Abstract
Background
Limited evidence is available on the association between estimated cardiorespiratory fitness (e-CRF) and incidence of cardiovascular disease (CVD) in Chinese population.
Methods
A total of 10,507 adults including 5084 men (48.4%) and 5423 (51.6%) women with a median age of 56.0 (25% quantile: 49, 75% quantile 63) years from the China Health and Retirement Longitudinal Study (CHARLS) was recruited in 2011 as baseline. The CVD incident events were followed-up until 2018. e-CRF was calculated from sex-specific longitudinal non-exercise equations and further grouped into quartiles. Cox proportional models were used to calculate hazard ratio (HR) and 95% confidence interval (CI) for incidence risks of CVD, heart disease and stroke.
Results
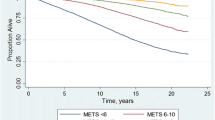
During a median follow-up of 7 years, a total of 1862 CVD, 1409 heart disease and 612 stroke events occurred. In fully adjusted models, each one MET increment of e-CRF was associated with lower risk of CVD (HR = 0.91, 95%CI = 0.85–0.96 for males, HR = 0.87, 95%CI = 0.81–0.94 for females). Compared with the Quartile (Q)1 group, the HRs (95%CI) of the Q2, Q3 and Q4 groups were 0.84 (0.63–1.03), 0.72 (0.57–0.91) and 0.66 (0.51–0.87) for CVD in males. Females had HRs of 0.79 (0.66–0.96) in Q2, 0.71 (0.57–0.88) in Q3 and 0.58 (0.45–0.75) in Q4 for CVD. The associations between e-CRF and heart disease and stroke were slightly weaker than that for CVD in both males and females.
Conclusions
Higher e-CRF decreases the incident risk of CVD, heart disease and stroke.
Similar content being viewed by others
Introduction
Cardiorespiratory fitness (CRF) is an integrated indicator that reflects the overall ability to acquire, transport and utilize oxygen during physical activity, which is quantified by maximal oxygen uptake [1]. Higher CRF is associated with lower risk of incidence of cardiovascular disease (CVD) and mortality from CVD [2, 3]. In a meta-analysis of 34 cohort studies, per 1 metabolic equivalent (MET) increase in CRF was associated with 13% (9–17%) lower risk of CVD mortality [4]. Cardiopulmonary exercise testing with ventilatory gas analysis at maximal effort is considered to be the gold standard of assessing CRF [5]. Alternatively, CRF can be measured by submaximal exercise tests using a specific physiological response with a standardized protocol (e.g., heart rate [HR], peak workload, amount of time or distance covered running or walking) [6]. These methods could provide relative accurate measurement of CRF, however, the use of them is limited as the high cost of specialized equipment, trained staff, a large indoor space, and a lot of time. Only individuals with relative healthy condition and normal exercise performance could complete the CRF test. Several non-exercise CRF estimation algorithms based on easily measured and self-reported variables have been developed to simplify the access to CRF data, especially in large population investigation. The application of CRF could be expanded to participants with limited mobility or chronic diseases.
Most algorithms were derived from cross-sectional studies with linear regression of age. Longitudinal data showed that CRF declines nonlinearly with aging [12, 13]. To estimate CRF more accurately, Jackson et al. developed a group of sex-specific longitudinal non-exercise equations accounting for the age-associated decline in CRF based on common physical parameters [14]. In Aerobics Center Longitudinal Study (ACLS), e-CRF based on longitudinal algorithms provided a valid prediction of all-cause mortality, CVD-related mortality and non-fatal CVD events, which was consistent with the results based on measured CRF [15]. Besides, e-CRF may add clinical value to the Framingham Risk Score to better predict long-term coronary heart disease (CHD) risk in males from ACLS [19]. Higher e-CRF estimated by longitudinal equations was associated with lower risk of all-cause and CVD mortality in both sexes derived from Third National Health and Nutrition Examination Survey [16]. In Norway population, e-CRF was a strong predictor of mortality from CVD an all-cause independent of traditional CVD risk factors [18]. Another study based on the same Norway population illustrated that e-CRF lower the risk of first acute myocardial infarction among females but not males [20]. Higher non-exercise CRF was related to lower death risk in women but not in men of Spanish older population [6]. The evidence on the association between e-CRF and inverse outcomes in Chinese population was limited. In Taiwan MJ Cohort, both e-CRF and its changes predicted the incidence of major biological CVD risk factors, especially hypertension and type 2 diabetes mellitus (T2DM) [21]. e-CRF was inversely associated with risk of all-cause and cause-specific mortality in The Rural Chinese Cohort Study [17]. However, no reports are available for the association between e-CRF and incidence of CVD including heart disease and stroke in Chinese population.
Thus, the aim of this study was to examine the associations between e-CRF and incidence of CVD, heart disease and stroke in a large prospective cohort in China. This study would provide evidence for application of e-CRF in different populations and inform future public health recommendations.
Methods
Study population
The data used in this study were downloaded from the China Health and Retirement Longitudinal Study (CHARLS, available at CHARLS (pku.edu.cn)). CHARLS is an ongoing national and household-based cohort survey of Chinese adults aged 45 years above. The baseline investigation started in 2011 and being followed up every 1–2 years. By now, CHARLS has been conducted for five Waves (in 2011, 2013, 2014, 2015 and 2018 respectively), among which the Wave1 (2011) and Wave4 (2015) collected blood samples and the Wave3 (2014) is the Life History Survey. Briefly, CHARLS was conducted using a multistage probability sampling and covered 28 provinces across Mainland China. Details about the research design and implementation process of CHARLS are available elsewhere [22]. The face-to-face interviews, anthropometric measurements and blood samples tests were conducted by trained staff following standard protocols. In this study, we used data in 2011 as baseline and followed up until 2018. [22]The primary aim of this study was to recruit subjects aged ≥ 45 years, but some subjects aged 18–44 years also attended the baseline survey. As several CRF estimation components were derived from anthropometric measurements, we included the individuals participated in biomarker measurement at least once in 2011, 2013 and 2015 to maximum the sample size. A nationally representative sample of the 15,292 subjects was recruited in Wave1. Subjects who met the following criteria were excluded: (1) missing data on e-CRF components (age, sex, BMI, WC, rHR, physical activity level, smoking), (2) had heart disease and stroke at baseline, (3) loss to follow-up. Ultimately, a total of 10,507 participants were include into this study. Ethics approval for the data collection was obtained from the Biomedical Ethics Review Committee of Peking University.
Background characteristics and anthropometric measurements
Participants were interviewed face-to-face using a structured questionnaire, which asked for demographic information (e.g., date of birth, sex, education attainment,), lifestyle data (e.g., smoking, alcohol use, physical activity), self-reported diseases (heart disease, stroke, hypertension, dyslipidemia, diabetes, and cancer) and use of medications for corresponding diseases. In this study, smoking/ drinking were defined as current or previous smoking/drinking. Each subject reported their amount of physical activity in a usual week including vigorous physical activity (VPA), moderate physical activity (MPA) and light physical activity (LPA). VPA makes participants breathe much harder than normal and may include heavy lifting, digging, plowing, aerobics, fast bicycling, and cycling with a heavy load. MVPA makes participants breathe somewhat harder than normal and may include carrying light loads, bicycling at a regular pace, or mopping the floor. LPA includes walking at work and at home, walking to travel from place to place, and any other walking that participants might do solely for recreation, sport, exercise, or leisure. Subjects who had conducted VPA/MPA/LPA for at least 10 min continuously in a usual week were further asked about the frequency and duration of VPA/MPA/LPA. Conducting a total of VPA or MPA greater than 4 days per week was considered as active physical activity.
Anthropometric indexes including weight, height, WC, rHR, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by trained staff following a standard protocol. BMI was calculated as weight (kg)/height (m) [2]. Hypertension was diagnosed by the following criteria: (1) SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg, (2) any treatment for hypertension, and (3) self-reported history of hypertension diagnosed by clinical physicians.
The whole blood and serum samples were collected from each subject after a 12-h overnight fasting. Biochemical variables including TG, total cholesterol (TC), HDL-C and low-density lipoprotein cholesterol (LDL-C) and fasting plasma glucose (FPG) were tested in metabolic examinations.
Assessment of e-CRF
e-CRF in METs was estimated using the sex-specific BMI models constructed by Jackson et al. [14]. These models have been validated in adults and the generated e-CRF have shown association with long-term health risk 6,15–17,21,23. The models are as follows:
Male: e-CRF (METs) = 21.2870 + (age × 0.1654) – (age 2 × 0.0023) – (BMI × 0.2318) – (WC × 0.0337) – (rHR × 0.0390) + (active physical activity × 0.6351) – (smoker × 0.4263);
Female: e-CRF (METs) = 14.7873 + (age × 0.1159) – (age 2 × 0.0017) – (BMI × 0.1534) – (WC × 0.0088) – (rHR × 0.0364) + (active physical activity × 0.5987) – (smoker × 0.2994).
Active physical activity = 1 if the participant is classified as active physical activity or 0 if not. Smoker = 1 if the participant is current or ever smoker or 0 if not.
Ascertainment of outcome
In this study, CVD includes heart disease and stroke. Heart disease was defined as heart attack, CHD, angina, congestive heart failure or other heart problems. Heart disease and stroke were assessed by asking the participant whether they had been diagnosed with any heart disease/stroke by a doctor and whether they had been under treatment because of heart disease/stroke?” Participants who reported either of the above were classified as incident heart disease/stroke cases. The diagnosis date of incident heart disease or stroke cases was determined by the date between the time of the survey that first recorded heart disease/stroke patients and the time of the previous round of survey.
Statistical analyses
After applying the algorithms of e-CRF, subjects were divided into four groups according to sex-specific quartile. Continuous variables were described as mean (standard deviation, [SD]) and were analyzed using the one-way ANOVA. Categorical variables were described as number (%) and were compared by chi-square test.
Cox regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of incidence of CVD, heart disease and stroke associated with e-CRF as categorical variable by quartile, with the first quartile (Q1) as reference group, and as continuous variable. Models were separately constructed for males, females and all participants. Age, resident area, smoking status, drinking status, hypertension, TG, HDL-C, FPG and drugs used to treat for hypertension, dyslipidemia and diabetes were adjusted. The follow-up time for each participant was defined as the duration from baseline date to the diagnosis date of heart disease and stroke or the end of following up (Wave4, 2018), whichever came first. The incidence rate of CVD, heart disease and stroke was calculated by the number of incident events divided by the person-years. Proportional hazards assumptions were tested using standard Schoenfeld residuals and no violation was observed. The linear trend was tested by modelling e-CRF as an ordinal variable. Sensitivity analyses were carried out by excluding subjects whose outcomes occurred in the first year of follow-up to avoid reverse causation and using the equations of e-CRF developed by Nes et al. [9]. Subgroup analysis was performed by age group (≤ 60 and > 60 years).
A two-tailed P < 0.05 was defined as statistical significance. All statistical analyses were conducted using R software (4.2.0).
Results
Baseline characteristics of the participants
10,507 participants from CHARLS were included in this study. The mean age of the subjects was 57.2 years (SD 9.7) and 5,432 (51.6%) were females. The mean levels of e-CRF were 10.16 METs (SD 2.01) among all participants, and were significantly higher in males (11.68 METs) than in females (8.74 METs). After a median of 7 year’s follow-up, a total of 1,862 CVD, 1,409 heart disease and 612 stroke events were recorded.
All the participants were classified into four groups according to the quartiles of e-CRF by male and female, respectively. Table 1 presents the baseline characteristics of the participants by quartile of e-CRF overall and stratified by sex. Subjects in higher quartile were younger, less likely to be smokers, lower in BMI, WC, rHR, TG, SBP, DBP, FPG, and higher in HDL-C and PA (P < 0.001). Similar results were found after stratified by sex. There was no significant difference in drinking status across e-CRF quartile (P = 0.214).
Association between e-CRF and incidence of CVD, heart disease and stroke
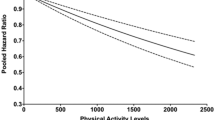
Table 2 describes the incident densities and adjusted HRs of incidence of CVD, heart disease and stroke with e-CRF overall and stratified by sex. Overall, e-CRF was inversely associated with the risk of CVD (HR 0.88, 95%CI [0.84–0.93]) after adjusting for covariates. Compared with the first quartile (Q1) of e-CRF, significantly decreased risks of CVD were found with the HR of 0.81 (95% CI: 0.71–0.93) for Q2, 0.71 (95% CI: 0.61–0.83) for Q3, and 0.61 (95% CI: 0.51–0.73) for Q4. After stratified by heart disease and stroke, similar results were found as CVD. Similar results were found with the association between e-CRF and risk of heart disease (HR 0.83, 0.77, 0.67 for Q2, Q3, Q4; HR 0.88 per 1-unit MET increase; p < 0.05) and stroke (HR 0.78, 0.65, 0.45 for Q2, Q3. Q4; HR 0.88 per 1-unit MET increase; p < 0.05).
After stratified by sex, the associations were consistent among males and females. When using e-CRF was continuous variable, each one MET increment in e-CRF was associated with lower risk of CVD (males: HR 0.91, 95%CI [0.85–0.96], females: 0.87 [0.81–0.94]), heart disease (males: 0.93 [0.86-1.00], females: 0.87 [0.80–0.94]) and stroke (males: 0.90 [0.82–0.99], females: 0.86 [0.78–0.98]) in both males and females. The effect of association for heart disease and stroke were slightly weaker than that for CVD when using e-CRF as an ordinal variable, especially for males.
Sensitivity analyses and subgroup analysis
The results of sensitivity analysis are presented in Table 3. The analysis that excluded the participants who developed events within one year after baseline and the analysis with e-CRF calculated by equations developed by Nes et al. both showed consistent results on the associations between e-CRF and incidence of CVD, heart disease and stroke [9]. Subgroup analysis that stratified by age (< 60 and ≥ 60 years) found significant associations for CVD and heart disease in both age groups, however the effect of association for stroke in subjects aged ≥ 60 years were slightly weaker.
Discussion
In this study, we explored the association between e-CRF and risk of CVD, heart disease and stroke with data from CHARLS, a representative cohort study from China. We found that higher level of e-CRF was associated with reduced risk of CVD, heart disease and stroke for both males and females. The associations between e-CRF and incidence of CVD, heart disease and stroke were robust after excluding participants who developed events within a year and stratifying participants into the mid-age (45–60 years) and the eldly (> 60 years). Furthermore, the associations based on CRF estimated by Nes et al. were consistent with that based on Jackson et al. [9, 14].
The reduction in CVD risk associated with increased e-CRF in our study are comparable to those studies that explored the association between measured CRF and CVD. In this study, each one MET increment in e-CRF was associated with a 9% (95%CI: 4-15%) and 13% (6-19%) lower risk of CVD, a 7% (0-14%) and 13% (6-20%) lower risk of heart disease and a 10% (1-18%) and 14% (2-22%) lower risk of stroke in males and females, respectively. In a meta-analysis including 84,323 participants based on 33 eligible studies, the risk of CHD/CVD decreased 15% (12-18%) as per 1-MET higher level of CRF [24]. Furthermore, the association remained robust after sex-specific and age-specific subgroup analyses, which is consistent with this study. Recently, a meta-analysis involving 392,240 individuals based on 18 prospective cohort studies showed the risk of CVD mortality was decreased by 13% (9-17%) with each one-MET increase in CRF and the dose-response associations were consistent in subgroup analyses across sex [4].
Studies looking at the associations between e-CRF and incidence of CVD are limited. Several studies have showed inconsistent associations between males and females. Compared to males with low e-CRF, males with high level of e-CRF estimated from longitudinal models were 29% less likely to develop CHD during a 12 years follow-up from ACLS prospective cohort, however females were excluded because of limited incident cases [19]. In another study based on ACLS cohort, both medium and high CRF were inversely with risks of non-fatal CVD events and CVD mortality in males. In females, only high e-CRF was significantly associated with non-fatal CVD risk [15]. In Nord-Trøndelage Health study (HUNT), e-CRF was associated with decreased risk of first acute myocardial infarction events among females but not in males [20]. In another HUNT study focusing on the association between e-CRF and risk of atrial fibrillation, the highest risk reduction of AF was 31% and 47% in the fourth quintile of eCRF when compared with the first quintile in males and females, respectively [25]. In our study, only the effect of association for heart disease and stroke were slightly weaker than that for CVD when using e-CRF as an ordinal variable, especially for males. Sex-specific algorithms or differences in included covariates and ethnicities may result in these inconsistent findings. Our study provided relative stable evidence for applying the longitudinal equations to estimate CRF and further exploring the associations between e-CRF and incidence of CVD events. All the variables in the estimating equations could be easily available in hospitals and large-population investigation. Considering the multiple limitations of measuring CRF, e-CRF obtained from non-exercise longitudinal algorithms can be a cost-effective way of assessing CRF as a useful and simple tool to identify subjects who are in high risks of CVD in routine clinical practice.
This study has several strengths. First, the data are from a nationally representative cohort with large sample size, rigorous research design and high-quality data. Second, compared with other models based on cross-sectional studies, the application of longitudinal equations may provide a more accurate estimation of e-CRF. Several limitations in this study should also be noted. First, the information of outcome was from self-reported questionnaire. Second, the effects of e-CRF for each kind of heart disease may be underestimated due to using overall heart disease as one outcome. Third, physical activity was from self-report, which may induce recall bias into the estimation of e-CRF. Lastly, other possible variables (muscle strength, maximal voluntary ventilation and maximal cardiac output) that may affect e-CRF aside from the components used in the longitudinal models were not examined in our study.
Conclusions
Non-exercise estimated CRF based on the commonly collected health indicators was inversely associated with the incidence of CVD, heart disease and stroke in both males and females in a representative sample of the Chinese population. The estimation method could be used to predict the risk of CVD events in clinical practice or large-population studies. Further research is needed to explore the associations between e-CRF changes and CVD events.
Data Availability
The authors thank the China Center for Economic Research, National School of Development, Peking University for providing the data. The data of CHARLS could be download from the website of CHARLS CHARLS (pku.edu.cn).
Abbreviations
- Q:
-
Quartile
- SD:
-
Standard deviation
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- rHR:
-
Resting heart rate
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- TG:
-
Triglycerides
- HDL-C:
-
High-density lipoprotein cholesterol
- FPG:
-
Fasting plasma glucose
- e-CRF:
-
Estimated cardiorespiratory fitness
- MET:
-
Metabolic equivalent
References
Ross R, Blair SN, Arena R, et al. Importance of assessing Cardiorespiratory Fitness in Clinical Practice: a case for fitness as a Clinical Vital sign: A Scientific Statement from the American Heart Association. Circulation. 2016;134(24):e653–99.
Kaminsky LA, Arena R, Ellingsen O, et al. Cardiorespiratory fitness and cardiovascular disease - the past, present, and future. Prog Cardiovasc Dis. 2019;62(2):86–93.
Al-Mallah MH, Sakr S, Al-Qunaibet A. Cardiorespiratory Fitness and Cardiovascular Disease Prevention: an update. Curr Atheroscler Rep. 2018;20(1):1.
Han M, Qie R, Shi X, et al. Cardiorespiratory fitness and mortality from all causes, cardiovascular disease and cancer: dose-response meta-analysis of cohort studies. Br J Sports Med. 2022;56(13):733–9.
Wang Y, Chen S, Lavie CJ, Zhang J, Sui X. An overview of non-exercise estimated cardiorespiratory fitness: estimation Equations, Cross-Validation and Application. J Sci Sport Exerc. 2019;1(1):38–53.
Martinez-Gomez D, Guallar-Castillón P, Hallal PC, Lopez-Garcia E, Rodríguez-Artalejo F. Nonexercise cardiorespiratory fitness and mortality in older adults. Med Sci Sports Exerc. 2015;47(3):568–74.
Balady GJ, Arena R, Sietsema K, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225.
Wier LT, Jackson AS, Ayers GW, Arenare B. Nonexercise models for estimating VO2max with waist girth, percent fat, or BMI. Med Sci Sports Exerc. 2006;38(3):555–61.
Nes BM, Janszky I, Vatten LJ, Nilsen TI, Aspenes ST, Wisløff U. Estimating V·O 2peak from a nonexercise prediction model: the HUNT Study, Norway. Med Sci Sports Exerc. 2011;43(11):2024–30.
Jurca R, Jackson AS, LaMonte MJ, et al. Assessing cardiorespiratory fitness without performing exercise testing. Am J Prev Med. 2005;29(3):185–93.
Cáceres JM, Ulbrich AZ, Panigas TF, Benetti M. Equações de predição da aptidão cardiorrespiratória de adultos sem teste de exercícios físicos. Revista Brasileira de Cineantropometria e Desempenho Humano 2012;14(3).
Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–82.
Jackson AS, Sui X, Hébert JR, Church TS, Blair SN. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. 2009;169(19):1781–7.
Jackson AS, Sui X, O’Connor DP, et al. Longitudinal cardiorespiratory fitness algorithms for clinical settings. Am J Prev Med. 2012;43(5):512–9.
Artero EG, Jackson AS, Sui X, et al. Longitudinal algorithms to estimate cardiorespiratory fitness: associations with nonfatal cardiovascular disease and disease-specific mortality. J Am Coll Cardiol. 2014;63(21):2289–96.
Zhang Y, Zhang J, Zhou J, et al. Nonexercise estimated Cardiorespiratory Fitness and Mortality due to all Causes and Cardiovascular Disease: the NHANES III Study. Mayo Clin Proc Innovations Qual Outcomes. 2017;1(1):16–25.
Zhao Y, Sun H, Qie R, et al. Association between cardiorespiratory fitness and risk of all-cause and cause-specific mortality. Eur J Clin Invest. 2022;52(7):e13770.
Nauman J, Nes BM, Lavie CJ, et al. Prediction of Cardiovascular Mortality by estimated Cardiorespiratory Fitness Independent of traditional risk factors: the HUNT study. Mayo Clin Proc. 2017;92(2):218–27.
Gander JC, Sui X, Hébert JR, et al. Addition of estimated cardiorespiratory fitness to the clinical assessment of 10-year coronary heart disease risk in asymptomatic men. Prev Med Rep. 2017;7:30–7.
Shigdel R, Dalen H, Sui X, Lavie CJ, Wisløff U, Ernstsen L. Cardiorespiratory Fitness and the risk of First Acute myocardial infarction: the HUNT study. J Am Heart Assoc. 2019;8(9):e010293.
Cabanas-Sánchez V, Artero EG, Lavie CJ, et al. Prediction of cardiovascular health by non-exercise estimated cardiorespiratory fitness. Heart. 2020;106(23):1832–8.
Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–8.
Wang Y, Chen S, Zhang J, et al. Nonexercise estimated Cardiorespiratory Fitness and All-Cancer Mortality: the NHANES III Study. Mayo Clin Proc. 2018;93(7):848–56.
Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–35.
Garnvik LE, Malmo V, Janszky I, Wisløff U, Loennechen JP, Nes BM. Estimated Cardiorespiratory Fitness and Risk of Atrial Fibrillation: the Nord-Trøndelag Health Study. Med Sci Sports Exerc. 2019;51(12):2491–7.
Chen G, Yi Q, Hou L et al. Transition of Hypertriglyceridemic-Waist Phenotypes and the Risk of Type 2 Diabetes Mellitus among Middle-Aged and Older Chinese: A National Cohort Study. International journal of environmental research and public health 2021;18(7).
Acknowledgements
All authors thank the CHARLS for providing data. We are grateful for those who designed, conducted and participated in this study.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Authors’ contributions.
Yuanjiao Liu and Jinghan Zhu contributed equally to this work. Yuanjiao Liu, Jinghan Zhu, Ziye Guo, Jiazhou Yu, Xuhui Zhang, Huiqing Ge and Yimin Zhu designed this study. Yuanjiao Liu and Jinghan Zhu analyzed the data. Ziye Guo and Jiazhou Yu did literature review and interpreted the result. Yuanjiao Liu and Jinghan Zhu wrote the manuscript. Xuhui Zhang, Huiqing Ge and Yimin Zhu provided revision of the manuscript. All authors read and approved the final manuscript for publication.
Author information
Authors and Affiliations
Contributions
Yuanjiao Liu and Jinghan Zhu contributed equally to this work. Yuanjiao Liu, Jinghan Zhu, Ziye Guo, Jiazhou Yu, Xuhui Zhang, Huiqing Ge and Yimin Zhu designed this study. Yuanjiao Liu and Jinghan Zhu analyzed the data. Ziye Guo and Jiazhou Yu did literature review and interpreted the result. Yuanjiao Liu and Jinghan Zhu wrote the manuscript. Xuhui Zhang, Huiqing Ge and Yimin Zhu provided revision of the manuscript. All authors read and approved the final manuscript for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Ethical approval for the study was granted by the Ethical Review Committee of Peking University and conducted by the National School for Development (China Centre for Economic Research) at Peking University following the Helsinki guideline. The Institutional Review Board (IRB) approval number for the main household survey, including anthropometrics, is IRB00001052-11015; the IRB approval number for biomarker collection, was IRB00001052-11014 [26]. Informed consent was obtained from all subjects involved in CHARLS; all participants signed written informed consent forms. All methods were carried out in accordance with relevant guidelines and regulation under above approval.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Y., Zhu, J., Guo, Z. et al. Estimated cardiorespiratory fitness and incident risk of cardiovascular disease in China. BMC Public Health 23, 2338 (2023). https://doi.org/10.1186/s12889-023-16864-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-16864-5