Abstract
Purpose
The incidence of early-onset colorectal cancer (EO-CRC), which occurs in people under age 50, has been increasing annually. The aim of this study was to provide an up-to-date estimate of the global EO-CRC burden.
Methods
We used Global Burden of Disease Study data and methodologies to describe changes in the EO-CRC burden from 1990 to 2019, including incidence, prevalence, mortality, and disability-adjusted life years (DALYs). The driving factors for cancer burden variation were further analyzed using decomposition analysis. Frontier analysis was used to visually demonstrate the potential for burden reduction in each country or region based on their development levels.
Results
The global EO-CRC incidence more than doubled, increasing from 95,737 (95% uncertainty interval (UI): 90,838–101.042) /100,000 in 1990 to 226,782 (95% UI: 207,495–248,604) /100,000 in 2019. Additionally, related deaths increased from 50,997 (95% UI: 47,692–54,410) /100,000 to 87,014 (95% UI: 80,259–94,339) /100,000, and DALYs increased from 256,1842 (95% UI: 239,4962–2,735,823) /100,000 to 4,297,573 (95% UI: 3,965,485–4,650,790) /100,000. Regarding age-standardized rates, incidence and prevalence increased significantly, while mortality and DALYs rate were basically unchanged. Decomposition analysis showed a significant increase in DALYs in the middle sociodemographic index (SDI) quintile region, in which aging and population growth played a major driving role. Frontier analysis showed that countries or regions with a higher SDI quintile tend to have greater improvement potential.
Conclusion
The current EO-CRC burden was found to be the greatest in the high-middle SDI quintile region and East Asia, which may need to adjust screening guidelines accordingly and introduce more effective interventions.
Similar content being viewed by others
Introduction
In 2019, colorectal cancer became the third most common cause of cancer deaths worldwide and the second leading cause of cancer-related disability-adjusted life years (DALYs) [1]. Early-onset colorectal cancer (EO-CRC) is defined as colorectal cancer diagnosed before the age of 50 and is reported that it accounts for about 10–12% of newly diagnosed colorectal cancer [2, 3]. Currently, around 50 is also the recommended age to start most screening programs, according to analyses of the cost-effectiveness for the sustainability of healthcare systems [4]. Although in many developed countries, the incidence of colorectal cancer in people above age 50 shows a stable or slightly decreasing trend, in recent decades, the incidence of EO-CRC has shown a continuous increasing trend in many countries, such as Australia, Canada, Denmark, and nine others [3, 5]. Based on previous long-term data, this upward trend dates back to the 1990s, at the earliest. It should be noted that studies have shown that the EO-CRC mortality rate is increasing annually, though it is decreasing in people over the age of 50 [6].
However, on the one hand, the reasons for the increasing incidence of EO-CRC have not been determined, and on the other hand, there is no reasonable explanation for why EO-CRC is often advanced and poorly differentiated when diagnosed [7]. Several findings suggest that many well recognized colorectal cancer risk factors, such as lifestyle westernization and tobacco and alcohol use, among others, play important roles in EO-CRC [8,9,10,11]. Some scholars believe that geographical and sociodemographic factors such as ethnicity and income are also associated with epidemiological changes in EO-CRC. Moreover, with the expansion of screening programs and the popularization of colonoscopy, the worldwide EO-CRC burden has changed dramatically [12, 13].
In conclusion, the global EO-CRC burden is ominous, and effective use of limited health resources requires an understanding of burden variance over time and across geographical locations as well as different factors’ roles in these changes. This study aimed to provide the latest estimates of the EO-CRC burden in 204 countries and regions worldwide. The relationship between the level of sociodemographic development and the EO-CRC burden was also studied. Finally, potential improvements in the EO-CRC burden were analyzed to identify those countries or regions where more work is needed.
Methods
Study population and data collection
The Global Burden of Disease (GBD) Study 2019 comprehensively assessed health loss in 204 countries and territories using the latest epidemiological data sources and improved standardized methods and found that health loss was caused by 369 diseases and injuries and 87 risk factors. In the present study, we obtained and analyzed GBD Study data on EO-CRC incidence, prevalence, mortality, and DALYs (< age 50) at the global, regional, and national levels.
The sociodemographic index (SDI), a comprehensive measure of education, economic, and fertility levels, including five levels corresponding to the five SDI quintiles (i.e., low, low-middle, middle, high-middle, and high), was also used.
All data for this study are available at: http://ghdx.healthdata.org/gbd-results-tool. Data analysis was completed on April 1, 2022. The Fujian Medical University Union Hospital (FMUUH) Institutional Review Board determined that the study did not require approval because it used publicly available data. All methods were carried out in accordance with relevant guidelines and regulations.
Statistical analysis
Previous studies have explained the methodologies of the GBD Study 2019 in detail [1, 14]. In the present study, a 95% uncertainty interval (UI) was calculated for each variable. All rates are reported per 100,000 population. All tests were two sided, and P values of less than .05 were considered significant.
Joinpoint regression analysis was performed to assess trends in the EO-CRC disease burden, using Joint Command Line Version 4.5.0.1, provided by the United States National Cancer Institute Surveillance Research Program. This software tracks trends in data over time and then fits the simplest model possible to the data by connecting several different line segments on a logarithmic scale. Average annual percentage changes (AAPCs) were calculated to assess trends, AAPCs is a geometrically weighted average of the different annual percentage changes from the joinpoint trend analysis, for which weights are equal to the length of each period during the specified time interval [15]. The 95% confidence interval (CI) was obtained from the linear regression model. For the AAPC value and 95% CI above zero, the corresponding age-standardized rate (ASR) showed an upward trend and vice versa. If the 95% CI of the AAPC included zero, the ASR was considered to be stable over time [16].
Decomposition analysis was used to visually demonstrate the role of the three factors driving changes in DALYs between 1990 and 2019 (i.e., aging, population, and epidemiology). Epidemiological changes refer to the underlying age and population-adjusted mortality and morbidity rates [17]. We applied frontier analysis to further assess the relationship between the EO-CRC burden and sociodemographic development. To produce a non-linear frontier, this frontier implies the lowest achievable burden determined according to development status. We used non-parametric data envelope analysis and referenced detailed descriptions in previous studies [18, 19]. The distance between the observed DALYs rate in a country and its frontier, defined as the effective difference, represents an unrealized health gain that exists based on the current level of development in the country or region. All statistical analyses and graphics were executed using R version 3.5.1.
Results
Overview of the Global Burden
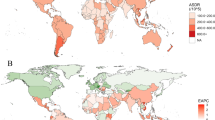
Figure 1 shows the age-standardized incidence of EO-CRC in 204 countries and territories. EO-CRC incidence more than doubled, rising from 95,737 cases (95% UI: 90,838–101,042) in 1990 to 226,782 cases (95% UI: 207,495–248,604) worldwide in 2019 (Table 1). During the same period, the age-standardized incidence rate increased from 2.95 (95% UI: 2.8–3.11) /100,000 in 1990 to 4.04 (95% UI: 3.7–4.43)/100,000 in 2019, with an average annual increase of 1.01% (95% confidence interval [1]: 0.76%–1.26%). By sex, the number of incidence cases in males 137,686 (95% UI: 121,841–155,610) was higher than that in females 89,096 (95% UI: 80,068–98,817), as was the age-standardized incidence rate, which increased faster in males than in females (males: AAPC = 1.43; 95% CI: 1.28–1.58; females: AAPC = 0.43; 95% CI: 0.27–0.59).
The incidence of early-onset colorectal cancer for both sexes in 204 countries and territories. A The age-standardized incidence of early-onset colorectal cancer in 2019; B The AAPC of age-standardized incidence of early-onset colorectal cancer from 1990 to 2019. AAPC, average annual percentage change
Figure 2 and Table 2 show the prevalence of EO-CRC in 1990 and 2019 for all locations, as well as the AAPC for 2009 and 2019. The number of EO-CRC cases increased by 169.5% in 2019 compared to 1990. The age-standardized prevalence rate increased from 16.31 (95% UI: 15.53–17.18) /100,000 in 1990 to 25.44 (95% UI: 23.24–27.9) /100,000 in 2019, with an average annual increase of 1.43% (95% CI: 1.34%–1.52%). The EO-CRC prevalence and rate of increase are much higher in males than in females.
The prevalence of early-onset colorectal cancer for both sexes in 204 countries and territories. A The age-standardized prevalence of early-onset colorectal cancer in 2019; B The AAPC of age-standardized prevalence of early-onset colorectal cancer from 1990 to 2019. AAPC, average annual percentage change
From 1990 to 2019, the number of worldwide deaths associated with EO-CRC increased from 50,997 (95% UI: 47,692–54,410) to 87,014 (95% UI: 80,259–94339; Table 3). However, the age-standardized mortality rate essentially remained flat over the same period (AAPC = 0.07%; 95% CI: -0.16–0.02). The worldwide EO-CRC age-standardized mortality rate and its changes are shown in Fig. 3. Regarding gender, the number of male deaths was 51,243 (95% UI: 45,960–56957) compared with 35,771 (95% UI: 32,388–39350) for females. The age-standardized death rate followed a similar trend. Evidently, men’s age-standardized mortality rate increased slightly each year (AAPC = 0.23; 95% CI: 0.07–0.38), but this was not the case for women (AAPC = -0.57; 95% CI: -0.71 – -0.44).
The mortality of early-onset colorectal cancer for both sexes in 204 countries and territories. A The age-standardized mortality of early-onset colorectal cancer in 2019; B The AAPC of age-standardized mortality of early-onset colorectal cancer from 1990 to 2019. AAPC, average annual percentage change
Worldwide DALYs caused by EO-CRC increased from 2,561,842 (95% UI: 239,4962–2735823) in 1990 to 4,297,573 (95% UI: 3,965,485–4,650,790) in 2019 (Table 4). The age-standardized DALYs rate was 77.7 (95% UI: 72.76–82.85) /100,000 in 1990 and 76.86 (95% UI: 70.91–83.19) /100,000 in 2019 (Fig. 4A) and has essentially been flat over the past 30 years (AAPC = -0.08; 95% CI: -0.16–0.01; Fig. 4B). Additionally, the number of DALYs in males was significantly higher than that in females, with an opposite trend (males: AAPC = 0.24; 95% CI: 0.09–0.39; females: AAPC = -0.55; 95% CI: -0.68 – -0.42).
The DALYs of early-onset colorectal cancer for both sexes in 204 countries and territories. A The age-standardized DALYs of early-onset colorectal cancer in 2019; B The AAPC of age-standardized DALYs of early-onset colorectal cancer from 1990 to 2019. ASR, age-standardized rate; AAPC, average annual percentage change; DALYs, Disability-Adjusted Life Years
EO-CRC Burden by SDI Quintile
Figure 5 shows EO-CRC incidence, prevalence, deaths, and DALYs grouped by SDI quintiles from 1990 to 2019. The middle SDI region had the most incidence cases (76,197; 95% UI: 67,037–86,572) and the most death cases (31,372; 95% UI: 28,038–34,858) and DALYs (155,0467; 95% UI: 138,8571–1,717,229). Regarding ASR, higher SDI quintiles tend to have higher age-standardized incidence rates, with the highest found in the high SDI quintiles region (6.13; 95% UI: 5.57–6.73/100, 000) (Fig. 6, Table 1). EO-CRC prevalence and variance across SDI quintiles are shown in Table 2 and Fig. 6. The highest age-standardized mortality rate was found in the high-middle SDI region (1.87; 95% UI: 1.69–2.08/100, 000). Regarding age-standardized DALYs, the high-middle SDI region ranked first (93.91; 95% UI: 84.98–103.82/100,000). Additionally, the EO-CRC burden was generally greater for males than for females in every SDI quintile (Fig. 6).
EO-CRC Burden in 21 GBD Regions
Among 21 GBD regions by SDI in 2019, East Asia had the highest EO-CRC incidence (91,083; 95% UI: 75,766–107,853), while high-income North America had the highest age-standardized incidence rate (7.18, 95% UI: 6.13–8.4/100,000; Table 1, Figure S1A). East Asia saw the most rapid increase in incidence rates (AAPC = 2.94; 95% CI: 2.7–3.18). Regarding the age-standardized prevalence rate, high-income North America (51.94; 95% UI: 44.67–60.39) and Australasia (51.94; 95% UI: 39.05–69.28) ranked first (Table 2, Figure S2A). East Asia had the most deaths (27,495; 95% UI: 22,938–32,625) and the highest age-standardized mortality rate (2.19; 95% UI: 1.83–2.59/100,000; Table 3, Figure S3A) as well as the highest age-standardized DALYs rate (111.28, 95% UI: 94.03–130.8; Table 4, Figure S4A). Central Latin America has the fastest growth rate in DALYs (AAPC = 1.26; 95% CI: 1.19–1.33).
EO-CRC Burden by Country or Region
In 2019, China had the most incidence cases (87,551; 95% UI: 72,074–104,615; Table S1), the most prevalence cases (591,944; 95% UI: 489,497–705,006; Table S2), the most death cases (26,320; 95% UI: 21,827–31,571; Table S3) and the most DALYs numbers (1,308,591; 95% UI: 1,096,385–1,556,940; Table S4; Figure S4B) in the world. Regarding ASR, the Chinese province of Taiwan had the highest incidence (13.19; 95% UI: 9.26–18.38/100,000; Figure S1B), prevalence (91.46; 95% UI: 64.15–127.86/100, 000; Figure S2B), mortality (3.46; 95% UI: 2.48–4.7/100,000; Figure S3B), and DALYs rates (173.31; 95% UI: 124.55–236.4/100,000; Fig. 4B) in the world. Additionally, Jamaica has shown the fastest cancer burden increase.
Decomposition analysis of age-standardized DALYs rates
The past 30 years have seen a significant global increase in DALYs, with the largest increase occurring in middle SDI quintile regions (Fig. 7). Aging and population growth accounted for 42.31% and 59.96%, of the worldwide increase in DALYs, respectively (Table S5), with the most significant aging contribution occurring in the high SDI quintile (370.5%), where population growth had the largest effect on DALYs growth (143.28%). The effect of epidemiological change on DALYs growth was negative (-2.27%) worldwide, and this effect was the most pronounced in the high SDI quintile (-413.78%). The effects of demography and epidemiology on DALYs differed across countries and regions (Table S5).
Changes in early-onset colorectal cancer DALYs according to population-level determinants of population growth, aging, and epidemiological change from 1990 to 2019 at the global level and by SDI quintile. The black dot represents the overall value of change contributed by all 3 components. For each component, the magnitude of a positive value indicates a corresponding increase in early-onset colorectal cancer DALYs attributed to the component; the magnitude of a negative value indicates a corresponding decrease in early-onset colorectal cancer DALYs attributed to the related component. SDI: Socio-demographic index; DALYs, Disability-Adjusted Life Years
Frontier analysis of age-standardized DALYs rates
The unrealized health gains of countries or regions at different levels of development during the period 1990–2019 are shown in Fig. 8A. Figure 8B and Table S6 show the DALYs burden and the effective difference in countries or regions with different sociodemographic development levels in 2019. With sociodemographic development, effective difference generally increased to some extent, indicating that countries or regions with a higher SDI have greater burden improvement potential (Fig. 8B).
Frontier analysis based on SDI and age-standardized early-onset colorectal cancer DALY rate in 2019. The frontier is delineated in solid black color; countries and territories are represented as dots. The top 15 countries with the largest effective difference (largest early-onset colorectal cancer DALYs gap from the frontier) are labeled in black; examples of frontier countries with low SDI (< 0.5) and low effective difference are labeled in blue (e.g., Somalia, Niger, Nepal, Bangladesh, and the Gambia), and examples of countries and territories with high SDI (> 0.85) and relatively high effective difference for their level of development are labeled in red (e.g., USA, Japan, Andorra, Monaco, Taiwan (province of China). Red dots indicate an increase in age-standardized early-onset colorectal cancer DALYs rate from 1990 to 2019; blue dots indicate a decrease in age-standardized early-onset colorectal cancer DALYs rate between 1990 and 2019. SDI: Socio-demographic index; DALYs: Disability-Adjusted Life Years
Risk factors of EO-CRC
In figure S5, we identified the contribution of 11 risk factors to DALYs due to EO-CRC for 21 GBD regions in 2019. Globally, a diet low in milk (32.8%), a diet low in whole grains (30.1%) played the main contributor to EO-CRC DALYs, while low physical activity (3.2%) and a diet low in fiber (4.5%) had less contribution to DALYs. Differences in regions’ development status could also affect the contribution of various risk factor to DALYs. In the high SDI region, a diet low in whole grains (31.2%) was the main risk factors. While in the low SDI region, the main risk factors was a diet low in calcium (39.8%). A diet low in calcium and milk were the main risk factor in Sub − Saharan Africa and Asia. In high-income North America, the main risk factor was a diet low in whole grains, while a diet low in milk was the main risk factor in the high-income Asia Pacific. (Figure S5).
Discussion
Similar to previous studies, [3] we found 136.9% new EO-CRC incidence worldwide in 2019 compared to 30 years ago. The global EO-CRC incidence generally increased, but the cause was unclear, possibly due to the birth cohort effect [20]. As a result of economic and industrial development, developing world lifestyles have gradually westernized, increasing the probability of exposure to behavioral risk factors including high red or processed meat intake, obesity, lack of involvement in sports, sedentariness, and premature smoking and drinking among those born in the second half of the 20th century [3, 5, 9, 21,22,23,24]. Studies have also shown that an increased EO-CRC risk in American women is associated with diet and lifestyle factors that lead to hyperinsulinemia [25]. Antibiotic use and changes in the gut microbiome may also play a role [26, 27].
The overall trend in age-standardized mortality rates worldwide was stable, and regions with a higher SDI quintile (high and high-middle SDI) had a decreasing mortality rate. Differences in global morbidity and mortality trends may be due to the normalization of screening tests (colonoscopy and fecal occult blood tests) and cancer registries. However, the corresponding increase in incidence due to the increased detection rate may be short-lived, as more adenomatous polyps will be excised during colonoscopy. Widespread use of early screening programs can significantly reduce mortality [28]. Improvements in medical technology, such as surgery, radiotherapy and chemotherapy, and targeted therapies, are also main reasons for the decline [29, 30].
In 2019, EO-CRC DALYs increased by 67.8% compared to 1990, with East Asia, Southeast Asia, and Southern Latin America bearing the heaviest burdens. Although developed regions have a higher EO-CRC incidence, we found that the increase in DALYs over the past 30 years occurred mainly in less developed regions, particularly middle- and low-SDI regions. We should pay attention to the frontier countries with low SDI in the frontier analysis, which showed excellent performance with limited resources. These countries’ practices and models could be valuable reference points for other countries with limited resources and a large burden. Conversely, some high SDI countries and regions such as Taiwan, Monaco, and the United States, performed poorly, suggesting that other factors have overwhelmed the health benefits of development. Future research is needed to further explore drivers in leading countries and barriers in lagging countries.
We found significantly more EO-CRC cases in men than in women, as well as age-standardized rates. Further, the gendered differences became more pronounced over time. According to the literature, men account for the majority of the colorectal cancer burden, mainly due to a higher prevalence of visceral fat, smoking, and drinking [31, 32]. In women, endogenous estrogens and oral contraceptives have been shown to reduce the cancer risk [33, 34]. We also found gendered differences in mortality, with EO-CRC-related mortality in males increasing and continuing to outpace that of females, while the female mortality rate declined, and women tended to have better survival outcomes [35]. Given the continued increase in the incidence of EO-CRC, recent recommendations to modify screening protocols are a good start. Additionally, the recommended age for screening could be lowered to 45 years, and the screening plan could be further modified for young adults with high-risk factors such as being male, smoking, obesity, a personal history of polyps or adenoma, and related family history [36].
The limitations of GBD research, as described in previous literature, are mainly in the following aspects. The first is that the inevitable loss of data severely affected the accuracy of the research. Inadequate cancer registries in some underdeveloped countries in Africa and Asia lead to underestimation of cases, as well as misdiagnosis or missed diagnosis due to poor health resources, although robust statistical methods were used to overcome this effect. Secondly, the data in this study are from different countries, which will inevitably lead to uneven quality, such as measurement bias, reporting delay, or disease misclassification. Finally, a GBD data lag occurred because the current estimates were calculated based on past trends and covariates [20, 37]. To balance, the limitation in GBD study, more international collaborations should be encouraged, including annual searching of available data with national collaborators. And data from more sources should be included in the statistics for cancer data, including epidemiological studies, vital registration systems, as well as cancer registries. In addition, the detailed cleaning, correction, and smoothing routines developed by GBD collaborators are also effective measures to overcome these limitations, including a series of advanced statistical modeling methods.
Conclusion
This study provided a comprehensive estimate of the global EO-CRC burden. Age-standardized incidence and prevalence rates increased sharply worldwide between 1990 and 2019, while age-standardized death and DALYs rates changed less dramatically. The EO-CRC burden varied significantly with gender, sociodemographic development, and geographical location. We found that areas with a higher level of sociodemographic development tend to have a higher burden. This study can provide a basis for the formulation of relevant policies and the rational allocation of limited resources.
Availability of data and materials
Publicly available datasets were analyzed in this study. The data can be found here: http://ghdx.healthdata.org/gbd-results-tool.
References
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22.
Stoffel EM, Murphy CC. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology. 2020;158(2):341–53.
Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, Jemal A. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68(12):2179–85.
Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol Oncol. 2019;13(2):109–31.
Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, Zadnik V, Pellisé M, Esteban L, Kaminski MF, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68(10):1820–6.
Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–64.
Chang DT, Pai RK, Rybicki LA, Dimaio MA, Limaye M, Jayachandran P, Koong AC, Kunz PA, Fisher GA, Ford JM, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol. 2012;25(8):1128–39.
Khan NA, Hussain M, ur Rahman A, Farooqui WA, Rasheed A, Memon AS. Dietary Practices, Addictive Behavior and Bowel Habits and Risk of Early Onset Colorectal Cancer: a Case Control Study. Asian Pac J Cancer Prev. 2015;16(17):7967–73.
Nimptsch K, Wu K. Is Timing Important? The Role of Diet and Lifestyle during Early Life on Colorectal Neoplasia. Curr Colorectal Cancer Rep. 2018;14(1):1–11.
Rosato V, Bosetti C, Levi F, Polesel J, Zucchetto A, Negri E, La Vecchia C. Risk factors for young-onset colorectal cancer. Cancer Causes Control. 2013;24(2):335–41.
Kim JY, Jung YS, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Choi KY, Park DI. Different risk factors for advanced colorectal neoplasm in young adults. World J Gastroenterol. 2016;22(13):3611–20.
Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130–60.
Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, Kuipers EJ. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–49.
GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–49.
Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–51.
Du M, Chen W, Liu K, Wang L, Hu Y, Mao Y, Sun X, Luo Y, Shi J, Shao K, et al. The Global Burden of Leukemia and Its Attributable Factors in 204 Countries and Territories: Findings from the Global Burden of Disease 2019 Study and Projections to 2030. J Oncol. 2022;2022:1612702.
Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, Maddukuri G, Tsai CY, Floyd T, Al-Aly Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94(3):567–81.
GBD 2015 Healthcare Access and Quality Collaborators. Electronic address: cjlm@uw.edu; GBD 2015 Healthcare Access and Quality Collaborators. Healthcare Access and Quality Index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990-2015: a novel analysis from the Global Burden of Disease Study 2015. Lancet. 2017;390(10091):231–66.
Xie Y, Bowe B, Xian H, Balasubramanian S, Al-Aly Z. Rate of Kidney Function Decline and Risk of Hospitalizations in Stage 3A CKD. Clin J Am Soc Nephrol. 2015;10(11):1946–55.
GBD 2019 Colorectal Cancer Collaborators. Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7(7):627–47.
Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713–32.
Young JP, Win AK, Rosty C, Flight I, Roder D, Young GP, Frank O, Suthers GK, Hewett PJ, Ruszkiewicz A, et al. Rising incidence of early-onset colorectal cancer in Australia over two decades: report and review. J Gastroenterol Hepatol. 2015;30(1):6–13.
Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH, Song M, He X, Fuchs CS, Ogino S, Willett WC, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 2019;5(1):37–44.
Patel P, De P. Trends in colorectal cancer incidence and related lifestyle risk factors in 15–49-year-olds in Canada, 1969–2010. Cancer Epidemiol. 2016;42:90–100.
Yue Y, Hur J, Cao Y, Tabung FK, Wang M, Wu K, Song M, Zhang X, Liu Y, Meyerhardt JA, et al. Prospective evaluation of dietary and lifestyle pattern indices with risk of colorectal cancer in a cohort of younger women. Ann Oncol. 2021;32(6):778–86.
Hofseth LJ, Hebert JR, Chanda A, Chen H, Love BL, Pena MM, Murphy EA, Sajish M, Sheth A, Buckhaults PJ, et al. Early-onset colorectal cancer: initial clues and current views. Nat Rev Gastroenterol Hepatol. 2020;17(6):352–64.
Zaborowski AM, Abdile A, Adamina M, Aigner F, d’Allens L, Allmer C, Álvarez A, Anula R, Andric M, Atallah S, et al. characteristics of early-onset vs late-onset colorectal cancer: a review. JAMA Surg. 2021;156(9):865–74.
Levin TR, Corley DA, Jensen CD, Schottinger JE, Quinn VP, Zauber AG, Lee JK, Zhao WK, Udaltsova N, Ghai NR, et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology. 2018;155(5):1383-1391.e1385.
Boland GM, Chang GJ, Haynes AB, Chiang YJ, Chagpar R, Xing Y, Hu CY, Feig BW, You YN, Cormier JN. Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Cancer. 2013;119(8):1593–601.
Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–73.
Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3(1):13.
Wilsnack RW, Wilsnack SC, Kristjanson AF, Vogeltanz-Holm ND, Gmel G. Gender and alcohol consumption: patterns from the multinational GENACIS project. Addiction. 2009;104(9):1487–500.
Murphy N, Strickler HD, Stanczyk FZ, Xue X, Wassertheil-Smoller S, Rohan TE, Ho GY, Anderson GL, Potter JD, Gunter MJ. A prospective evaluation of endogenous sex hormone levels and colorectal cancer risk in postmenopausal women. J Natl Cancer Inst. 2015;107(10):djv210.
Luan NN, Wu L, Gong TT, Wang YL, Lin B, Wu QJ. Nonlinear reduction in risk for colorectal cancer by oral contraceptive use: a meta-analysis of epidemiological studies. Cancer Causes Control. 2015;26(1):65–78.
Yang Y, Wang G, He J, Ren S, Wu F, Zhang J, Wang F. Gender differences in colorectal cancer survival: a meta-analysis. Int J Cancer. 2017;141(10):1942–9.
Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, Etzioni R, McKenna MT, Oeffinger KC, Shih YT, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250–81.
GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17–30.
Acknowledgements
The authors appreciate the works by the Global Burden of Disease Study 2019 collaborators. Thanks to Xiao Ming (Xiaoming_room@hotmail.com) for his work in the GBD database. His excellent sharing of GBD database analysis procedure and other public database, makes it easier for us to explore the GBD database.
Funding
Joint Funds for the innovation of Science and Technology, Fujian province (2017Y9038, 2019Y9101, 2020Y9071); Natural Science Foundation of Fujian Province (2020J011030, 2022J01753); Fujian Provincial Health Technology Project (2020GGB022, 2020CXA025); Medical Science Research Foundation of Beijing Medical and Health Foundation(B20062DS); Bethune Charitable Foundation (X-J-2018–004).
Author information
Authors and Affiliations
Contributions
HP, ZZha, SH, YH and PC conceived of and designed the project. HP and YD collected the data. ZZha, ZZhe analysed and interpreted the data. HP and YD drafted the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Fujian Medical University Union Hospital (FMUUH) Institutional Review Board determined that the study did not require ethics approval and consent to participate because it used publicly available data.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. Age-standardized incidence rate at the global, regional, and national level. (A) Age-standardized incidence s rate of early-onset colorectal cancer globally and for 21 GBD regions by SDI, 1990–2019. (B) Age-standardized incidence rates of early-onset colorectal cancer for 204 countries and territories in 2019. The black line represents the expected age-standardized incidence rate of rheumatic heart disease based solely on SDI. GBD: Global Burden of Diseases, Injuries, and Risk Factors Study; SDI: Socio-demographic index; DALYs, Disability-Adjusted Life Years.
Additional file 2:
Figure S2. Age-standardized prevalence rate at the global, regional, and national level. (A) Age-standardized prevalence rate of early-onset colorectal cancer globally and for 21 GBD regions by SDI, 1990–2019. (B) Age-standardized prevalence rates of early-onset colorectal cancer for 204 countries and territories in 2019. The black line represents the expected age-standardized prevalence rate of rheumatic heart disease based solely on SDI. GBD: Global Burden of Diseases, Injuries, and Risk Factors Study; SDI: Socio-demographic index; DALYs, Disability-Adjusted Life Years.
Additional file 3:
Figure S3. Age-standardized mortality rate at the global, regional, and national level. (A) Age-standardized mortality rate of early-onset colorectal cancer globally and for 21 GBD regions by SDI, 1990–2019. (B) Age-standardized mortality rates of early-onset colorectal cancer for 204 countries and territories in 2019. The black line represents the expected age-standardized mortality rate of rheumatic heart disease based solely on SDI. GBD: Global Burden of Diseases, Injuries, and Risk Factors Study; SDI: Socio-demographic index; DALYs, Disability-Adjusted Life Years.
Additional file 4:
Figure S4. Age-standardized DALYs rate at the global, regional, and national level. (A) Age-standardized DALYs rate of early-onset colorectal cancer globally and for 21 GBD regions by SDI, 1990–2019. (B) Age-standardized DALYs rates of early-onset colorectal cancer for 204 countries and territories in 2019. The black line represents the expected age-standardized DALYs rate of rheumatic heart disease based solely on SDI. GBD: Global Burden of Diseases, Injuries, and Risk Factors Study; SDI: Socio-demographic index; DALYs, Disability-Adjusted Life Years.
Additional file 5:
Figure S5. Percentage of age-standardised DALYs due to early-onset colorectal cancer attributable to risk factors for 21 GBD regions, both sexes, 2019. GBD: Global Burden of Diseases, Injuries, and Risk Factors Study; SDI: Socio-demographic index; DALYs, Disability-Adjusted Life Years.
Additional file 6:
Table S1. Incidence of early-onset colorectal cancer in 1990 and 2019 with AAPC from 2009 and 2019 at countries/territories level, both sexes.
Additional file 7:
Table S2. Prevalence of early-onset colorectal cancer in 1990 and 2019 with AAPC from 2009 and 2019 at countries/territories level, both sexes.
Additional file 8:
Table S3. Mortality of early-onset colorectal cancer in 1990 and 2019 with AAPC from 2009 and 2019 at countries/territories level, both sexes.
Additional file 9:
Table S4. DALY of early-onset colorectal cancer in 1990 and 2019 with AAPC from 2009 and 2019 at countries/territories level, both sexes.
Additional file 10:
Table S5. Changes in DALYs number according to population-level determinants and causes from 1990 to 2019.
Additional file 11:
Table S6. Frontier DALYs, and effective difference by country or territory.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pan, H., Zhao, Z., Deng, Y. et al. The global, regional, and national early-onset colorectal cancer burden and trends from 1990 to 2019: results from the Global Burden of Disease Study 2019. BMC Public Health 22, 1896 (2022). https://doi.org/10.1186/s12889-022-14274-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-022-14274-7