1164/rccm.201308-1483ST.
Nurmi HM, Kettunen H, Suoranta S, Purokivi MK, Kärkkäinen MS, Selander TA, et al. Several high-resolution computed tomography findings associate with survival and clinical features in rheumatoid arthritis-associated interstitial lung disease. Respir Med. 2018;134:24–30. https://doi.org/10.1016/j.rmed.2017.11.013.
Mononen ME, Kettunen H, Suoranta S, Kärkkäinen M,S., Selander TA, Purokivi MK, et al. Several specific high-resolution computed tomography patterns correlate with survival in patients with idiopathic pulmonary fibrosis. J Thorac Dis. 2021;13(4):2319–30. https://doi.org/10.21037/jtd-20-1957.
Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MDL, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax. 2003;58(4):339–43. https://doi.org/10.1136/thorax.58.4.339.
Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: The UCSD Shortness of Breath Questionnaire. Chest. 1998;113(3):619–24. https://doi.org/10.1378/chest.113.3.619.
Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85:25–31. https://doi.org/10.1016/S0954-6111(06)80166-6.
George PM, Spagnolo P, Kreuter M, Altinisik G, Bonifazi M, Martinez FJ, et al. Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. The Lancet Respir Med. 2020;8(9):925–34. https://doi.org/10.1016/S2213-2600(20)30355-6.
Chen T, Tsai APY, Hur SA, Wong AW, Sadatsafavi M, Fisher JH, et al. Validation and minimum important difference of the UCSD Shortness of Breath Questionnaire in fibrotic interstitial lung disease. Respir Res. 2021;22(1):202. https://doi.org/10.1186/s12931-021-01790-0.
Raj AA, Pavord DI, Birring SS: Clinical Cough IV:What is the Minimal Important Difference for the Leicester Cough Questionnaire? In Pharmacology and Therapeutics of Cough. Edited by Chung KF, Widdicombe J. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009:311–320.
Swigris JJ, Brown KK, Behr J, du Bois RM, King TE, Raghu G, et al. The SF-36 and SGRQ: validity and first look at minimum important differences in IPF. Respir Med. 2010;104(2):296–304. https://doi.org/10.1016/j.rmed.2009.09.006.
Shipe ME, Deppen SA, Farjah F, Grogan EL. Developing prediction models for clinical use using logistic regression: an overview. J Thorac Dis. 2019;11:S574-84. https://doi.org/10.21037/jtd.2019.01.25.
Mononen M, Saari E, Hasala H, Kettunen H, Suoranta S, Nurmi H, et al. Risk factors of clinically significant complications in transbronchial lung cryobiopsy: A prospective multi-center study. Respir Med. 2022;200. https://doi.org/10.1016/j.rmed.2022.106922.
Edey A, Devaraj A, Barker R, Nicholson A, Wells A, Hansell D. Fibrotic idiopathic interstitial pneumonias: HRCT findings that predict mortality. Eur Radiol. 2011;21(8):1586–93. https://doi.org/10.1007/s00330-011-2098-2.
Jacob J, Bartholmai BJ, Rajagopalan S, Kokosi M, Nair A, Karwoski R, et al. Mortality prediction in idiopathic pulmonary fibrosis: evaluation of computer-based CT analysis with conventional severity measures. Eur Respir J. 2017;49(1):1601011. https://doi.org/10.1183/13993003.01011-2016.
Crews MS, Bartholmai BJ, Adegunsoye A, Oldham JM, Montner SM, Karwoski RA, et al. Automated CT analysis of major forms of interstitial lung disease. J Clin Med. 2020;9(11):3776. https://doi.org/10.3390/jcm9113776.
Takei R, Arita M, Kumagai S, Ito Y, Tokioka F, Koyama T, et al. Radiographic fibrosis score predicts survival in systemic sclerosis-associated interstitial lung disease. Respirology. 2018;23(4):385–91. https://doi.org/10.1111/resp.13175.
Ito Y, Arita M, Kumagai S, Takei R, Noyama M, Tokioka F, et al. Radiological fibrosis score is strongly associated with worse survival in rheumatoid arthritis-related interstitial lung disease. Mod Rheumatol. 2019;29(1):98–104. https://doi.org/10.1080/14397595.2018.1442170.
Bowman WS, Newton CA, Linderholm AL, Neely ML, Pugashetti JV, Kaul B, et al. Proteomic biomarkers of progressive fibrosing interstitial lung disease: a multicentre cohort analysis. The Lancet Respir Med. 2022;18. https://doi.org/10.1016/S2213-2600(21)00503-8.
Margraf A, Zarbock A. Platelets in inflammation and resolution. J Immunol. 2019;203(9):2357–67. https://doi.org/10.4049/jimmunol.1900899.
Crooks MG, Fahim A, Naseem KM, Morice AH, Hart SP. Increased platelet reactivity in idiopathic pulmonary fibrosis is mediated by a plasma factor. PloS one. 2014;9(10):e111347. https://doi.org/10.1371/journal.pone.0111347.
Kowal-Bielecka O, Kowal K, Lewszuk A, Bodzenta-Lukaszyk A, Walecki J, Sierakowski S. Beta thromboglobulin and platelet factor 4 in bronchoalveolar lavage fluid of patients with systemic sclerosis. Ann Rheum Dis. 2005;64(3):484–6. https://doi.org/10.1136/ard.2004.022970.
Carrington R, Jordan S, Wong YJ, Pitchford SC, Page CP. A novel murine model of pulmonary fibrosis: the role of platelets in chronic changes induced by bleomycin. J Pharmacol Toxicol Methods. 2021;109:107057. https://doi.org/10.1016/j.vascn.2021.107057.
Bellou V, Belbasis L, Evangelou E. Tobacco smoking and risk for pulmonary fibrosis: A prospective cohort study from the UK biobank. Chest. 2021;160(3):983–93. https://doi.org/10.1016/j.chest.2021.04.035.
Bae W, Lee C, Lee J, Kim YW, Han K, Choi SM. Impact of smoking on the development of idiopathic pulmonary fibrosis: results from a nationwide population-based cohort study. Thorax. 2022;77(5):470–6. https://doi.org/10.1136/thoraxjnl-2020-215386.
King T, Tooze J, Schwarz M, Brown K, Cherniack R. Predicting survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164(7):1171–81. https://doi.org/10.1164/ajrccm.164.7.2003140.
Antoniou KM, Hansell DM, Rubens MB, Marten K, Desai SR, Siafakas NM, et al. Idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(2):190–4. https://doi.org/10.1164/rccm.200612-1759OC.
Kärkkäinen M, Kettunen H, Nurmi H, Selander T, Purokivi M, Kaarteenaho R. Effect of smoking and comorbidities on survival in idiopathic pulmonary fibrosis. Respir Res. 2017;18(1):160. https://doi.org/10.1186/s12931-017-0642-6.
Kishaba T, Nagano H, Nei Y, Yamashiro S. Clinical characteristics of idiopathic pulmonary fibrosis patients according to their smoking status. J Thorac Dis. 2016;8(6):1112–20. https://doi.org/10.21037/jtd.2016.03.89.
Mena-Vázquez N, Rojas-Gimenez M, Romero-Barco C, Manrique-Arija S, Hidalgo Conde A, Arnedo Díez de Los Ríos, Rocío, et al. Characteristics and predictors of progression interstitial lung disease in rheumatoid arthritis compared with other autoimmune disease: A retrospective cohort study. Diagnostics (Basel, Switzerland). 2021;11(10):1794. https://doi.org/10.3390/diagnostics11101794.
Nurmi HM, Purokivi MK, Kärkkäinen MS, Kettunen H, Selander TA, Kaarteenaho RL. Variable course of disease of rheumatoid arthritis-associated usual interstitial pneumonia compared to other subtypes. BMC Pulm Med. 2016;16(1):107. https://doi.org/10.1186/s12890-016-0269-2.
Acknowledgements
The authors wish to thank Leena Tuomisto, Elsa Nylund, Johan Söderström, Aki Vainio, Heikki Pautola, Sumu Lehtola, Markku Pekonen, Päivi Torkko, Anssi Ukkonen, Severi Seppälä, Minna Tommola, and Kirsi Hämäläinen for aiding with the data collection.
Funding
This study was supported by the Foundation of the Finnish Anti-Tuberculosis Association, Väinö and Laina Kivi Foundation, The Research Foundation of the Pulmonary Diseases, Jalmari and Rauha Ahokas Foundation, and The Respiratory Foundation of the Kuopio Region. The funding had no role in the design of the study neither in collection, analysis, and interpretation of data or writing the manuscript.
.
Author information
Authors and Affiliations
Contributions
MM: Conceptualization, Data curation, Formal analysis and interpretation, Methodology, Investigation, Writing - original draft, Visualization, Funding acquisition, Final draft approval; ES: Conceptualization, Data curation, Investigation, Writing - review & editing, Funding acquisition, Final draft approval; HH: Data curation, Investigation, Writing - review & editing, Final draft approval; H-PK: Data curation, Investigation, Writing - review & editing, Final draft approval; SS: Data curation, Investigation, Writing - review & editing, Final draft approval; HN: Data curation, Investigation, Writing - review & editing, Final draft approval; MK: Data curation, Investigation, Writing - review & editing, Final draft approval; TS: Formal analysis and interpretation, Methodology, Writing - review & editing, Final draft approval; JR: Data curation, Writing - review & editing, Final draft approval; TU: Data curation, Writing - review & editing, Final draft approval; JL: Data curation, Writing - review & editing, Final draft approval; HK: Conceptualization, Formal analysis and interpretation, Methodology, Writing - review & editing, Final draft approval; RK: Conceptualization, Methodology, Writing - review & editing, Supervision, Project administration, Funding acquisition, Final draft approval; MP: Conceptualization, Methodology, Writing - review & editing, Supervision, Project administration, Resources, Funding acquisition, Final draft approval. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Research Ethics Committee of the Northern Savo Hospital District (statement 80/2014) and Tampere University Hospital (R15149), and the study was conducted in compliance with the Declaration of Helsinki (as revised in 2013). A written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
MM: personal consulting fee, congress travel costs and a lecture fee from Boehringer Ingelheim, congress travel cost from Roche, outside the submitted work. ES: personal consulting fee and congress travel cost from Boehringer Ingelheim, congress travel cost from Novartis Finland and Orion Pharma, owns personal stocks from Orion LTD, outside the submitted work. SS: owns personal stocks from Merck & Co, Faron Pharmaceuticals, CRISPR Theraupeutics, and 3 M Co. outside the submitted work. HN: personal lecture fees from Boehringer Ingelheim and Roche, congress travel cost from Boehringer Ingelheim, Sanofi-Genzyme, and Chiesi, outside the submitted work. MK: participation on GlaxoSmithKline advisory board, outside the submitted work. HK: owns personal stocks from Orion LTD, outside the submitted work. RK: personal consulting and lecture fees, advisory board member Boehringer Ingelheim, lecture fee from Roche, congress travel cost from Orion Pharma, virtual congress travel cost from Roche and Novartis, advisory board member MSD, outside the submitted work. MP: personal lecture fee, congress travel cost and advisory board member Boehringer Ingelheim, lecture fee Roche, congress travel cost Orion Pharma, outside the submitted work. HH, H-PK, TS, JR, JL, and TU declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Describing the inclusion and exclusion criteria and the transbronchial lung cryobiopsy protocol in detail as well as including. Tables S1–2 Describing immunosuppressive therapiesand causes of death in detail.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mononen, M., Saari, E., Hasala, H. et al. Reticulation pattern without honeycombing on high-resolution CT is associated with the risk of disease progression in interstitial lung diseases. BMC Pulm Med 22, 313 (2022). https://doi.org/10.1186/s12890-022-02105-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02105-9