Abstract
Background
Pneumocystis jirovecii pneumonia (PCP) with acute respiratory failure can result in development of pneumothorax during treatment. This study aimed to identify the incidence and related factors of pneumothorax in patients with PCP and acute respiratory failure and to analyze their prognosis.
Methods
We retrospectively reviewed the occurrence of pneumothorax, including clinical characteristics and results of other examinations, in 119 non-human immunodeficiency virus patients with PCP and respiratory failure requiring mechanical ventilator treatment in a medical intensive care unit (ICU) at a tertiary-care center between July 2016 and April 2019.
Results
During follow up duration, twenty-two patients (18.5%) developed pneumothorax during ventilator treatment, with 45 (37.8%) eventually requiring a tracheostomy due to weaning failure. Cytomegalovirus co-infection (odds ratio 13.9; p = 0.013) was related with occurrence of pneumothorax in multivariate analysis. And development of pneumothorax was not associated with need for tracheostomy and mortality. Furthermore, analysis of survivor after 28 days in ICU, patients without pneumothorax were significantly more successful in weaning from mechanical ventilator than the patients with pneumothorax (44% vs. 13.3%, p = 0.037). PCP patients without pneumothorax showed successful home discharges compared to those who without pneumothorax (p = 0.010).
Conclusions
The development of pneumothorax increased in PCP patient with cytomegalovirus co-infection, pneumothorax might have difficulty in and prolonged weaning from mechanical ventilators, which clinicians should be aware of when planning treatment for such patients.
Similar content being viewed by others
Background
The incidence of Pneumocystis jirovecii pneumonia (PCP) in patients without human immunodeficiency virus (HIV) has increased as more patients receive chemotherapy or immunosuppressive agents [1]. The disease progress in these patients is rapid, and the prognosis is worse compared to that of PCP patients with HIV [2]. Furthermore, when complicated by respiratory failure, the prognosis is poor and mortality rate is high [3].
Pneumothorax is one of the complications of PCP [4]. The prevalence of pneumothorax ranged from 13 to 61% in PCP patients with and without HIV in previous study [5]. There are several studies on the association between the occurrence of pneumothorax in PCP and prognosis in patients with HIV [6,7,8]. In one study of patients without HIV, development of pneumothorax was related to poor prognosis, including high acutely physiology and chronic health evaluation (APACHE) III scores, prolonged positive pressure ventilation, and intubation delay, in patients with acute respiratory failure complicating PCP [9]. And, the management of patients with PCP was more difficult when pneumothorax develops [10]. However, the relationship between pneumothorax and prognosis of PCP with acute respiratory failure in patients without HIV remains unclear [5].
The aim of this study was to analyze the incidence and related risk factors of pneumothorax in non-HIV patients with PCP and acute respiratory failure, and to further identify the clinical impact in those patients.
Methods
Study population
In this study, we retrospectively investigated the medical records of 1,210 patients who were admitted to a medical intensive care unit at a tertiary care university hospital in South Korea between July 2016 and April 2019. Patients with PCP without HIV who needed mechanical ventilation due to respiratory failure in ICU care were included. Three criteria were applied for the diagnosis of PCP: (1) immunocompromised status from chemotherapy, immunosuppressant usage, or long term use of steroid with clinical symptoms of pneumonia, such as cough, sputum, fever, and dyspnea; (2) P. jirovecii DNA must be confirmed using polymerase chain reaction (PCR) assays from patient sputum samples, endotracheal aspirates, or bronchoalveolar lavage fluids; and (3) radiologic finding from chest computed tomography (CT) representing typical patterns of PCP, including bilateral interstitial opacities, ground glass opacities, or septal thickening must be evident. P. jirovecii PCR-positive patients who did not receive treatment for PCP were excluded due to possible false positivity.
Data collection
Patient baseline characteristics, laboratory findings, and information on disease severity, such as APACHE II score, Sequential Organ Failure Assessment (SOFA) score, and Simplified Acute Physiology Score (SAPS) II, were collected from data obtained within 24 h of ICU admission. We reviewed the mechanical ventilator (MV) parameters, including respiratory rate, tidal volume (mL/predicted body weight), peak inspiratory pressure, positive expiratory end pressure (PEEP), dynamic driving pressure (the difference between peak inspiratory pressure and PEEP) within 24 h after MV initiation. We also reviewed peak inspiratory pressure three days after- and maximal peak pressure within a week after MV initiation. Concomitant Cytomegalovirus (CMV) antigenemia was decided through a quantitative PCR test. The cutoff value for clinically meaningful positive CMV PCR result was higher or equal 1500 copies/mL [11].
Occurrence of pneumothorax was evaluated via radiologic manifestations with chest radiography and CT. We investigated the prognosis of patients, including successful MV weaning outcomes, ICU length of stay, 28-day mortality and in-hospital mortality. Approval for this study was provided by the institutional review board of Yongin Severance Hospital (IRB 9-2021-0045). The need for informed consent was waived due to the retrospective nature of this study. This study was conducted in accordance with the tenets set by the Declaration of Helsinki.
Management of PCP
All study patients were administered intravenous or oral trimethoprim (15–20 mg/kg per day) and sulfamethoxazole (75–100 mg/kg per day) as first-line treatment for PCP. The duration of planned treatment was three weeks. We changed to second-line treatment using primaquine (15–30 mg/day) and clindamycin (1800 mg/day) or pentamidine (4 mg/kg per day) in patients who did not present clinical improvement. Twenty-one (17.6%) patients were treated with second-line medication due to no clinical improvement or side effects of the first-line medication. In addition, all patients were administered adjuvant corticosteroid (40 mg prednisolone twice daily for five days, followed by 40 mg prednisolone twice daily for five days, after which 20 mg prednisolone twice daily for 11 days was given). Patients requiring MV treatment for more than two weeks due to MV weaning failure underwent tracheostomy.
Statistical analysis
Categorical variables were presented as frequencies and percentages with comparisons done using a chi-square test. Continuous variables were presented as means and standard deviations if the distribution was normal, and as interquartile ranges (IQRs) if the distribution was not normal. For comparisons of continuous variables, a Student’s t-test and Mann–Whitney U test were used. A multivariable logistic regression analysis was performed with pre-specified covariates. Odds ratios (ORs) and 95% confidence intervals (CIs) were also calculated. A p-value < 0.05 was considered significant for all analysis. All data were analyzed statistically using IBM SPSS version 25.0 software (IBM Corp., Armonk, NY, USA).
Results
Study flow
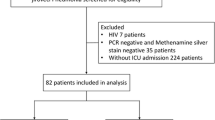
A total of 160 patients with clinically confirmed PCP and on treatment for it were enrolled. Forty one patients who had respiratory failure, but had not received MV treatment and had undergone tracheostomy before ICU admission were excluded; therefore, 119 patients were finally included in our study (Fig. 1).
Baseline characteristics of the study participants
The baseline characteristics of study patients are summarized in Table 1. Among the 119 patients reviewed, 76 (63.9%) were male and 43 (36.1%) were female. Median age was 65.0 (IQR, 56.0–72.0) years. Median APACHE II score was 26.5 (IQR, 20.0–32.0). Thirty-four patients (28.6%) were smokers, and 31 (26.1%) had underlying chronic lung diseases, including airway disease (n = 9, 7.3%) and interstitial lung disease (n = 22, 18.5%). The main cause of immunodeficiency was a solid cancer (n = 37, 31.1%), followed by immunosuppressive agent use (n = 27, 22.7%), and hematologic malignancy (n = 22, 18.5%). Forty-one patients (34.5%) needed continuous renal replacement therapy (CRRT) because of acute kidney injury. Concomitant CMV antigenemia was noted in 75 patients (63.0%).
Factors and outcomes associated with occurrence of pneumothorax
In total, 22 patients (18.5%) developed pneumothorax during MV treatment, with the median time from initiating ventilator management to development of pneumothorax being 8.0 days (IQR, 1.5–16.0). Table 2 shows comparisons of associated factors and outcomes, according to occurrence of pneumothorax. On univariate analysis, low body mass index (BMI), underlying airway disease, renal failure requiring CRRT, low procalcitonin level, low SOFA score, and CMV antigenemia were associated with the development of pneumothorax in patients with PCP and respiratory failure. However, there was no significant difference in MV parameters within 24 after MV initiation, including tidal volume, peak pressure, and dynamic driving pressure. Peak pressure at three days after MV initiation and maximal peak pressure seven days after MV initiation did not show statistical significance between those with and without pneumothorax. Furthermore, there was no significant difference in proportion of patients who had undergone tracheostomy and mortality rate, according to presence or absence of pneumothorax.
Age, sex, and variables related to pulmonary involvement, including BMI, underlying airway disease, and CMV antigenemia, with a p-value < 0.05 in the univariate analysis were used in the multivariate analysis. Low BMI (OR, 1.2; 95% 67 CI, 1.011–1.356) and CMV antigenemia (OR 13.9; 95% CI, 1.737–111.072) remained significant risk factors in the multivariate analysis.
The 28-day mortality in ICU and prognosis of PCP with respiratory failure
The 28-day mortality rate in ICU was 62.2% (n = 45). The in-hospital mortality rate was 71.4% (n = 85). Patients with renal failure requiring CRRT were at significantly higher mortality(p = 0.001). High disease severity, including high APACHE II score (p = 0.045), SOFA score (p < 0.001), and SAPS II (p = 0.015), was related to poor outcomes in patients with PCP. Regarding MV parameters, the peak pressure three days after admission (p = 0.003) and maximal peak pressure (p = 0.009) were higher in 28-day mortality group than in 28-day survivor groups. In the multivariate analysis, renal failure requiring CRRT (OR, 6.645; 95% CI, 1.967–22.446) was significantly associated with poor prognosis in patients with PCP and respiratory failure. (Table 3).
Clinical impact of pneumothorax in surviving PCP patients.
Of 119 patients, 45 (37.8%) underwent tracheostomy due to weaning failure. The need for tracheostomy for long term MV management did not significantly differ between patients with- and without pneumothorax (Table 2).
We further investigated on the 74 study patients who survived more than 28 days in ICU. Among them, 15 (20.3%) developed pneumothorax. There was no significant difference in tracheostomy rates between those with and without pneumothorax (Table 4). However, patients without pneumothorax were significantly more successful in weaning from MV than were patients with pneumothorax (44% vs. 13.3%, p = 0.037). The ICU length of stay was also longer in patients with pneumothorax, although there was no statistically significant difference (p = 0.068). Patients with PCP and pneumothorax were significantly associated with a poor prognosis, with a mortality of 33.3% (p = 0.048), and only two patients (13.3%) were eventually discharged home, compared to 25 (51.0%) successful home discharges among PCP patients without pneumothorax (p = 0.010, Table 4).
Discussion
This study described the incidence and related factors of pneumothorax in patients with PCP and their prognosis. In addition, we analyzed survival outcomes in patients with PCP and acute respiratory failure. The development of pneumothorax in PCP was not associated with increased 28-day mortality; however, patients with pneumothorax had difficulty and prolonged MV weaning. Among several factors, CMV co-infection was associated with the development of pneumothorax.
There are several complications associated with PCP [12], and pneumothorax occurs with prevalence of 5–20% [13]. The pathogenesis of pneumothorax in PCP was unclear, but had suggested to the tissue destruction from direct tissue toxicity by the pathogens, over-distention of bronchioles, and prolonged existence of macrophage with increased elastase and other enzymes [14]. As a results, cyst and bullae were formed, and parenchymal lung was vulnerable status to development of pneumothorax [6]. Pneumothorax is also a common complication during ventilator treatment, with a reported incidence of 4–15% [15,16,17]. Patients with acute respiratory distress syndrome are more vulnerable to the occurrence of pneumothorax, with further higher risks in patients with underlying lung disease, than those who do not have this syndrome [18]. In our study population of patients with acute respiratory failure requiring MV care, which is a risk factor for pneumothorax [19], the incidence of pneumothorax was 18.5% (22/119), which is high. Furthermore, underlying lung diseases, including airway disease and interstitial lung disease, showed relation to increased occurrence of pneumothorax in the univariate analysis, in accordance with a previous report [20].
We found that MV parameters were not related to the development of pneumothorax. Some studies showed that ventilator parameters, such as peak airway pressure, tidal volume, and PEEP, had no correlation with increased risk of pneumothorax [21,22,23], although earlier studies had reported relevance [24]. Miller et al. reported that protective lung strategies had an effect on decreasing barotrauma [25]. We had managed patients with tidal volumes of 6–7 mg/kg and peak pressures lower than 35 cmH2O, according to the lung protective strategy [26]. We showed that respiratory mechanics did not significantly affect the development of pneumothorax in patients with PCP when this lung protective strategy was applied. Furthermore, in one study, Boussarsar et al. said that barotrauma during MV care was more strongly associated with underlying lung conditions and compliance than with MV parameters [27].
In immunocompromised patients, PCP commonly presents with co-infection of other pathogens, especially CMV [28]. The reports on effect and outcome of CMV co-infection with PCP are controversial [29, 30], although there are several researches stating that concurrent infection of CMV is related to increased mortality and poor prognosis [31, 32]. Our study showed that CMV antigenemia was not associated with a high 28-day mortality rate; however, patients with CMV antigenemia were significantly associated with increased occurrence of pneumothorax. In our study populations, the effect of CMV reactivation on PCP prognosis is meaningful because the seropositivity of CMV in Koreans was reported as high as 94.1% [33]. Cook et al. suggested that CMV reactivation could cause abnormal cytokine/chemokine expression, resulting in pulmonary fibrosis in an animal model [34]. In one prior research on histopathological findings in 12 deceased patients with PCP, three had evidence of CMV co-infection, and two of them presenting with pulmonary fibrosis [35]. Furthermore, structural changes in lung parenchyma, including fibrosis, is one of proposed mechanisms of pneumothorax and increases vulnerability to the occurrence of pneumothorax [36].
Occurrence of PCP in patients without HIV has poorer progress and higher mortality than that in patients with HIV [37]. In our study, the in-hospital mortality rate was high at 71.4%, will only 28 patients (23.5%) achieving MV weaning. Pneumothorax in patients with PCP with- or without HIV was difficult to treat and had a worse prognosis than pneumothorax from other etiologies [9, 10, 19]. However, in our study, the development of pneumothorax was not associated with increased 28-day mortality. In a previous study, acute respiratory failure requiring invasive MV was found to be a risk factor for increased mortality; therefore, the effect of pneumothorax was likely to be lessened because all patients enrolled our study already had respiratory failure [37]. However, we found that development of pneumothorax in patients with PCP who required invasive MV procedures made weaning difficult.
Our study had several limitations. First, this study was retrospectively conducted at a single center. However, we enrolled a large number of patients with PCP and respiratory failure without HIV requiring invasive MV procedures. Furthermore, since all enrolled patients were applied MV, the bias of applying MV could be reduced. Second, we did not acquire microbiological findings from patient samples for diagnosis of PCP but only diagnosed via P. jirovecii PCR assays. However, the sensitivity and specificity of this assay for detecting P. jirovecii were comparable to those of microscopic staining [38]. Furthermore, we included only patients who were treated for PCP with typical symptoms of pneumonia and characteristic radiological findings. Third, all patients did not perform the chest CT and followed up chest CT. So, we did not identify the cystic changes of lung parenchyme that could affect the occurrence of pneumothorax in PCP patients. Fourth, the duration of air-leak that could affect the prognosis of PCP patients with pneumothorax and several parameter of mechanical ventilator including plateau pressure were not evaluate because our analysis was retrospective. Finally, in patients discharged to long-term outpatient care after survival, it was not possible to investigate final success of MV weaning.
Nevertheless, our study also had some strengths. We analyzed a large number of patients with PCP and acute respiratory failure, and found related factor of pneumothorax. ICU clinicians might predict that patients with PCP and CMV antigenemia have increased risk of pneumothorax. Additionally, development of pneumothorax in patients with PCP could be a predictive factor of delayed MV weaning and poor final outcome. These results could be helpful to the real clinical field ICU clinician. However, further prospective studies are needed to validate our findings.
Conclusion
The results of this study suggest that patients with PCP who develop pneumothorax might have difficulty and delayed weaning from MV. Concomitant CMV antigenemia could be a predictive factor for pneumothorax occurrence. Therefore, clinicians need to closely observe the occurrence of pneumothorax in patient with PCP and CMV antigenemia and should anticipate that MV weaning may be difficult in such patients.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PCP:
-
Pneumocystis jirovecii pneumonia
- ICU:
-
Intensive care unit
- HIV:
-
Human immunodeficiency virus
- APACHE:
-
Acutely physiology and chronic health evaluation
- PCR:
-
Polymerase chain reaction
- CT:
-
Computed tomography
- SOFA:
-
Sequential Organ Failure Assessment
- SAPS:
-
Simplified Acute Physiology Score
- MV:
-
Mechanical ventilator
- PEEP:
-
Positive expiratory end pressure
- IQRs:
-
Interquartile ranges
- ORs:
-
Odds ratios
- CIs:
-
Confidence intervals
- CRRT:
-
Continuous renal replacement therapy
- CMV:
-
Cytomegalovirus
- BMI:
-
Body mass index
References
Varthalitis I, Aoun M, Daneau D, Meunier F. Pneumocystis carinii pneumonia in patients with cancer: an increasing incidence. Cancer. 1993;71(2):481–5.
Kim SJ, Lee J, Cho YJ, Park YS, Lee CH, Yoon HI, Lee SM, Yim JJ, Lee JH, Yoo CG, et al. Prognostic factors of Pneumocystis jirovecii pneumonia in patients without HIV infection. J Infect. 2014;69(1):88–95.
Boonsarngsuk V, Sirilak S, Kiatboonsri S. Acute respiratory failure due to Pneumocystis pneumonia: outcome and prognostic factors. Int J Infect Dis. 2009;13(1):59–66.
Tumbarello M, Tacconelli E, Pirronti T, Cauda R, Ortona L. Pneumothorax in HIV-infected patients: role of Pneumocystis carinii pneumonia and pulmonary tuberculosis. Eur Respir J. 1997;10(6):1332–5.
Kotani T, Katayama S, Miyazaki Y, Fukuda S, Sato Y, Ohsugi K. Risk Factors for the Mortality of Pneumocystis jirovecii Pneumonia in Non-HIV Patients Who Required Mechanical Ventilation: a Retrospective Case Series Study. Biomed Res Int. 2017;2017:7452604.
McClellan MD, Miller SB, Parsons PE, Cohn DL. Pneumothorax with pneumocystis carinii pneumonia in AIDS: incidence and clinical characteristics. Chest. 1991;100(5):1224–8.
Bedos J-P, Dumoulin J-L, Gachot B, Veber B, Wolff M, Regnier B, Chevret S. Pneumocystis carinii pneumonia requiring intensive care management: survival and prognostic study in 110 patients with human immunodeficiency virus. Crit Care Med. 1999;27(6):1109–15.
Coker RJ, Moss F, Peters B, McCarty M, Nieman R, Claydon E, Mitchell D, Harris JR. Pneumothorax in patients with AIDS. Respir Med. 1993;87(1):43–7.
Festic E, Gajic O, Limper AH, Aksamit TR. Acute respiratory failure due to pneumocystis pneumonia in patients without human immunodeficiency virus infection. Chest. 2005;128(2):573–9.
Ingram RJ, Call S, Andrade A, White C, Wheeler D. Management and outcome of pneumothoraces in patients infected with human immunodeficiency virus. Clin Infect Dis. 1996;23(3):624–7.
Weinberg A, Hodges TN, Li S, Cai G, Zamora MR. Comparison of PCR, antigenemia assay, and rapid blood culture for detection and prevention of cytomegalovirus disease after lung transplantation. J Clin Microbiol. 2000;38(2):768–72.
Teruya K, Yasuoka A, Yamaguchi M, Yasuoka C, Yamamoto Y, Genka I, Tachikawa N, Kikuchi Y, Oka S. Complications during clinical courses of Pneumocystis carinii pneumonia in patients with acquired immunodeficiency syndrome. Intern Med. 2001;40(3):221–6.
Ko Y, Jeong B-H, Park HY, Koh W-J, Suh GY, Chung MP, Kwon OJ, Jeon K. Outcomes of Pneumocystis pneumonia with respiratory failure in HIV-negative patients. J Crit Care. 2014;29(3):356–61.
Eng RHK, Bishburg E, Smith SM. Evidence for destruction of lung tissues during pneumocystis carinii infection. Arch Intern Med. 1987;147(4):746–9.
Petersen GW, Baier H: Incidence of pulmonary barotrauma in a medical ICU. CritCare Med. 1983, 11(2).
Zwillich CW, Pierson DJ, Creagh CE, Sutton FD, Schatz E, Petty TL. Complications of assisted ventilation: a prospective study of 354 consecutive episodes. Am J Med. 1974;57(2):161–70.
De Latorre FJ, Klamburg J, Leon C, Soler M, Rius J. Incidence of Pneumothorax and Pneumomediastinum in patients with aspiration pneumonia requiring ventilatory support. Chest. 1977;72(2):141–4.
Gammon RB, Shin MS, Groves RH Jr, Hardin JM, Hsu C, Buchalter SE. Clinical risk factors for pulmonary barotrauma: a multivariate analysis. Am J Respir Crit Care Med. 1995;152(4 Pt 1):1235–40.
She WH, Chok KSH, Li IWS, Ma KW, Sin SL, Dai WC, Fung JYY, Lo CM. Pneumocystis jirovecii-related spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema in a liver transplant recipient: a case report. BMC Infect Dis. 2019;19(1):66–66.
Gammon RB, Shin MS, Buchalter SE. Pulmonary barotrauma in mechanical ventilation. Patterns and risk factors. Chest. 1992;102(2):568–72.
Weg JG, Anzueto A, Balk RA, Wiedemann HP, Pattishall EN, Schork MA, Wagner LA. The relation of pneumothorax and other air leaks to mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):341–6.
Brochard L, Roudot-Thoraval F, Roupie E, Delclaux C, Chastre J, Fernandez-Mondéjar E, Clémenti E, Mancebo J, Factor P, Matamis D et al: Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med 1998, 158(6):1831–8.
Hsu C-W, Sun S-F. Iatrogenic pneumothorax related to mechanical ventilation. World J Crit Care Med. 2014;3(1):8–14.
Petersen GW, Baier H. Incidence of pulmonary barotrauma in a medical ICU. Crit Care Med. 1983;11(2):67–9.
Miller MP, Sagy M. Pressure characteristics of mechanical ventilation and incidence of pneumothorax before and after the implementation of protective lung strategies in the management of pediatric patients with severe ARDS. Chest. 2008;134(5):969–73.
Brower RG, Ware LB, Berthiaume Y, Matthay MA. Treatment of ARDS. Chest. 2001;120(4):1347–67.
Boussarsar M, Thierry G, Jaber S, Roudot-Thoraval F, Lemaire F, Brochard L. Relationship between ventilatory settings and barotrauma in the acute respiratory distress syndrome. Intensive Care Med. 2002;28(4):406–13.
Chou CW, Lin FC, Tsai HC, Chang SC. The impact of concomitant pulmonary infection on immune dysregulation in Pneumocystis jirovecii pneumonia. BMC Pulm Med. 2014;14:182.
Kim T, Moon SM, Sung H, Kim MN, Kim SH, Choi SH, Jeong JY, Woo JH, Kim YS, Lee SO. Outcomes of non-HIV-infected patients with Pneumocystis pneumonia and concomitant pulmonary cytomegalovirus infection. Scand J Infect Dis. 2012;44(9):670–7.
Miles PR, Baughman RP, Linnemann CC Jr. Cytomegalovirus in the bronchoalveolar lavage fluid of patients with AIDS. Chest. 1990;97(5):1072–6.
Korkmaz Ekren P, Töreyin ZN, Nahid P, Doskaya M, Caner A, Turgay N, Zeytinoglu A, Toz S, Bacakoglu F, Guruz Y, et al. The association between Cytomegalovirus co-infection with Pneumocystis pneumonia and mortality in immunocompromised non-HIV patients. Clin Respir J. 2018;12(11):2590–7.
Töreyin ZN, Ekren P, Caner A, Töz S, Gürgün A, Uluer H, Bacakoglu F. Prognosis of pneumocystis jirovecii pneumonia in non-HIV patients. Eur Respir J. 2013;42(Suppl 57):P2759.
Choi SR: Changes in Cytomegalovirus Seroprevalence in Korea for 21 Years: a Single Center Study. 2018, 25(3):123–131.
Cook CH, Zhang Y, Sedmak DD, Martin LC, Jewell S, Ferguson RM. Pulmonary cytomegalovirus reactivation causes pathology in immunocompetent mice. Crit Care Med. 2006;34(3):842–9.
Shaddock EJ, Richards GA, Murray J: Lung fibrosis in deceased HIV-infected patients with Pneumocystis pneumonia. 2012 2012, 13(2):4.
Zantah M, Dominguez Castillo E, Townsend R, Dikengil F, Criner GJ. Pneumothorax in COVID-19 disease- incidence and clinical characteristics. Respir Res. 2020;21(1):236.
Roux A, Canet E, Valade S, Gangneux-Robert F, Hamane S, Lafabrie A, Maubon D, Debourgogne A, Le Gal S, Dalle F, et al. Pneumocystis jirovecii pneumonia in patients with or without AIDS France. Emerg Infect Dis. 2014;20(9):1490–7.
Robert-Gangneux F, Belaz S, Revest M, Tattevin P, Jouneau S, Decaux O, Chevrier S, Le Tulzo Y, Gangneux JP. Diagnosis of Pneumocystis jirovecii pneumonia in immunocompromised patients by real-time PCR: a 4-year prospective study. J Clin Microbiol. 2014;52(9):3370–6.
Acknowledgements
Not applicable.
Funding
This work was supported by the Korea Medical Device Development Fund grant funded by the Korean government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project Number: 202011B26).
Author information
Authors and Affiliations
Contributions
Conceptualization: J.S.C., S.H.L., Data curation: J.S.C., S.H.L., S.H.Y., A.Y.L., S.Y.K., S.H.L., K.S.C., E.Y.K., J.Y.J., Y.A.K., M.S.P., Y.S.K., Formal analysis: J.S.C., M.C.K., C.H.S., S.H.L, Funding acquisition: A.Y.L., K.S.C., Y.S.K., S.H.L., Investigation: J.S.C., S.H.K., M.C.K., C.H.S., S.R.K., B.H.P., E.H.L., S.H.L, Methodology: J.S.C., S.H.L, Supervision: E.H.L., M.S.P., Y.S.K., S.H.L., Writing – original draft: J.S.C., S.H.L, Writing – review & editing: All authors reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval for this study was provided by the institutional review board of Yongin Severance Hospital (IRB 9-2021-0045). The need for informed consent was waived due to the retrospective nature of this study. This study was conducted in accordance with the tenets set by the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Choi, J.S., Kwak, S.H., Kim, M.C. et al. Clinical impact of pneumothorax in patients with Pneumocystis jirovecii pneumonia and respiratory failure in an HIV-negative cohort. BMC Pulm Med 22, 7 (2022). https://doi.org/10.1186/s12890-021-01812-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-021-01812-z