Abstract
Background
The newly described inflammatory burden index (IBI) reflects a patient’s inflammatory burden. This study aimed to estimate the association between IBI, osteoarthritis (OA), and all-cause mortality in patients with OA.
Methods
We extracted the data of adults from the National Health and Nutrition Examination Survey database between 1999 and 2018. After using appropriate survey weights to correct for sample bias, we conducted multivariate logistic regression analyses to explore the association between IBI and OA across three models: in the unadjusted model, partially adjusted model (adjusting age, sex, race, education level, marital status, PIR, BMI, smoking status, drinking status, stroke, CVD, DM, and hypertension) and fully adjusted model (which included additional variables: HBA1C, ALT, AST, BUN, TC, and HDL). And the odds ratios (OR) and 95% confidence intervals (CI) were calculated. Similarly, using comparable survey weights and covariates adjustments, we employed Cox proportional hazards regression analysis to investigate the association between IBI and all-cause mortality in the other 3 models. The Cox proportional hazards regression models were fitted to calculate the hazard ratios (HR) and 95% CI of the association between IBI and all-cause mortality. A restricted cubic spline (RCS) was used to explore the nonlinear relationships between association effects. Subgroup analysis was performed to validate the reliability of their effects.
Results
In total, 22,343 eligible participants were included. Multiple logistic regression models revealed that participants with the highest IBI had 2.54 times (95%CI, 2.23, 2.90)) higher risk of OA than those with the lowest IBI in Model 1, whereas the OR was 1.21 (95%CI, 1.03, 1.42) in Model 2 and 1.23 (95%CI,1.05, 1.45) in Model 3. Multiple Cox regression models showed participants with the highest IBI had 186% (95%CI, 1.50, 2.31) times risk of developing all-cause death than those with the lowest IBI in Model 1. This trend remained stable in Models 2 (HR,1.54; 95%CI,1.22, 1.95) and 3 (HR, 1.41; 95%CI, 1.10, 1.80). The RCS revealed a significant positive association between IBI and OA risk. With respect to the association between IBI and all-cause mortality, a slight decrease in mortality was observed from the lowest quartile to the second quartile of IBI, and the mortality risk increased with increasing IBI. Subgroup analyses showed that age, cardiovascular disease, and hypertension were pivotal in the association of IBI with all-cause mortality, whereas the association of IBI with OA remained stable after stratification by other factors such as sex, race, education level, marital, smoking, and drinking status, hypertension, and most serological indices.
Conclusions
This study provides evidence of a positive association between IBI, OA, and all-cause mortality. IBI may be a promising signature for assessing the inflammatory burden in patients with OA, which, in turn, is conducive to precise references for high-risk population recognition, anti-inflammatory guidance, and reducing mortality intervention.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is the most common chronic bone and joint wound that does not heal and chiefly affects the knees, hips, and hand joints of middle-aged and elderly people [1,2,3]. A recent report estimated that 240 million individuals suffer from symptomatic OA globally, including 10% of males and 18% of females aged 60 years and above [4]. Compared to non-OA patients, patients with OA are more likely to be sedentary have more complications, and have a 20% higher mortality rate after age adjustment [5, 6]. OA is characterized by progressive pain, joint stiffness, swelling, and deformity, and imposes a substantial burden on families, society, and the healthcare system because of its high disability rate and poor quality of life [7]. In the United States (US), approximately 800 billion USD was spent on OA in 2016 [8]. Current treatment strategies for OA include aerobic exercise, strength training, pain medication, and joint replacement [9, 10]. In the absence of disease-specific drugs and the small-to-moderate curative effects of symptom-relieving treatments, the focus should be prevention via early identification and proactive intervention of modifiable risk factors.
Activation of inflammatory responses is pivotal to the pathogenesis of OA in joints, involving inflammatory cell infiltration, inflammatory mediator production, and innate and adaptive immunity activation. However, the current understanding of the causes and how to link these specific changes to cartilage damage is limited [11]. A recent review indicated that the interplay of various infiltrating immune cells and other cells that reside in OA can aggravate a vicious joint cycle, leading to a high inflammatory burden in patients with OA, which promotes the development of a poor outcome [12]. Increased expression of proinflammatory cytokines such as C-reactive protein (CRP) and inflammatory cells such as neutrophils and lymphocytes can be observed in the synovial tissue, synovial fluid, and peripheral blood of patients with OA [13,14,15]. Based on these indicators, a novel biomarker, the inflammatory burden index (IBI), has been proposed to quantify the degree of inflammation and has great clinical applicability. Xie et al. initially developed the IBI to evaluate the inflammatory load and predict the prognosis of cancers in a multicenter study of 6,359 patients with different cancers, confirming that IBI is a feasible prognostic biomarker for cancer [16]. Ding et al. used an external prospective cohort of 103 patients with locally advanced gastric cancer to verify the generalization of the IBI [17]. Similar findings have been reported in patients with non-small cell lung cancer [18]. In a multicenter Chinese cohort study, 295 patients with acute ischemic stroke treated with endovascular thrombectomy demonstrated that a higher IBI level was associated with an increased risk of a 90-day adverse outcome [19]. Although an increasing number of inflammatory biomarkers have been identified to indicate the severity of inflammation and predict the prognosis of OA, the optimal inflammatory indicators for comprehensive evaluation of the inflammatory burden still need to be explored and applied clinically.
Previous studies have utilized a variety of demographic, lifestyle, and health-related variables to investigate arthritis and related health outcomes. Common variables across these studies include age, sex, race/ethnicity, education, BMI, physical activity, smoking status, alcohol consumption, diabetes, hypertension, and various biochemical markers [20,21,22,23,24]. The findings highlight the multifactorial nature of OA, influenced by demographic factors, lifestyle choices, and comorbid conditions. However, the relationship between the IBI and OA has not yet been elucidated. Directed acyclic graphs (DAGs) are powerful tools used in epidemiological research to model potential causal relationships and identify confounding factors. They help in understanding and illustrating assumptions about the relationships between variables. The use of DAGs in studies involving the National Health and Nutrition Examination Survey (NHANES) data can clarify the causal pathways and control for biases effectively.
This study aims to fill this gap by investigating the associations of IBI with OA and mortality while controlling for potential confounding factors. We constructed a DAG to model these relationships, identifying and adjusting for the variables. By leveraging data from the NHANES database, we aim to comprehensively analyze the IBI-OA and IBI-mortality relationships, enhancing our understanding of the inflammatory mechanisms underlying OA and informing potential therapeutic interventions.
Methods
Study participant selection
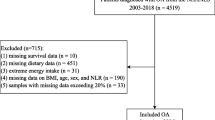
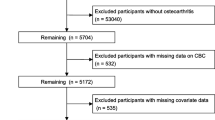
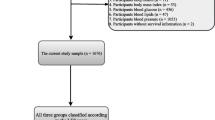
The NHANES is a publicly available database (https://wwwn.cdc.gov/nchs/nhanes/ ) that is used to assess and monitor the health and nutritional status of the US population by collecting demographic data, self-report questionnaires, and physical examination and serological test results [25]. As a constantly updated cross-sectional survey, the NHANES applies a stratified, multistage probability sampling design rather than a simple sample design. Initially, 101,316 participants were recruited from the NHANES between 1999–2018(https://wwwn.cdc.gov/nchs/nhanes/). After removing those who were aged < 18 years (n = 42,112), were pregnant (n = 1,670), did not know whether they had arthritis or their arthritis information was missing (n = 4,115), were diagnosed with arthritis of unknown specific type (n = 4,885), were diagnosed with rheumatoid (n = 2,938), psoriatic (n = 90), or other type of arthritis (n = 1,494), and those with missing data on IBI (n = 21,669), finally, 22,343 eligible participants were selected for the current study. As previously reported [26], this study explicitly adhered to the STROBE guidelines in terms of questionnaires, covariates definition, data collection and standardization, ethical standards, and statistical analyses, to enhance the reliability and validity of our findings [27]. There are various methods to handle missing data, such as mean imputation, median imputation, multiple imputation (MI) [28], and K-Nearest Neighbors (KNN) Imputation [29]. MI is a method for handling missing data by creating multiple complete datasets to estimate missing values, followed by analysis and aggregation of the results. The process involves the following steps: initially, identify which variables have missing values and the patterns of these missing values (e.g., missing at random, not missing at random), and generate missing value distribution plots for each variable. Using the Multiple Imputation by Chained Equations (MICE) algorithm, generate 10 imputed datasets, where missing values are estimated based on information from other variables. Conduct statistical analyses independently on each of the generated imputed datasets, performing various sensitivity analyses to validate the robustness of the results and the efficacy of the MI method in handling missing data. Finally, combine the analysis results from the 10 imputed datasets to obtain final estimates and confidence intervals, accounting for the uncertainty of the imputations and providing more reliable statistical inferences [28, 30].Finally, 10 iterations of multiple imputation were conducted to generate 10 complete datasets (Dataset 1 to Dataset 10), reflecting the uncertainty of missing values.
Participants were approved for participation in the NHANES project by the National Center for Health Statistics Research Ethics Review Board and informed consent was obtained from all participants. Thus, this study was exempt from ethical approval by the Ethics Review Board of the Civil Aviation General Hospital.
Covariate selection
The serological indices collected from the mobile examination center (MEC) included glycated hemoglobin (HbA1c), aspartate aminotransferase (AST), alanine transaminase (ALT), blood urea nitrogen (BUN), total cholesterol (TC), low-density lipoprotein (LDL), and high-density lipoprotein (HDL). These serological indices combined with self-reported age and physical activity (PA) were considered continuous covariates. The categorical covariates included Sex: female and male; Race: Mexican American, non-Hispanic black, non-Hispanic white and other; Education level: below high school, high school and above high school; Marital status: other and married; Body mass index (BMI): <18.5 kg/m2, 18.5–25 kg/m2, and ≥ 25 kg/m2; Poverty to income ratio (PIR): <1.3, 1.3–3.5, and ≥ 3.5; Drinking status: never (individuals who have consumed fewer than 12 drinks in their lifetime), mild (individuals who consume alcohol infrequently or in small quantities, defined as fewer than one drink per week), moderate (individuals who consume alcohol regularly but in moderate amounts, defined as up to one drink per day for women and up to two drinks per day for men), heavy (individuals who consume alcohol in large quantities, defined as more than one drink per day for women and more than two drinks per day for men), and former (individuals who have consumed at least 12 drinks in their lifetime but have not consumed any alcohol in the past year); Smoking status: never (individuals who have smoked fewer than 100 cigarettes in their lifetime), ever (individuals who have smoked at least 100 cigarettes in their lifetime but had quit smoking at the time of the survey), and current (individuals who have smoked at least 100 cigarettes in their lifetime and were still smoking at the time of the survey). Except for BMI data from the physical examination of the MEC, the remaining categorical variable data were acquired from the corresponding questionnaire parts or in-person interviews. The clinical disease status (cardiovascular disease (CVD), stroke, diabetes mellitus (DM), and hypertension) was obtained using a self-reported questionnaire, medication use, and indicator measurements. For more information on how to interpret, access, and calculate each covariate, please refer to the official NHANES analytic guidelines.
IBI calculation
Outcome measure
In this study, one of the outcomes was the risk of OA. To evaluate OA, the study participants were subjected to a dual-question assessment concerning arthritis. Firstly, if they answered “yes” to the question “Has a doctor ever told you that you have arthritis?”, they were confirmed as arthritis (MCQ160a). Next, to further discriminate types of arthritis, those who responded “yest” were questioned on “What type of arthritis?” (MCQ195, MCQ190, MCQ191), with response options including “Rheumatoid arthritis,” “Osteoarthritis,” “Psoriatic arthritis,” “Other,” “Refused,” and “Do not know.” Those who responded with “Osteoarthritis” were considered to have OA. More detailed information please search MCQ160a, MCQ195, MCQ190, MCQ191 in the Search Term of the Variable Keyword Search in the website: https://wwwn.cdc.gov/nchs/nhanes/search/default.aspx. Another outcome was the all-cause mortality in patients with OA, defined as death from any cause. Mortality data were acquired by correlating the NHANES data with the latest (2019) National Death Index data (https://www.cdc.gov/) by matching specifically personalized labels such as name, sex, and date of birth. The period from the date of the participants’ interview by the staff of the Centers for Disease Control and Prevention to the date of death was considered as the follow-up time.
Statistical analysis
All statistical analyses were conducted using the R software version 4.2.1. A two-sided P < 0.05 was considered statistically significant. This study employed weighted methods to minimize significant fluctuations in the NHANES dataset, which utilizes a complex, multi-stage sampling design to ensure accurate and representative estimates of the U.S. population. Sampling weights were adjusted for selection probability and response rates, specifically using wtmec4 year *2/6 for the 1999–2002 cycles and wtmec2 year *1/6 for the 2003–2010 cycles. The analysis incorporated strata (SDMVSTRA) and clusters (SDMVPSU) to account for the survey’s stratification and clustering, thereby enhancing the precision and reliability of variance estimates. Adhering to NHANES analysis guidelines, these methods were employed to mitigate significant dataset fluctuations and provide unbiased results. Supplementary Table S1 presents the non-weighted baseline characteristic analysis. In the descriptive statistics analysis, survey-weight mean ± standard error (SE) was used to summarize the continuous variables, and survey-weighted percentage ± SE was used to summarize the categorical variables. In the inferential statistical analysis, continuous variables were analyzed using a survey-weighted analysis of variance, whereas categorical variables were analyzed using the survey-weighted chi-square test. The LDL and PA variables were not subjected to subsequent analyses because the ratios of missing values were > 10%.
Next, we conducted logistic regression analyses using the ‘stats’ package to assess the association between IBI and OA outcomes. Model 1 was not adjusted for any variables. Model 2 was adjusted for nine demographic variables (age, sex, race, education level, marital status, PIR, BMI, smoking status, and drinking status) and four clinical disease status variables (stroke, CVD, DM, and hypertension). Model 3 was adjusted for all variables except LDL and PA. The odds ratio (OR) and 95% confidence intervals (95% CI) were estimated in the three models using quartiles of the IBI to classify the participants into four groups, with the Q1 group denoting the reference. Besides, the ORs for the 10 input datasets were also conducted and aggregated to obtain an overall estimate, visualizing by a forest plot.
To evaluate the association between IBI and all-cause mortality, we constructed three Cox proportional hazards models using the ‘survival’ package to calculate hazard ratios (HR) and 95% CI. Adjustments for potentially confounding variables in each model were similar to those in the aforementioned logistic regression models. Similarly, we calculated the HRs for the 10 input datasets and got an overall estimate, which was visualized by a forest plot. We employed the Archer-Lemeshow test to assess the goodness-of-fit for design-based regression models and utilized the Area Under the Receiver Operating Characteristic Curve (AUC) to evaluate the model’s performance [28].
Additionally, we conducted restricted cubic spline (RCS) analyses using the ‘rms’ package for model building and the ‘ggrcs’ package for creating plots to clarify the nonlinear connections between IBI and OA, and between IBI and all-cause mortality. To verify the stability of the relationships between IBI and OA and between IBI and all-cause mortality, we also performed subgroup analyses by stratifying the baseline characteristics (age, sex, race, education, marital status, PIR, BMI, stroke, CVD, DM, hypertension, drinking status, smoking status, HBA1C, ALT, AST, BUN, TC, and HDL) into subgroups. An interaction test was used to assess statistical significance, and a forest map was used to visualize the effects. For RCS and similar plots, it is conventional to display the actual data. We have not found any studies presenting weighted data in such plots [30].
Results
Baseline characteristics of the study participants
Figure 1 illustrated the participant selection process. A total of 22,343 adult participants from the NHANES between 1999 and 2018 were included and divided into four groups: Q1, Q2, Q3, and Q4 based on quartiles of the IBI.
The distribution of missing values by IBI quartile is shown in Fig.S1. Variables with no missing values included sex, age, race, and DM. Variables with low missing values were CVD (1), hypertension (10), smoke (19), stroke (21), HBA1c (41), and education (32). Variables with moderate missing values included BUN (150), TC (107), HDL (108), ALT (206), AST (208), marital status (339), and BMI (406). Variables with high missing values were family PIR (1737) and alcohol (1760).
Participants in the highest IBI quartile were more likely to be older, female, and non-Hispanic white (Table 1). Further, they had a less stable marital status and worse lifestyle habits, including heavy drinking, ever and current smoking, and overweight status (BMI ≥ 25 kg/m2). Compared to the lowest IBI quartile, those in the highest IBI quartile also presented with higher levels of HbA1c, ALT, BUN, and TC and had a greater risk of clinical diseases such as stroke, CVD, DM, and hypertension. On the contrary, participants with a lower IBI quartile had a higher education level, family income (PIR ≥ 3.5), and HDL level. However, the PA showed no statistically significant differences among the four IBI groups.
From DAG (Fig. 2), we found that most covariates including AGE, SEX, RACE, BMI, SMOKE, ALCOHOL.USER, DM, HYPERTENSION, HBA1C, ALT, AST, BUN, TC, and HDL influence the IBI, whereas AGE, SEX, RACE, EDUCATION, MARITAL STATUS, FAMILY PIR, BMI, STROKE, CVD, SMOKE, DM, HYPERTENSION and ALCOHOL affect the OA. The interplay among them was intricate, for example, “SEX → IBI and SEX → OA” signified that sex might affect IBI and OA, reflecting disparities in inflammatory responses and susceptibility to OA between females and males. And “HDL → IBI and HDL → OA” implied that HDL might influence systemic inflammation and the risk of developing OA.
DAG of IBI and OA with Detailed Factors. This DAG illustrated the potential causal relationships and confounding factors associated with the IBI and OA. Each node represents a variable that may influence IBI and OA. Arrows indicate the direction of influence or causal relationship between variables. DAG, Directed Acyclic Graph; IBI, Inflammatory Burden Index; OA, Osteoarthritis; EDUCATION, Education Level; FAMILY PIR, Family Income to Poverty Ratio; BMI, Body Mass Index; CVD, Cardiovascular disease; SMOKE, Smoking; DM, Diabetes Mellitus; HBA1C, Hemoglobin A1c; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; BUN, Blood Urea Nitrogen; TC, Total Cholesterol; HDL, High-Density Lipoprotein. The variable, IBI, is the primary exposure variable. The variables, OA and MORTALITY, are the outcome
Association of IBI with OA
The multiple logistic regression models showed an association between IBI and OA (Table 2). When the IBI was analyzed as a continuous variable, the association between IBI and the risk of OA was not significant, regardless of whether confounding variables were adjusted for (all ORs: 1, all P < 0.05). When the IBI was further converted to categorical variables (quartiles), the participants in the highest quartile of IBI had a 2.54 times higher risk of OA compared those in the lowest quartile of IBI in Model 1 (95% CI: 2.23–2.90, P < 0.01), whereas in Model 2 the value was 1.21 (95% CI: 1.03–1.42, P < 0.05), and 1.23 in Model 3 (95% CI: 1.05–1.45, P < 0.05).
Figure 3A displayed the pooled results from the 10 imputed datasets, indicating that higher IBI levels are significantly associated with increased odds of developing OA.
Association of IBI with all-cause mortality
Table 3 shows the association between IBI and all-cause mortality using multiple Cox proportional hazards regression models. The effect size was not obvious when we estimated the relationship between continuous IBI and all-cause mortality in the three fitted models (all HRs: 1, all P < 0.05). Once the IBI was converted to a categorical variable (quartiles) and input into the models, a significant dose-response relationship was observed. Specifically, the participants in the highest quartile of the IBI had an 86% increased risk of developing all-cause death compared with those in the lowest quartile (95% CI: 1.50–2.31, P < 0.01) in Model 1. This trend remained stable in Model 2 with an HR of 1.54 (95% CI: 1.22–1.95, P < 0.01), and Model 3 with an HR of 1.41 (95% CI: 1.10–1.80, P < 0.01).
Figure 3B presented the pooled results from the 10 imputed datasets, indicating that higher IBI levels were significantly associated with increased mortality risk in OA individuals. The results from the multiple imputation and sensitivity analysis indicate that the data distribution is consistent before and after imputation, demonstrating stable results (Fig. 3 and Supplementary Table S3).
Restricted cubic spline analyses
We performed an RCS analysis to assess the nonlinear relationship between the two effects. We observed a significant positive association between continuous IBI and the risk of OA (P = 0.01 for nonlinearity) (Fig. 4), and the area between the blue dashed lines suggests a 95% CI around the point estimate. When the IBI was converted to a categorical variable, this trend persisted, particularly in the conversion from the third quartile to the highest IBI quartile, as shown in Fig. S1.
Restricted cubic spline plot illustrating the non-linear association between continuous inflammatory burden index (IBI) and the risk of osteoarthritis (OA) in the entire study population. The solid line represents the hazard ratio, while the dotted line denotes the 95% confidence interval. Fig. S1 shows the risk for OA according to IBI quartiles in the entire study population
The association between continuous IBI and the risk of all-cause mortality is depicted by Fig. 5; a higher IBI value was correlated with a greater risk of all-cause mortality (P < 0.01 for nonlinearity), especially to the left of the inflection point. However, this positive linkage was not stable when categorical IBI was fitted into the models. As shown in Fig. S2, the risk of all-cause mortality decreased slightly between the lowest and second quartiles of the IBI, and the risk of all-cause mortality increased gradually with an increase in IBI, which was statistically significant (P < 0.01 for nonlinearity).
Restricted cubic spline plot demonstrating the non-linear relationship between continuous IBI and the risk of all-cause mortality among individuals with OA. The solid line signifies the hazard ratio, and the dotted line indicates the 95% CI. Fig. S2 shows the risk for all-cause mortality according to IBI quartiles in the OA population
Subgroup analyses
To further verify the stability of the positive associations between the IBI and OA and IBI and all-cause mortality, subgroup analyses were conducted. Figure 6 shows that the positive effects of IBI and OA were not affected by the stratification of sex, race, education level, marital status, PIR, BMI, smoking status, drinking status, hypertension, ALT, AST, BUN, TC, or HDL levels (all P for interaction > 0.05). In contrast, a significant interaction was observed in the stratification of age, CVD, DM, and HbA1c levels (all P < 0.05). However, the participants who were ≥ 65 years old, or had CVD, DM, or HbA1c level > 6% showed no statistical significance, indicating that the impacts of stratification by age, CVD, DM, and HbA1c need to be further verified.
The stability of the positive association between IBI and all-cause mortality is shown in Fig. 7. This effect was independent of sex, race, education level, marital status, PID, BMI, stroke, smoking status, DM, HbA1c, ALT, AST, BUN, and TC (all P > 0.05). Whether this effect was affected by different subgroups of drinking status is controversial because participants without a drinking status showed no statistical significance. Surprisingly, this interaction was evident after stratification by age, stroke, CVD, and hypertension. In other words, the participants aged < 65 years old had a higher risk of all-cause mortality compared to those aged ≥ 65 years old. Patients with stroke were also inclined to develop all-cause mortality compared to those without stroke, whereas those with CVD and hypertension presented a lower risk of all-cause mortality than those without CVD and hypertension.
Discussion
This cross-sectional study not only evaluated the association of inflammatory burden (described by the IBI) with OA but also estimated the relationship between the IBI and all-cause mortality among patients with OA. Among the 22,343 eligible participants from the NHANES between 1999 and 2018, we found that a higher IBI was associated with a greater risk of OA in multiple logistic regression models. Based on the three Cox models, we observed a positive relationship between the IBI and all-cause mortality. The RCS revealed nonlinear relationships between these effects. Subgroup analyses showed that age, CVD, and hypertension exerted pivotal influences on the association between IBI and all-cause mortality, whereas the association between IBI and OA remained stable after stratification by sex, race, education level, marital status, PIR, BMI, smoking status, drinking status, hypertension, ALT, AST, BUN, TC, and HDL.
OA was once thought to be a simple wear and tear disorder; instead, it has now been accepted as a complex and low-grade inflammatory condition involving mechanics and immunity. A growing number of researchers are committed to identifying easily accessible, timely, and reliable laboratory indicators in clinical practice as biomarkers for OA. Despite being a novel inflammatory indicator, the IBI has not been investigated in patients with OA, or all-cause mortality of patients with OA. Our findings suggested that a higher IBI was associated with a greater risk of OA and all-cause mortality, indicating that the IBI is a reliable inflammatory biomarker for predicting OA and all-cause mortality. Unlike what we had found, Zhou et al. found that the systemic inflammatory response index was a noninvasive biomarker of OA in a large-sample retrospective cohort [31]. The neutrophil-to-lymphocyte ratio (NLR) has been previously identified as a preventative biomarker of knee OA severity [32]. Bay-Jensen et al. reported that the serum CRP metabolite is a biomarker for the early identification of patients with OA with an inflammatory phenotype [33]. Xie et al. first developed the blood-based tool, IBI, to assess the inflammatory burden in multiple cancers [16]. Their subsequent investigation further suggested that IBI had the highest accuracy in predicting the prognosis of colorectal cancer among the various systemic inflammatory signatures [34]. Moreover, IBI was considered a promising prognostic biomarker in patients after endovascular thrombectomy for acute ischemic stroke [19]. One possible reason for these differences is that the IBI integrates the advantages of two indexes simultaneously. CRP is commonly used to detect inflammation in the body [35], whereas the NLR accounts for immune responses [36]. The IBI can evaluate the inflammatory and immune status from a holistic perspective of a patient’s health [37]. Additionally, the IBI can reflect the dynamic crosstalk between inflammatory and immune responses [38].
However, the mechanisms linking inflammation to OA and all-cause mortality remain unclear. In OA pathogenesis, the infiltrative neutrophils of synovial fluids release a series of cytokines such as IL-1, IL-8, TNF-β, and extended inflammatory responses, which promote the progression of the acute inflammatory process into chronic inflammation [39,40,41]. Chronic inflammation can contribute to cartilage loss, further aggravating OA [42]. In addition, in diseases associated with an increased risk of death, such as DM, chronic inflammation can worsen and exacerbate disease progression [43]. Moreover, patients with OA often present with joint pain that can activate the hypothalamic-pituitary-adrenal (HPA) axis [44]. The activation of the HPA axis leads to alterations in peripheral blood cell numbers, thereby suppressing inflammation [45]. Meanwhile, the sympathetic nervous system is activated in combination with the HPA axis, stimulating increased levels of cortisol and catecholamines [46, 47]. The progression of many chronic diseases, including CVD, DM, stroke, and cancer, is strongly related to these hormones and is linked to a greater risk of all-cause mortality [38]. Future investigations with adequate sample sizes and follow-up cohorts are urgently required to elucidate the underlying mechanisms of the association of IBI with OA and all-cause mortality.
In clinical practice, this study highlighted the importance of the IBI for clinicians, especially those in orthopedics and rheumatology, emphasizing the need to consider the dynamic interplay between inflammation and immune responses. The IBI offers a new, reliable biomarker based on existing laboratory tests that can enhance the accuracy of diagnosis and prognosis for patients with OA. Integrating IBI into routine clinical practice could ultimately optimize treatment strategies, help clinicians identify patients at heightened risk of OA or all-cause mortality, enhance patient management, and allocate healthcare resources more effectively. For future research, especially randomized controlled trials (RCTs), can be designed based on this study. These trials can validate our findings and establish a causal relationship between IBI and OA outcomes. Furthermore, this study focuses on the importance of exploring the potential mechanisms underlying the association between IBI, OA, and overall mortality, providing deeper insights into the biological pathways involved.
This study has several limitations. First, some of the basic characteristics of this study were obtained based on questionnaires or in-person interviews, and recall bias was inevitable. Second, although the confounding variables in the study were adjusted for, other potential or unmeasured confounding factors such as bowel, diet, and sleeping habits were still difficult to eliminate. Third, the application of DAG could clarify the causal pathway and effectively control the bias, but more robust studies may be needed in the future studies to elucidate the causal relationship between IBI and OD. Finally, our findings were obtained from US adult civilians; whether they can be extended to other countries or ethnic or special groups needs to be further validated in global, multiethnic, or diverse populations.
Conclusions
In conclusion, this cross-sectional study provides the latest evidence of a positive association between IBI, OA, and all-cause mortality in patients with OA. Our findings suggest that the IBI may be a promising signature for assessing the inflammatory burden in patients with OA, which, in turn, is conducive to precise references for high-risk population recognition, anti-inflammatory guidance, and reducing mortality intervention. Future research is urgently needed to illustrate the association of IBI with OA and mortality, as well as to elucidate the potential mechanisms of these effects.
Data availability
The cohort generated during the current study are available in the NHANES database(https://wwwn.cdc.gov/nchs/nhanes/Default.aspx). The cohort used and analyzed in this study are available from the corresponding author upon reasonable request.
Abbreviations
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- BUN:
-
Blood urea nitrogen
- CI:
-
Confidence interval
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular disease
- DAG:
-
Directed Acyclic Graph
- DM:
-
Diabetes mellitus
- HbA1c:
-
Glycated hemoglobin
- HDL:
-
High-density lipoprotein
- HPA:
-
Hypothalamic-pituitary-adrenal
- HR:
-
Hazard ratios
- IBI:
-
Inflammatory burden index
- LDL:
-
Low-density lipoprotein
- MEC:
-
Mobile examination center
- NHANES:
-
National Health and Nutrition Examination Survey
- NLR:
-
Neutrophil-to-lymphocyte ratio
- OA:
-
Osteoarthritis
- OR:
-
Odds ratio
- PA:
-
Physical activity
- PIR:
-
Poverty to income ratio
- RCS:
-
Restricted cubic spline
- SE:
-
Standard error
- TC:
-
Total cholesterol
- US:
-
United States
References
Xia B, Di C, Zhang J, Hu S, Jin H, Tong P. Osteoarthritis pathogenesis: a review of molecular mechanisms. Calcif Tissue Int. 2014;95(6):495–505.
Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–99.
Huston P. Why osteoarthritis of the knee is called a wound that does not heal and why Tai Chi is an effective treatment. Front Med (Lausanne). 2023;10:1208326.
Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 2022;30(2):184–95.
Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325(6):568–78.
Wang Y, Nguyen UDT, Lane NE, Lu N, Wei J, Lei G, Zeng C, Zhang Y. Knee osteoarthritis, potential mediators, and risk of all-cause mortality: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2021;73(4):566–73.
Chen B, Wang L, Xie D, Wang Y. Exploration and breakthrough in the mode of chondrocyte death - A potential new mechanism for osteoarthritis. Biomed Pharmacother. 2023;170:115990.
Dieleman JL, Baral R, Birger M, Bui AL, Bulchis A, Chapin A, Hamavid H, Horst C, Johnson EK, Joseph J, et al. US Spending on Personal Health Care and Public Health, 1996–2013. JAMA. 2016;316(24):2627–46.
Vargas Negrín F, Medina Abellán MD, Hermosa Hernán JC, de Felipe Medina R. [Treatment of patients with osteoarthritis]. Aten Primaria. 2014;46(Suppl 1Suppl 1):39–61.
Li Y, Zhao J, Guo S, He D. siRNA therapy in osteoarthritis: targeting cellular pathways for advanced treatment approaches. Front Immunol. 2024;15:1382689.
Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1825–34.
Nedunchezhiyan U, Varughese I, Sun AR, Wu X, Crawford R, Prasadam I. Obesity, inflammation, and Immune System in Osteoarthritis. Front Immunol. 2022;13:907750.
Griffin TM, Scanzello CR. Innate inflammation and synovial macrophages in osteoarthritis pathophysiology. Clin Exp Rheumatol. 2019;37(Suppl 120):57–63.
Hsueh MF, Zhang X, Wellman SS, Bolognesi MP, Kraus VB. Synergistic roles of macrophages and neutrophils in Osteoarthritis Progression. Arthritis Rheumatol. 2021;73(1):89–99.
Jin X, Beguerie JR, Zhang W, Blizzard L, Otahal P, Jones G, Ding C. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(4):703–10.
Xie H, Ruan G, Ge Y, Zhang Q, Zhang H, Lin S, Song M, Zhang X, Liu X, Li X, et al. Inflammatory burden as a prognostic biomarker for cancer. Clin Nutr. 2022;41(6):1236–43.
Ding P, Wu H, Liu P, Sun C, Yang P, Tian Y, Guo H, Liu Y, Zhao Q. The inflammatory burden index: a promising prognostic predictor in patients with locally advanced gastric cancer. Clin Nutr. 2023;42(2):247–8.
Xie H, Ruan G, Wei L, Deng L, Zhang Q, Ge Y, Song M, Zhang X, Lin S, Liu X, et al. The inflammatory burden index is a superior systemic inflammation biomarker for the prognosis of non-small cell lung cancer. J Cachexia Sarcopenia Muscle. 2023;14(2):869–78.
Du M, Xu L, Zhang X, Huang X, Cao H, Qiu F, Lan W, Jiang H. Association between Inflammatory Burden Index and unfavorable prognosis after endovascular thrombectomy in Acute ischemic stroke. J Inflamm Res. 2023;16:3009–17.
Xia F, Li Q, Luo X, Wu J. Identification for heavy metals exposure on osteoarthritis among aging people and machine learning for prediction: a study based on NHANES 2011–2020. Front Public Health. 2022;10:906774.
Wang J, Fan J, Yang Y, Moazzen S, Chen D, Sun L, He F, Li Y. Vitamin D Status and Risk of All-Cause and Cause-Specific Mortality in Osteoarthritis Patients: Results from NHANES III and NHANES 2001–2018. Nutrients 2022, 14(21).
Xu Y, Wu Q. Trends and disparities in osteoarthritis prevalence among US adults, 2005–2018. Sci Rep. 2021;11(1):21845.
Deng X, Tan Y. A national cross-sectional analysis of selenium intake and risk of osteoarthritis: NHANES 2003–2016. Front Public Health. 2022;10:1047605.
Yu G, Lin Y, Dai H, Xu J, Liu J. Association between serum 25-hydroxyvitamin D and osteoarthritis: a national population-based analysis of NHANES 2001–2018. Front Nutr. 2023;10:1016809.
Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, Hirsch R, Burt VL, Johnson CL. National Health and Nutrition Examination Survey: sample design, 2007–2010. Vital Health Stat 2 2013(160):1–23.
Lee YB, Lee JH, Kang MJ, Choi JY, Kim JW, Yu DS, Han KD, Park YG. Association between allergic diseases and ophthalmologic diseases, including cataracts and glaucoma, using the Korean National Health and Nutrition Examination Survey 2010–2012: a STROBE-compliant article. J Dermatol. 2018;45(4):463–7.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7.
Issanov A, Karim ME, Aimagambetova G, Dummer TJB. Does Vaccination protect against human papillomavirus-related cancers? Preliminary findings from the United States National Health and Nutrition Examination Survey (2011–2018). Vaccines (Basel) 2022, 10(12).
Rahman SA, Huang Y, Claassen J, Heintzman N, Kleinberg S. Combining Fourier and lagged k-nearest neighbor imputation for biomedical time series data. J Biomed Inf. 2015;58:198–207.
He L, Xie X, Xue J, Xie H, Zhang Y. Association of the systemic immune-inflammation index with all-cause mortality in patients with arteriosclerotic cardiovascular disease. Front Cardiovasc Med. 2022;9:952953.
Zhou Q, Liu J, Xin L, Hu Y, Qi Y. Systemic inflammation response index as an emerging biomarker in osteoarthritis patients: a bibliometric and large sample retrospective investigation. Clin Exp Rheumatol 2023.
Taşoğlu Ö, Bölük H, Şahin Onat Ş, Taşoğlu İ, Özgirgin N. Is blood neutrophil-lymphocyte ratio an independent predictor of knee osteoarthritis severity? Clin Rheumatol. 2016;35(6):1579–83.
Bay-Jensen AC, Bihlet A, Byrjalsen I, Andersen JR, Riis BJ, Christiansen C, Michaelis M, Guehring H, Ladel C, Karsdal MA. Serum C-reactive protein metabolite (CRPM) is associated with incidence of contralateral knee osteoarthritis. Sci Rep. 2021;11(1):6583.
Xie H, Ruan G, Wei L, Zhang H, Ge Y, Zhang Q, Song M, Zhang X, Liu X, Lin S, et al. Comprehensive comparative analysis of prognostic value of serum systemic inflammation biomarkers for colorectal cancer: results from a large multicenter collaboration. Front Immunol. 2022;13:1092498.
Sproston NR, Ashworth JJ. Role of C-Reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754.
Zhang B, Lin L, Yuan F, Song G, Chang Q, Wu Z, Miao Z, Mo D, Huo X, Liu A. Clinical application values of neutrophil-to-lymphocyte ratio in intracranial aneurysms. Aging. 2021;13(4):5250–62.
Song Z, Lin F, Chen Y, Li T, Li R, Lu J, Han H, Li R, Yang J, Li Z, et al. Inflammatory Burden Index: Association between Novel systemic inflammatory biomarkers and prognosis as Well as in-hospital complications of patients with Aneurysmal Subarachnoid Hemorrhage. J Inflamm Res. 2023;16:3911–21.
He C, Wu D, Wei X, Li Y, Liao Y, Yang D. Association between inflammatory burden index and all-cause mortality in the general population aged over 45 years: data from NHANES 2005–2017. Nutr Metab Cardiovasc Dis 2023.
Abbink JJ, Kamp AM, Nieuwenhuys EJ, Nuijens JH, Swaak AJ, Hack CE. Predominant role of neutrophils in the inactivation of alpha 2-macroglobulin in arthritic joints. Arthritis Rheum. 1991;34(9):1139–50.
Clements KM, Ball AD, Jones HB, Brinckmann S, Read SJ, Murray F. Cellular and histopathological changes in the infrapatellar fat pad in the monoiodoacetate model of osteoarthritis pain. Osteoarthritis Cartilage. 2009;17(6):805–12.
Ross TN, Kisiday JD, Hess T, McIlwraith CW. Evaluation of the inflammatory response in experimentally induced synovitis in the horse: a comparison of recombinant equine interleukin 1 beta and lipopolysaccharide. Osteoarthritis Cartilage. 2012;20(12):1583–90.
Wang M, Li S, Zhang L, Tian J, Ma J, Lei B, Xu P. Injectable Bioactive Antioxidative one-component polycitrate hydrogel with anti-inflammatory effects for Osteoarthritis Alleviation and Cartilage Protection. Adv Healthc Mater 2023:e2301953.
Tateya S, Kim F, Tamori Y. Recent advances in obesity-induced inflammation and insulin resistance. Front Endocrinol (Lausanne). 2013;4:93.
Nishimura H, Kawasaki M, Suzuki H, Matsuura T, Baba K, Motojima Y, Yamanaka Y, Fujitani T, Ohnishi H, Tsukamoto M, et al. The neurohypophysial oxytocin and arginine vasopressin system is activated in a knee osteoarthritis rat model. J Neuroendocrinol. 2020;32(8):e12892.
Heinzel S, Marchingo JM, Horton MB, Hodgkin PD. The regulation of lymphocyte activation and proliferation. Curr Opin Immunol. 2018;51:32–8.
Daniela M, Catalina L, Ilie O, Paula M, Daniel-Andrei I, Ioana B. Effects of Exercise Training on the autonomic nervous system with a focus on anti-inflammatory and antioxidants effects. Antioxid (Basel) 2022, 11(2).
Hall JM, Cruser D, Podawiltz A, Mummert DI, Jones H, Mummert ME. Psychological stress and the cutaneous Immune response: roles of the HPA Axis and the sympathetic nervous system in atopic dermatitis and psoriasis. Dermatol Res Pract. 2012;2012:403908.
Acknowledgements
Not applicable.
Funding
Capital’s Funds for Health Improvement and Research(2024-1G-3101).
Author information
Authors and Affiliations
Contributions
ZX and YW drafted the manuscript and designed the charts. ZX and CS collected and analyzed the data. ZX, XZ and PY designed the study. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Participants were approved for participation in the NHANES project by the National Center for Health Statistics Research Ethics Review Board and informed consent was obtained from all participants. Thus, this study was exempt from ethical approval by the Ethics Review Board of the Civil Aviation General Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiong, Z., Xu, W., Wang, Y. et al. Inflammatory burden index: associations between osteoarthritis and all-cause mortality among individuals with osteoarthritis. BMC Public Health 24, 2203 (2024). https://doi.org/10.1186/s12889-024-19632-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19632-1