Abstract
Background
Acute respiratory distress syndrome (ARDS) has high mortality and is mainly related to the circulatory failure.Therefore, real-time monitoring of cardiac function and structural changes has important clinical significance.Transthoracic echocardiography (TTE) is a simple and noninvasive real-time cardiac examination which is widely used in intensive care unit (ICU) patients.The purpose of this study was to analyze the effect of TTE on the prognosis of ICU patients with ARDS.
Methods
The data of ARDS patients were retrieved from the MIMIC-III v1.4 database and patients were divided into the TTE group and non-TTE group. The baseline data were compared between the two groups. The effect of TTE on the prognosis of ARDS patients was analyzed through multivariate logistic analysis and the propensity score (PS). The primary outcome was the 28-d mortality rate. The secondary outcomes included pulmonary artery catheter (PAC) and Pulse index continuous cardiac output (PiCCO) administration, the ventilator-free and vasopressor-free days and total intravenous infusion volume on days 1, 2 and 3 of the mechanical ventilation. To illuminate the effect of echocardiography on the outcomes of ARDS patients,a sensitivity analysis was conducted by excluding those patients receiving either PiCCO or PAC. We also performed a subgroup analysis to assess the impact of TTE timing on the prognosis of patients with ARDS.
Results
A total of 1,346 ARDS patients were enrolled, including 519 (38.6%) cases in the TTE group and 827 (61.4%) cases in the non-TTE group. In the multivariate logistic regression, the 28-day mortality of patients in the TTE group was greatly improved (OR 0.71, 95%CI 0.55–0.92, P = 0.008). More patients in the TTE group received PAC (2% vs. 10%, P < 0.001) and the length of ICU stay in the TTE group was significantly shorter than that in the non-TTE group (17d vs.14d, P = 0.0001). The infusion volume in the TTE group was significantly less than that of the non-TTE group (6.2L vs.5.5L on day 1, P = 0.0012). Importantly, the patients in the TTE group were weaned ventilators earlier than those in the non-TTE group (ventilator-free days within 28 d: 21 d vs. 19.8 d, respectively, P = 0.071). The Kaplan–Meier survival curves showed that TTE patients had significant lower 28-day mortality than non-TTE patients (log-rank = 0.004). Subgroup analysis showed that TTE after hemodynamic disorders can not improve prognosis (OR 1.02, 95%CI 0.79–1.34, P = 0.844).
Conclusion
TTE was associated with improved 28-day outcomes in patients with ARDS.
Similar content being viewed by others
Introduction
Acute respiratory distress syndrome (ARDS) is associated with adverse clinical outcomes and has an approximate overall mortality rate of 40%, despite the most standard treatment. ARDS patients have been shown to have a good tolerance to relative hypoxemia. However, their survival rate is not necessarily increased by the improvement of oxygenation [1]. ARDS patients frequently suffer from circulatory failure, which is independently related to death [2] and often accompanied by hemodynamic instability, such that more than 60% of the patients suffer from hemodynamic disorders, among which 65% need to use catecholamine drug [3, 4]. ARDS causes shock according to the following three factors: (1) pulmonary arterial pressure (PAP) elevation caused by vasoconstriction due to microthrombosis, arterial remodeling and hypoxia, acidosis and/or inflammatory mediators; (2) the impact of mechanical ventilation on the function of the right ventricle (RV); (3) RV failure caused by abnormal tissue oxygen demand and hemodynamic disorders due to sepsis, which probably results from insufficient preload or excessive afterload. Assessing the volume status and correcting hemodynamic disorders helps to avoid the second attack based on hypoxemia and represents an important part of the ARDS treatment [5].
Hemodynamic monitoring of ARDS patients contributes to developing reasonable therapeutic regimens and improving the prognosis. There are many methods for hemodynamic monitoring and evaluation of heart function, among which pulse index continuous cardiac output (PiCCO), pulmonary artery catheter (PAC) and echocardiography are the three most commonly used methods and help to guide treatment adjustments. However, the invasive nature of monitoring and the requirements for equipment, technology, and operators severely limit the PiCCO and PAC clinical application, while there are also hidden dangers of complications such as blood flow infection, arrhythmia, pulmonary embolism, pulmonary arterioles rupture and hemorrhage, airbag rupture, catheter knot, etc. during the placement of the catheter, and are expensive. Moreover, studies have shown that PiCCO-based fluid management does not improve outcomes compared to central venous pressure (CVP)-based fluid management [6]. Likewise, a prospective cohort study have shown PAC was even associated with increased mortality and increased utilization of resources [7]. Echocardiography, a minimally invasive and repeatable hemodynamic monitoring tool, has become increasingly essential in the management of ARDS because it can not only help differentiate the causes of shock, but it can also provide real-time information of volume status and cardiac function. It is the best bedside method to repeatedly assess cardiac function, and the rapid progress in ultrasonic techniques for critical diseases has made it possible to achieve early diagnosis and prognostic evaluation of ARDS patients. Doctors can judge the disease severity and promptly adjust the treatment plan with the help of transthoracic echocardiography (TTE).
Despite these advances, the rationality of TTE for ARDS patients has not yet been assessed, and active adjustment of the treatment plan based on the results of ultrasonography is not always achieved by doctors [8]. In addition, preoperative echocardiography was found unable to reduce mortality and the length of stay for patients undergoing non-cardiac surgery [9]. Some studies also suggested that TTE cannot improve the Acute Physiology and Chronic Health Evaluation II(APACHEII) score for the risk of death in critically ill patients [10].
In this study, we perform a retrospective analysis to observe whether TTE can affect the short-term prognosis and related indexes of intensive care unit (ICU) patients with ARDS. This work provides a definite basis for the rational use of TTE in clinical practice, reducing the overuse of it and optimizing the allocation of medical resources.
Methods
Study population
The data in this study came from the MIMIC-III database. MIMIC-III is an open access medical database, jointly released by the Massachusetts Institute of Technology(MIT) Laboratory for Computational Physiology, Beth Israel Deaconess Medical Center, Philips Medicine and the National Institutes of Health [11]. It contains the hospitalization information of more than 50,000 patients admitted to Beth Israel Deaconess Medical Center from June 2001 to October 2012, including the vital signs, drugs, laboratory test results, clinical observation results, records made by nurses, fluid balance, imaging reports, length of stay and survival data.
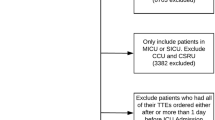
All the patients in the database were screened. Inclusion criteria were as follows: (1) adults aged ≥ 18 years old, with complete medical records, including ultrasound reports during the ICU stay; (2) Length of ICU stay ≥ 72 h; (3) Meeting the Berlin criteria for ARDS [12]. ARDS is defined as follows: acute attack, oxygenation index ≤ 300 mmHg, bilateral infiltration on chest X-ray and which the lung edema pattern could not be explained by heart failure or fluid overload. We use the following coding book of the database to define ARDS population that met Berlin definition: (1) Onset of ARDS is acute—we assume this as our cohort is only recently mechanically ventilated patients (i.e. exclude trach) (2) Bilateral opacities—we parse the free-text radiology reports for mention of opacities/infiltrates (3) PEEP > 5cmH2O. To exclude cardiogenic lung edema, we first excluded patients with heart failure on admission, and then use the coding book of the database to extract the information about ARDS patients, including echocardiography, and reviewed. Based on the oxygenation index, ARDS is classified into mild, moderate and severe degrees. For patients admitted to the ICU several times, only the data related to the first ICU admission were considered.
Data extraction
Rather than the assessment of volume and cardiac function in ARDS patients, echocardiography can also be used for the routine assessment of ICU admission. Thus, only the patients who underwent echocardiography within 24 h of mechanical ventilation were enrolled in the study. The enrolled ARDS patients were divided into Echo_1 group and non-Echo_1 group according to whether echocardiography was performed within 24 h of mechanical ventilation. In addition, in order to assess the timing of echocardiography, the patients were also divided into the Echo_2 group and non-Echo_2 group according to whether echocardiography was performed within 24 h after shock (Fig. 1). The variables we extracted or calculated including the baseline characteristics, comorbidities, mean vital signs within 24 h after entering the ICU, and parameters on the first day of mechanical ventilation. The first 24 h after entering the ICU (ie the baseline value) and the extreme values during the ICU stay [ie the maximum and minimum values]. All patients were evaluated for the severity score of organ dysfunction within 24 h after admission.The details of collected data are listed in Additional file 1: Table S1.
The value of lactic acid was not collected in more than 30% of patients in this cohort. Thus, using it directly as a covariate would result in a large number of missing values. Therefore, it was used as a covariate for stratification.
Outcome indexes
The primary outcome in this study was the 28-d post-admission mortality rate. The secondary outcomes included PAC and PiCCO administration, the ventilator- and vasopressor-free days within 28 d after ICU admission and total intravenous infusion volume on days 1, 2 and 3 of the mechanical ventilation.
Statistical methods
The data were expressed as mean ± standard deviation, median of interquartile range and ratio (absolute and relative frequency) according to the actual conditions. Continuous data was assessed for normal distribution by Skewness-kurtosis test (sktest) and compared using the Student's t test or Mann–Whitney test, while categorical variables were compared using the χ2 test or Fisher’s exact test. Statistical analyses were conducted using Stata (version 15.0, StataCorp, College Station, Texas). The propensity score (PS) of patients undergoing TTE was assessed using the psestimate command to minimize the imbalance of variables between the TTE and non-TTE groups [13]. With PS as the weight, the weighted queue was generated by the inverse probability weighting (IPW) model. A P < 0.05 was considered to be statistically significant [14].
Multivariate logistic regression was performed on the association between echocardiography and mortality using full models to evaluate the independent effect of echocardiography on the prognosis of patients with ARDS. The final models was built using a stepwise backward elimination with a significance level of 0.05. Potential multicollinearity was tested using a variance inflation factor, with a value of ≥ 5 indicating multicollinearity. Additionally, the log-rank test in the Kaplan-Meter survival analysis was used to compare the different survival rates between each group To exclude the confounding effect of PiCCO and PAC, we performed the sensitivity analysis by excluding patients who receiving either PiCCO or PAC.
Subgroup analysis
For the Echo_2 group, we performed a propensity score to balance the baseline differences between the two groups, and then performed a χ2 test and multivariate logistic regression to clarify the relationship between TTE timing and 28-d mortality.
Results
Among the 46,476 ICU patients with 61,532 data elements (times of ICU admission) in the MIMIC-III v1.4 database, the Berlin criteria were met in 2,224 elements, and 1,346 patients were finally enrolled in the study and divided into the Echo_1 group: 519 (38.56%) cases, including 219 women (42.2%), and non-Echo_1 group: 827 (61.44%) cases, including 334 women (40.4%). The characteristics of this cohort are shown in Table 1. The simplified acute physiology score(SAPS) and Sequential Organ Failure Assessment(SOFA) scores were higher in the Echo_1 group than those in non-Echo_1 group (SAPS: 44 points vs. 40 points, respectively, P < 0.001) (SOFA: 7 points vs. 6 points, respectively, P < 0.001). Besides, the proportions of patients receiving mechanical ventilation (MV) and using vasoactive drugs (VD) were significantly higher in Echo_1 group than those in non-Echo_1 group (MV: 89.4% vs. 84.0% respectively) (VD: 51.6% vs. 42.8% respectively). It can be seen that the patients undergoing TTE have severer disease than those without TTE (see Table 1 for details). Before PS matching, there were differences in the following parameters between the Echo_1 and non-Echo_1 groups: oxygenation index, positive end expiratory pressure(PEEP), systolic blood pressure, mean arterial pressure(MAP), plateau pressure, Ca2+, pH, age, respiratory rate, ARDS severity, ICU type, admission type and the presence or absence of sepsis, SOFA scores, SAPS II score, Oxford Acute Severity of Illness Score (OASIS) and Elixhauser comorbidity score [15,16,17,18] (Table 1). After PS matching (1:1) between 440 patients undergoing echocardiography and 440 patients without echocardiography, the imbalance between the Echo_1 and non-Echo_1 groups was significantly reduced (Additional file 2: Fig. S1), and all baseline variables were comparable between the two groups (Table 1). After matching, the 28-d mortality rate significantly declined in the Echo_1 group compared with the non-Echo_1 group (25.9% vs. 35.2%, respectively, P = 0.003). The infusion volume in the TTE group was significantly smaller than that in non-TTE group on day 1 (6.1 L vs. 5.5 L, respectively, P = 0.028) and day 3 (3.2 L vs. 2.7 L, respectively, P = 0.008). The TTE group received more PAC(2% vs. 10%, P < 0.001), whereas there were no differences on the use of PiCCO between the two groups. The time of using vasopressor and ventilator was significantly shorter in the TTE group than in the non-TTE group (vasopressor-free days within 28 d: 26.8 d vs. 27.3 d, respectively, P = 0.033, ventilator-free days within 28 d: 17 d vs. 19.8 d, respectively, P = 0.0202). No statistically significant differences were observed in other secondary endpoints between the two groups (Table 2).
The univariate logistic regression analysis for 28-d mortality was shown in Additional File 3: Table S2. There was a significant association between patients’ variables (Blood urea nitrogen, Arterial pH.
Plateau pressure, SOFA score, Malignancy, Temperature, Mean respiratory rate, Systolic blood pressure, Age) and 28-d mortality. In multivariate logistic regression analysis, after adjusting for the listed clinical confounders, we found that TTE patients had a significantly lower 28-day mortality risk than non-TTE patients (OR 0.71, 95% CI 0.55–0.91, P = 0.007) (Table 3).
Kaplan–Meier curves showed that TTE was strongly associated with improved survival (P = 0.004 by log-rank test, Fig. 2).
Subgroup analysis
Before PS matching, there were differences between the Echo_2 group and non-Echo_2 group in the following parameters: the oxygenation index, PEEP, systolic blood pressure, MAP, plateau pressure, peak airway pressure, blood urea nitrogen(BUN), weight, minute volume, pH, SOFA score, SAPSII, OASIS, respiratory rate, ARDS severity, ICU type, lactic acid and the presence or absence of sepsis (Additional file 4: Table S3). After PS matching (1:1) between 314 patients undergoing echocardiography and 316 patients without echocardiography, the imbalance between the Echo_2 and non-Echo_2 groups was significantly reduced and all baseline variables were comparable between the two groups (Additional file 5: Fig S2). After matching, there was no significant difference in the 28-d mortality rate between the Echo_2 and non-Echo_2 groups (32.9% vs. 29.9%, respectively, P = 0.418). The analysis results of secondary endpoints revealed a significantly shorter length of stay in the TTE group than that in the non-TTE group (12.5 d vs. 11 d, respectively, P = 0.017), while other secondary endpoints showed no statistically significant differences between the two groups (Table 4) (Additional file 5: Fig. S2).
In multivariable logistic regression analysis, after adjusting for the clinical confounders listed, we found that TTE patients compared with non-TTE patients had not association with the 28-d mortality (OR 1.02, 95%CI 0.79–1.34, P = 0.844)(Table 3).
Senitivity analysis
To illuminate the impact of echocardiography on the outcomes of ARDS patients, we performed a sensitivity analysis after excluding patients received with PAC or PiCCO. There was a significant difference in the 28-d mortality rate between the TTE and non-TTE groups (27.2% vs. 35.9%, respectively, P = 0.011). Kaplan–Meier curves showed that TTE was strongly associated with improved survival (P = 0.017 by log-rank test, Fig. 3).
Discussion
ARDS is a life-threatening pulmonary disease with a poor prognosis and an increased mortality rate [19, 20]. Such an adverse outcome may result from circulatory failure rather than hypoxemia [2]. Therefore, hemodynamic monitoring plays a very important role in the management of ARDS patients. Clinically widely used hemodynamic monitoring strategies, including CVP, PAC, and PiCCO. However, when CVP is within a relatively normal range, the ability of prediction to guide fluid management is limited [21]. Therefore, it is not reasonable to use CVP alone to monitor hemodynamics [22] As for PAC, previous studies have shown no significant improvement on mortality [23,24,25] and even more complications than central venous catheter guided therapy [26]. The risk of the PiCCO cannulation limits its use in critically ill or high-risk patients with complex and severe hemodynamic impared [27] Furthermore, it does not improve patient outcomes compared with CVP [6], 28] This is because the decisive question is how clinicians use the information obtained for subsequent management. Critical ultrasound examination is a rapid, non-invasive and reproducible operation, with a dynamic and visual presentation of the results, combining the monitoring results and diagnosis and treatment thoughts for critical disease. It plays an increasingly important role in the clinical diagnosis and treatment of ARDS.
Previous studies mainly focused on its diagnosis and treatment of ARDS, while the influencing factors for its prognosis and the value of ultrasonography for the prognostic evaluation have rarely been studied. It is of great significance to determine the actual application value of the auxiliary examination for the patients. First, there should be good reasoning for the patients requiring examination. Second, unnecessary examination and occupation of the medical resources should be avoided to ease the economic burden on the individuals, society and country. Therefore, continuous testing in clinical practice is needed for any new technique to determine its application value. The MIMIC-III database, established based on the electronic medical record, is a continuously updated dynamic data system, which reflects the diagnosis and treatment process of critically ill patients and has been commonly used by the researchers of intensive care medicine [29].To the best of our knowledge, this is the first study assessing the effect of echocardiography on the prognosis of ARDS patients. In this work, patients undergoing TTE had a higher disease severity score and more comorbid conditions, suggesting a severer disease degree. It was found that the 28-d mortality rate of patients undergoing TTE significantly declined after adjusting the confounding factors. Several hypotheses were proposed to explain the survival benefit, and some variables were compared between the TTE and non-TTE groups. The infusion volume in the TTE group was smaller at day 1 and day 3 after ventilation. Vasopressors were used more often in the TTE group, probably because TTE promotes the timely use of vasopressors. Patients in the TTE group also stopped taking vasopressors earlier than those in non-TTE group, which could be related to several factors according to the analysis of PS matching. First, studies have shown that acute pulmonary heart disease occurs in 20–25% of ARDS patients [30], and many obstructive factors, including hypoxia-induced pulmonary vasospasm, hypercapnia, high airway pressure, inflammatory factor-induced vasoconstriction and lung volume collapse cause an increased pulmonary vascular resistance, which greatly affects the right heart function and pulmonary circulatory resistance [31]. During positive pressure ventilation, pulmonary vessels are compressed by the stretched alveoli, which leads to increased pulmonary artery resistance and obviously reduces the pulmonary circulation blood flow [32]. The increase in PEEP raises the pulmonary vascular resistance, which leads to right heart dysfunction and may eventually result in the occurrence and development of shock. Echocardiography assessment of the right heart function during treatment can reveal the major cause of hemodynamic involvement or instability, because the status of the right heart involvement greatly varies among different types of shock, which directly affects the development and implementation of the clinical therapeutic regimen. Second, in terms of fluid management, right heart enlargement can be caused by an acute increase in the blood volume. Acute right heart enlargement occurs when there is a failure of compensatory fluid discharge due to renal insufficiency or low MAP. This results in left ventricular diastolic restriction through the ventricular septum and the pericardium, increasing the left ventricular filling pressure, and thus the extravascular lung water [33].The fluid management strategy test of the ARDS Collaboration in 2006 well established that patients with ARDS can mostly benefit from the conservative fluid management strategy through shock correction (vasopressor dependence), keeping the circulatory stability and guaranteeing organ perfusion. Although the conservative strategy does not reduce the 60-d mortality, it can shorten the duration of mechanical ventilation and length of ICU stay and ameliorate oxygenation without increasing the incidence of other organ dysfunction [34]. Moreover, a systematic review and meta-analysis covering 2051 patients with sepsis and/or ARDS in 11 randomized trials in 2017 found no significant difference in the mortality rate between the restricted fluid management group and routine treatment group [35]. However, the ventilator-free duration was found to be significantly increased and the length of ICU stay was significantly shortened in the restricted fluid management group. Our study showed that the infusion volume was smaller in the TTE group, which may have contributed to the improved survival. Third, vasoactive drugs are an important treatment means to lower the pulmonary circulatory pressure. As the distribution of related receptors varies, attention should be paid to the different effects of vasoactive drugs on pulmonary circulation and systemic circulation. Generally, vasodilators may also affect the systemic circulation when they act on the pulmonary circulation, a contradiction that may be sharper in severely ill patients. On the one hand, vasodilators expand the pulmonary artery and lower the pulmonary circulatory resistance, which supports the restoration of the right heart function and reduces the central venous pressure (CVP). On the other hand, vasodilators reduce the systemic circulation pressure, leading to circulatory instability. In particular, when less obvious decline in PAP and obvious decline in the systemic circulation pressure are observed when vasodilators are used, the transseptal pressure will be altered, which results in a leftward shift of the interventricular septum, a significant decrease in the left ventricular end-diastolic volume and a decline in the cardiac output. As a result, the systemic circulation pressure is further decreased, leading to an autonomous vicious cycle of the right heart [36]. It has been confirmed that these drugs can be applied under the guidance of tricuspid annular plane systolic excursion(TAPSE), right/left ventricular area ratio and eccentricity index [30]. The above-mentioned discussion may include the reasons for the improved mortality of ARDS patients by ultrasonography.
In addition, the patients were divided into the Echo_2 group and non-Echo_2 group according to the time of ultrasonography. Unlike the Echo_1 group, patients in the Echo_2 group showed no improvement regarding the clinical outcome, with no significant difference in the 28-d mortality between the Echo_2 group and control group. It is believed that ultrasonography should be performed as soon as possible on ARDS patients to reduce the mortality rate to the largest extent. However, no relevant studies have been presented. Hence, larger-scale prospective randomized controlled trials should be performed in the future to determine the timing of ultrasonography for ARDS patients. Although early assessment will not necessarily help to avoid further lung injury throughout the course of the disease, it can enable us to adjust the ventilation strategy, thereby ameliorating the prognosis. Based on our experience, we can suggest that ALI or ARDS patients receive ultrasonography at the time of ICU admission, and regular review should be performed according to the disease condition. Echocardiography at admission can provide valuable information, not only about the current clinical conditions, but also about preexisting diseases (e.g., severe right ventricular hypertrophy suggests the presence of chronic lung disease). If the condition is stable, then echocardiography should be performed at least once a week before weaning and after extubation (i.e., fluid therapy monitoring). Moreover, echocardiographic can assist in determining the cause (or auxiliary factor) of the progressive respiratory failure, such as systolic pulmonary artery pressure(sPAP) elevation, right ventricular dilatation, progressive right or left ventricular failure or individualized treatment. Re-examination (ventilation and non-ventilation) should be performed whenever right ventricular dilation or dysfunction is developed (even by conventional echocardiography), other options (i.e., inhalation of nitric oxide and prone position) should also be considered, and patients should be monitored more closely using echocardiography.
Our study had some limitations. First, despite the large sample size of real data, the data came from a single medical center, and there may be deviations among the subjects in the medical level, habits and population. Second, this was a retrospective analysis, so a large amount of data might have been eliminated, and there might have been a selection bias due to the lack of key information and other reasons during data extraction. Although TTE is a non-invasive and convenient operation, it has a poor repeatability. We could not assess the consistency of TTE in this study, resulting in a measurement bias. Therefore, we suggest designing a prospective multi-center study based on similar studies to further observe the effects of TTE on patients with severe ARDS. Third, although the 28-d mortality rate was explored, some significant outcome variables were not considered in the analysis, including long-term mortality and ICU readmission. Finally, the MIMIC-III database included the cases before 2012. Some studies suggest expanding the Berlin criteria for ARDS to include patients who have undergone high-flow nasal oxygen therapy (at least 30 L/min) and meet other standards in the Berlin criteria [37], which may lead to a result deviation in this study.
Conclusion
TTE was associated with improved 28-day outcomes of critically ill ARDS patients. Its mechanism remains to be explored, but under the guidance of the TTE results, we suggest that it may be related to the evaluation of right heart function and adjustment of fluid and vasoactive drugs. In future work, more large-scale prospective studies are needed to explore the influencing mechanism of ultrasound to the long-term prognosis of patients with ARDS and the timing of ultrasound use.
Availability of data and materials
The datasets analysed during the current study are available in the MIMIC-III repository, https://physionet.org/content/mimiciv/0.4/.
Abbreviations
- MIMIC-III:
-
The medical information mart for intensive care III
- ARDS:
-
Acute respiratory distress syndrome
- TTE:
-
Transthoracic echocardiography
- ICU:
-
Intensive care unit
- PS:
-
Propensity score
- OR:
-
Odds ratio
- PAP:
-
Pulmonary arterial pressure
- RV:
-
Right ventricle
- CT:
-
Computer tomography
- APACHEII:
-
Acute physiology and chronic health evaluation II
- MIT:
-
Massachusetts institute of technology
- IPW:
-
Inverse probability weighting
- SAPS:
-
Simplified acute physiology score
- SOFA:
-
Sequential organ failure assessment
- MV:
-
Mechanical ventilation
- VD:
-
Vasoactive drugs
- PEEP:
-
Positive end expiratory pressure
- MAP:
-
Mean arterial pressure
- OASIS:
-
Oxford acute severity of illness score
- BUN:
-
Blood urea nitrogen
- CVP:
-
Central venous pressure
- TAPSE:
-
Tricuspid annular plane systolic excursion
- sPAP:
-
Systolic pulmonary artery pressure
- CHF:
-
Congestive heart failure
- AFIB:
-
Atrial fibrillation
- CKD:
-
Chronic kidney disease
- COPD:
-
Chronic obstructive pulmonary disease
- CAD:
-
Coronary artery disease
- SpO2:
-
Pulse oxygen saturation
- PO2:
-
Oxygen partial pressure
- PCO2:
-
Carbon dioxide partial pressure
- PAC:
-
Pulmonary artery catheter
- PiCCO:
-
Pulse index continuous cardiac output
References
Mehdi Mezidi CG. Conservative versus liberal oxygenation targets for mechanically ventilated patients-a pilot multicenter randomized controlled trial. J Thorac Dis. 2016;8(3):307–10. https://doi.org/10.21037/jtd.2016.02.47.
Antoine Vieillard-Baron EG, Valente Elisabeth, Brun-Buisson Christian. Predictors of mortality in acute respiratory distress syndrome. Focus on the role of right heart catheterization. Am J Respir Crit Care Med. 2000;161:1597–601.
McAuley DF, Laffey JG, O’Kane CM, Perkins GD. Simvastatin in the acute respiratory distress syndrome. N Engl J Med. 2014;371:1695–703. https://doi.org/10.1056/NEJMoa1403285.
Charron C, Bouferrache K, Caille V, Castro S, Aegerter P, Page B, Jardin F, Vieillard-Baron A. Routine prone positioning in patients with severe ARDS_ feasibility and impact on prognosis. Intensive Care Med. 2011;37:785–90. https://doi.org/10.1007/s00134-011-2180-x.
Repesse X, Charron C, Vieillard-Baron AX, Repessé CC, Vieillard-Baron A. Right ventricular failure in acute lung injury and acute respiratory distress syndrome. Min Anestesiologica. 2012;78(8):941–8.
Zhongheng Zhang HN. Zhixian Q (2015) Effectiveness of treatment based on PiCCO parameters in critically ill patients with septic shock and_or acute respiratory distress syndrome_ a randomized controlled trial. Intensive Care Med. 2015;41:444–51. https://doi.org/10.1007/s00134-014-3638-4.
Connors AF Jr, Speroff T, Dawson NV. The effectiveness of right heart catheterization in the initial care of critically ill patients SUPPORT Investigators. JAMA. 1996;276:889–97. https://doi.org/10.1001/jama.276.11.889.
Matulevicius SA, Rohatgi A, Das SR, Price AL, Reimold SC. Appropriate use and clinical impact of transthoracic echocardiography. JAMA Intern Med. 2013;173:1600. https://doi.org/10.1001/jamainternmed.2013.8972.
Wijeysundera DN, Beattie WS, Karkouti K, Neuman MD, Austin PC, Laupacis A. Association of echocardiography before major elective non-cardiac surgery with postoperative survival and length of hospital stay_ population based cohort study. BMJ. 2011;342:d3695. https://doi.org/10.1136/bmj.d3695.
Sawchuk CW, Wong DT, Kavanagh BP, Siu SC. Transthoracic echocardiography does not improve prediction of outcome over APACHE II in medical-surgical intensive care. Can J Anaesth. 2003;50:305–10. https://doi.org/10.1007/BF03017803.
Johnson AE, Pollard TJ, Shen L, Lehman LWH, Feng M, Ghassemi M, Moody B, Szolovits P, Anthony Celi L, Mark RG. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. https://doi.org/10.1038/sdata.2016.35.
Force TADT. Acute respiratory distress syndrome_ the Berlin Definition. JAMA. 2012;307:2526–33. https://doi.org/10.1001/jama.2012.5669.
Imbens G. Matching methods in practice__three examples. J Hum Resour. 2014;50(2):373–418. https://doi.org/10.3386/w19959.
Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–64. https://doi.org/10.1093/aje/kwn16.
Johnson AE, Kramer AA, Clifford GD. A new severity of illness scale using a subset of acute physiology and chronic health evaluation data elements shows comparable predictive accuracy. Crit Care Med. 2013;41(1):711–8. https://doi.org/10.1097/CCM.0b013e31828a24fe.
van Walraven C, Austin PC, Jennings A. A modification of the elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–33. https://doi.org/10.1097/MLR.0b013e31819432e5.
Takala J-LVRMJ. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction_failure. Intensive Care Med. 1996;22:707–10. https://doi.org/10.1007/BF01709751.
Knaus WA, Wagner DP, Draper EA. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. CHEST. 1991;100:1619–36. https://doi.org/10.1378/chest.100.6.1619.
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, Van Haren F, Larsson A, McAuley DF, Ranieri M. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. https://doi.org/10.1001/jama.2016.0291.
Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–304. https://doi.org/10.1056/NEJMoa1011802.
Eskesen TG, Wetterslev M, Perner A. Systematic review including re-analyses of 1148 individual data sets of central venous pressure as a predictor of fluid responsiveness. Intensive Care Med. 2016;42:324–32. https://doi.org/10.1007/s00134-015-4168-4.
Cecconi M, De Backer D, Antonelli M. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European society of intensive care medicine. Intensive Care Med. 2014;40:1795–815. https://doi.org/10.1007/s00134-014-3525-z.
Christian Richard JW, Anguel N. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome_ a randomized controlled trial. JAMA. 2003;290(20):2713–20. https://doi.org/10.1001/jama.290.20.2713.
Rhodes A, Cusack RJ, Newman PJ. A randomised, controlled trial of the pulmonary artery catheter in critically ill patients. Intensive Care Med. 2002;28:256–64. https://doi.org/10.1007/s00134-002-1206-9.
Shah MR, Hasselblad V, Stevenson LW. Impact of the pulmonary artery catheter in critically ill patients_ meta-analysis of randomized clinical trials. JAMA. 2005;294:1664–70. https://doi.org/10.1001/jama.294.13.1664.
Network ACT. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–24. https://doi.org/10.1056/NEJMoa061895.
Litton E, Morgan M. The PiCCO monitor_ a review. Anaesth Intensive Care. 2012;40:393–409. https://doi.org/10.1177/0310057X1204000304.
Zhong Yuanbo WJ, Fei S. ICU management based on PiCCO parameters reduces duration of mechanical ventilation and ICU length of stay in patients with severe thoracic trauma and acute respiratory distress syndrome. Ann Intensive Care. 2016. https://doi.org/10.1186/s13613-016-0217-6.
Weeks HL, Beck C, McNeer E, Williams ML, Bejan CA, Denny JC, Choi L. medExtractR_ A targeted, customizable approach to medication extraction from electronic health records. J Am Med Inform Assoc. 2020;27:407–18. https://doi.org/10.1093/jamia/ocz207.
Sundar Krishnan GAS. Acute right ventricular dysfunction_ real-time management with echocardiography. Chest. 2015;147(3):835–46. https://doi.org/10.1378/chest.14-1335.
Greyson CR. Pathophysiology of right ventricular failure. Crit Gare Med. 2008;36(Suppl.):S57–65. https://doi.org/10.1097/01.CCM.0000296265.52518.70.
Francois Jardin AVB. Right ventricular function and positive pressure ventilation in clinical practice from hemodynamic subsets to respirator settings. Intensive Care Med. 2003;29(9):1426–34. https://doi.org/10.1007/s00134-003-1873-1.
Mitchell JR, Whitelaw WA, Sas R, et al. RV filling modulates LV function by direct ventricular interaction during mechanical ventilation. Am J Physiol Heart Circ Physiol. 2005;289(2):H549-557. https://doi.org/10.1152/ajpheart.01180.2004.
The National Heart L, Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–75. https://doi.org/10.1097/sa.0b013e318149f920.
Silversides JA, Major E, Ferguson AJ, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 2017;43:155–70. https://doi.org/10.1007/s00134-016-4573-3.
Hrymak C, Strumpher J, Jacobsohn E. Acute RV failure in ICU: assessment and management. Can J Cardiol. 2017;33(1):61–71. https://doi.org/10.1016/j.cjca.2016.10.030.
Matthay MA, Thompson BT, Ware LB. The Berlin definition of acute respiratory distress syndrome: should patients receiving high-flow nasal oxygen be included? Lancet Respir Med. 2021;9(8):933–6.
Acknowledgements
We would like to thank the Massachusetts Institute of Technology and the Beth Israel Deaconess Medical Center for the MIMIC project.
Funding
This research was funded by Shaanxi Provincal key research and development project "The Application of Right Heart Protective Ventilation Strategy in Patients with Acute Respiratory Distress Syndrome" (Project Number: 2021SF-257).
Author information
Authors and Affiliations
Contributions
DDR and ZY conceptualized the research aims, planned the analyses, and guided the literature review. DDR extracted the data from the MIMIC-IV database. DDR, WC and WY participated in data analysis and interpretation. DDR wrote the first draft of the paper and the other authors provided comments and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Access to the database for research was approved by the Institutional Review Boards of the Massachusetts Institute of Technology (Cambridge, MA, USA) and the Beth Israel Deaconess Medical Center.
All patients’ information in the MIMIC-III database is anonymous, and informed consent is not required; this study is eligible for exemption after being reviewed by the Ethics Committee of Shannxi Provincial People’s Hospital. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Table S1.
Additional file 2.
Figure S1.
Additional file 3.
Table S2.
Additional file 4.
Table S3.
Additional file 5.
Figure S2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dong, D., Wang, Y., Wang, C. et al. Effects of transthoracic echocardiography on the prognosis of patients with acute respiratory distress syndrome: a propensity score matched analysis of the MIMIC-III database. BMC Pulm Med 22, 247 (2022). https://doi.org/10.1186/s12890-022-02028-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02028-5