Abstract
Background
Increasing evidence suggests that leptin is involved in the pathology of autism spectrum disorder (ASD). In this study, our objective was to investigate the levels of leptin in the blood of children with ASD and to examine the overall profile of adipokine markers in ASD through meta-analysis.
Methods
Leptin concentrations were measured using an enzyme-linked immunosorbent assay (ELISA) kit, while adipokine profiling, including leptin, was performed via meta-analysis. Original reports that included measurements of peripheral adipokines in ASD patients and healthy controls (HCs) were collected from databases such as Web of Science, PubMed, and Cochrane Library. These studies were collected from September 2022 to September 2023 and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Standardized mean differences were calculated using a random effects model for the meta-analysis. Additionally, we performed meta-regression and explored heterogeneity among studies.
Results
Our findings revealed a significant increase in leptin levels in children with ASD compared to HCs (p = 0.0319). This result was consistent with the findings obtained from the meta-analysis (p < 0.001). Furthermore, progranulin concentrations were significantly reduced in children with ASD. However, for the other five adipokines analyzed, there were no significant differences observed between the children with ASD and HCs children. Heterogeneity was found among the studies, and the meta-regression analysis indicated that publication year and latitude might influence the results of the meta-analysis.
Conclusions
These findings provide compelling evidence that leptin levels are increased in children with ASD compared to healthy controls, suggesting a potential mechanism involving adipokines, particularly leptin, in the pathogenesis of ASD. These results contribute to a better understanding of the pathology of ASD and provide new insights for future investigations.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by early impairments in social communication and interaction, as well as restricted and repetitive behaviors [1, 2]. It affects approximately 1% of the global population, with a higher prevalence in males than in females [3]. Both genetic and environmental factors have been suggested to contribute to the development of ASD [4].
Recent studies have associated elevated plasma leptin levels in early childhood with a higher incidence of autism, as observed in the Boston Birth Cohort [5]. Leptin, originally known for its role in regulating food intake and energy balance [6], has gained increased attention in the field of psychiatry [7]. Leptin is in the same protein family as interleukin (Il)-6, an inflammatory cytokine, and its dysregulation has been associated with psychopathology, and evidence suggests that this relationship is related to leptin’s inflammatory function [8,9,10]. It has been implicated in various psychiatric disorders such as schizophrenia [11, 12], major depressive disorder [13], and bipolar disorder [14, 15]. The role of leptin in ASD has also become a subject of interest, but the underlying mechanisms remain to be determined. Other adipokines, which are inflammatory mediators mainly secreted by adipose tissue and some immune cells, have also garnered increasing attention in ASD research.
Adipokines have the potential to impact immune responses and are believed to play a role in the pathophysiology of ASD [16]. Several studies have reported alterations in adipokine levels in ASD, including downregulation of adiponectin [17], among others. However, the findings regarding adipokines in ASD are inconsistent, possibly due to differences in patient populations, variations in methodologies, or small sample sizes lacking statistical power.
To address these discrepancies and clarify the relationship between adipokines and autism, this study aimed to investigate serum leptin levels in ASD patients and HCs, and to conduct a systematic review of the literature. This comprehensive review, the first of its kind, will assess studies that have measured adipokines in both ASD patients and control groups. By doing so, we hope to identify potential differences in adipokine profiles associated with the development of ASD and shed light on the possible involvement of adipokines in the pathogenesis of autism.
Material and method
Subjects and sample collection
A total of 42 patients with autism spectrum disorder (ASD) were recruited from Hunan Provincial Maternal and Child Healthcare Hospital. The diagnosis of ASD was made according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Additionally, 42 healthy control (HC) subjects without psychiatric illnesses, as evaluated by trained psychiatrists, were also recruited from the same hospital via advertisement. The age of the enrolled children was between 3–7 years old, and there was no other medical history. Autistic children with a history of seizure or psychotic disorders, severe head injury, or any other acute or chronic mental disease, inflammatory illness, or comorbidities were excluded. Control participants with a history of any immunological or infectious illness; or metabolic, psychiatric, or neurological disorders were also excluded. The demographic and clinical characteristics of the patients and controls are presented in Fig. 1A. Written informed consent was obtained from all participants, and the study protocol was approved by the Ethics Committee at Minzu University of China, adhering to the principles of the Declaration of Helsinki. Peripheral blood was collected from all participants in the morning, between 7 and 8 AM. Blood samples were collected in anticoagulation tubes, and serum samples were obtained by centrifugation at 5,000 rpm for 15 min. The samples were stored at -80 °C until further analysis.
Serum leptin levels in ASD patients and HC subjects. A Demographic and clinical characteristics of patients and controls. B Serum leptin levels in ASD patients and HC subjects. C Serum leptin levels in male ASD patients and male HC subjects. D Serum leptin levels in female ASD patients and female HC subjects. E ROC curves evaluating the accuracy of serum leptin levels in differentiating between ASD patients and HC subjects. * p < 0.05, ** p < 0.01, *** p < 0.001
Leptin concentration measurement
Leptin levels in peripheral blood were measured using a commercially available ELISA kit (Wuhan Eiaab Science Incorporated Company, Wuhan, China), following the manufacturer's instructions.
Article selection process
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [18]. Two investigators systematically searched PubMed, Web of Science, and Cochrane Library from September 2022 to September 2023. The search was conducted using specific keywords related to autism spectrum disorder and adipokines: (autism or autistic disorder or ASD) and (leptin or ghrelin or adiponectin or soluble leptin receptor or lipocalin or resistin or insulin or omentin or obestatin or osteopontin or chemerin or visfatin or vaspin or apelin or progranulin or dipeptidyl peptidase 4 or retinol-binding protein 4 or adipokines). Only peer-reviewed English articles published between January 1971 and January 2023 were included for analysis. The inclusion criteria involved studies that provided serum or plasma adipokine data for children with ASD and controls. Studies were excluded if they lacked necessary data, involved animal models, lacked a healthy control group, used samples other than serum or plasma, or did not involve patients with ASD.
Data extraction
Data extraction included potential adipokine concentrations with standard deviation (SD), sample size, and P values for effective size (ES) generation. Information such as country, gender, age, sampling source, publication year, assay type, and diagnosis were also recorded for each included study (eTable 1). The quality of the included studies was assessed using the Newcastle–Ottawa Quality Assessment Scale (eTable 2).
Data analysis
Comprehensive Meta-analysis software was used to analyze the extracted data. When necessary, sample size and P values were utilized for ES generation due to a lack of direct data on adipokine concentrations. The random-effects model was employed when significant between-study heterogeneity was present, while the fixed-effect model was used otherwise. Hedge's g statistic analysis, sensitivity analysis, I2 statistic, Cochrane Q test, meta-regressions, and Egger's test were performed as previously described [19]. Statistical significance was set at P < 0.05, except for the Cochrane Q test, where P < 0.10 was considered statistically significant.
Results
Investigation of serum leptin concentrations in ASD patients
The demographic and clinical characteristics of patients and controls are presented in Fig. 1A. The ELISA assay revealed that serum leptin levels were significantly increased in ASD patients compared to HC subjects (Mean difference = 5.589, 95% confidence interval (CI) of difference = 0.4946 to 10.68, p = 0.0319) (Fig. 1B). Further analysis showed a significant reduction in serum leptin levels in male ASD patients (Mean difference = 9.194, 95% CI of difference = 0.2013 to 18.19, p = 0.0453) (Fig. 1C), while no significant difference was observed in female ASD patients (Mean difference = -2.270, 95% CI of difference = -7.607 to 3.068, p = 0.3955) (Fig. 1D). Receiver Operating Characteristic curve (ROC) analysis indicated that leptin demonstrated limited accuracy in differentiating between ASD patients and controls, with an area under curve (AUC) of 0.660 (95% CI: 0.542—0.778) (Fig. 1E).
Meta-analysis of adipokines
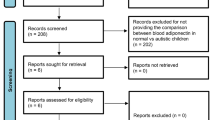
After thorough screening, a total of 19 articles were included in the meta-analysis, providing data on 2196 participants, including 740 ASD subjects and 1456 healthy controls. The distribution of the literature selection process is presented in Fig. 2.
Differences in peripheral marker concentrations
Significant differences in peripheral adipokine concentrations were observed between ASD and HC groups for two analytes. Leptin levels in the blood were significantly higher in ASD children compared to HC children (Hedges g (95% CI): 1.310 (0.684 to 1.936), Q = 149.362, p < 0.001, 11 studies) (Table 1 and Fig. 3A). Conversely, progranulin concentrations were significantly decreased in ASD children compared to controls (Hedges g (95% CI): -0.778 (-1.130 to -0.427), Q = 0.022, p = 0.882, 2 studies) (Table 1 and Fig. 3B). However, adiponectin, insulin, ghrelin, resistin, and dipeptidyl peptidase-4 did not show significant associations with ASD (Table 1). Further investigation of heterogeneity was conducted for the leptin analysis due to the limited number of studies available for progranulin. High levels of heterogeneity were observed in the plasma leptin analysis (I2 = 93.905; 9 articles) (Fig. 3C). However, adiponectin, insulin, ghrelin, resistin and dipeptidyl peptidase-4 did not show the significantly difference in ASD children compared to HC children (Fig. 4A-E), which may require further researches.
Forest plots illustrating the effect sizes for leptin (A) and progranulin (B) in ASD patients compared to HC subjects. Forest plot demonstrating pooled results and heterogeneity analysis for leptin levels between ASD patients and HC subjects, stratified by source of sampling (plasma). Heterogeneity: Q = 131.262, p < 0.001, I2 = 93.905, 9 studies
Investigation of heterogeneity
Meta-regression analysis indicated a significant association between publication year and leptin concentrations (p = 0.0065) (Fig. 5A). Additionally, latitude was found to be a confounding factor in the regression analysis (p = 0.0009) (Fig. 5B). However, age and gender did not demonstrate a significant effect on the study outcomes (Fig. 5C, D). No evidence of publication bias was detected through funnel plots, Egger's test, or trim and fill tests (eFigure).
Meta-regression analysis examining the relationship between leptin levels and publication year (A), latitude (B), age (C), and gender (D). Association between publication year (Regression coefficient [SE]: -0.0399 [0.0147], 95% CI: -0.0687 to -0.0112, p = 0.0065), latitude (Regression coefficient [SE]: -0.0409 [0.0123], 95% CI: -0.0650 to -0.0167, p = 0.0009), age (Regression coefficient [SE]: -0.0071 [0.0079], 95% CI: -0.0226 to 0.0083, p = 0.3651), and gender (Regression coefficient [SE]: -0.0058 [0.0035], 95% CI: -0.0127 to -0.0011, p = 0.0972) with effect size (Hedges's g) for leptin levels
Discussion
This study examined the significant increase in serum leptin levels in ASD patients compared to healthy controls. Additionally, a meta-analysis of 19 studies with a total sample size of 740 patients and 1456 controls revealed alterations in leptin and progranulin levels in individuals with ASD. The analysis highlights that the significant difference in leptin levels primarily focuses on plasma and suggests a potential influence of publication year and latitude on the observed heterogeneity. It is important to note that the results for progranulin present limitations, requiring further studies for confirmation.
It is widely recognized that children with ASD have a higher risk of overweight or obesity compared to those with typical development [20,21,22]. This study explored the role of leptin, an adipocyte-derived factor, in ASD and its potential involvement in the development of the disorder. While previous research supports the critical role of leptin as a regulator of food intake and energy expenditure, recent studies have proposed that dysregulation of leptin is a mechanism for psychopathologies, including ASD [23]. This proposal is based on the observed comorbidity between obesity and various mental illnesses [24]. Moreover, inflammation is posited to be a potential mechanism through which leptin may impact ASD, given its pro-inflammatory cytokine properties [25, 26]. Additionally, altered leptin levels have been associated with structural and functional brain changes, including lower brain weight, reduced myelin content, neuronal soma size, and synaptic protein levels [27,28,29]. Notably, studies have observed a higher concentration of leptin in the anterior cingulate gyrus of individuals with autism compared to controls [30]. Furthermore, leptin has been found to suppress serotonin synthesis, suggesting another potential biological pathway through which leptin may impact ASD [31].
As brain samples are challenging to obtain, researchers often examine peripheral leptin levels to elucidate on the underlying mechanisms involved. A study comparing 70 children diagnosed with ASD to 99 age-matched control children reported a significant increase in leptin levels in autism, with higher levels observed in early onset cases compared to regressive autism [32]. Similarly, another study demonstrated increased leptin levels in autistic patients over a one-year period [23]. Consistent with these findings, our study also revealed a significant increase in leptin levels in ASD patients compared to healthy controls. Furthermore, we analyzed the relationship between gender and leptin concentration, finding higher leptin levels in boys with ASD compared to healthy boys, while no significant difference was observed in girls. Our results, combined with the meta-analysis findings, highlight the potential of leptin as an important focus in ASD research. However, our ROC curve did not exhibit high specificity or sensitivity for leptin, likely due to the small sample size. Although elevated peripheral leptin may not serve as a unique biomarker for ASD, the consistent increasing trend in ASD patients provides confidence in its potential significance. To explore changes in other adipokines, we conducted a meta-analysis, which revealed decreased levels of progranulin in ASD compared to control, while other adipokines did not exhibit significant differences. This lack of significant findings may be attributed to the limited research on adipokines in the context of ASD, emphasizing the need for further studies to identify multiple factors and establish new diagnostic approaches for ASD.
Although the significant results remained robust when considering factors such as age, IQ, BMI, and medication status, heterogeneities were observed among studies investigating leptin in ASD [32]. The sampling source (serum or plasma) did not account for these heterogeneities. Meta-regressions conducted in our study indicate that publication year and latitude significantly influence the associations between leptin and ASD. This analysis partially addresses the between-study heterogeneities through subgroup and meta-regression analyses, which strengthens the present meta-analysis. Moreover, it is important to acknowledge that other adipokines can also modulate brain functions [33]. Previous research has shown changes in the levels of ghrelin and adiponectin in ASD children [17, 34]. However, the limited research investigating the relationship between ASD and adipokines poses challenges in explaining the underlying mechanisms involved. In our study, we identified only two studies reporting changes in progranulin levels in ASD patients, underscoring the need for increased attention to adipokines in future research endeavors.
Despite these findings, it is essential to acknowledge the inherent limitations of this study. The first limitation is the small sample size, although the meta-analysis approach helps to confirm the trend of leptin level changes. The second limitation is the scarcity of studies focusing on adipokines in the context of ASD, highlighting the need for further development in future meta-analyses. Last, while this study investigated changes in adipokine concentrations in the peripheral blood of ASD children, the extent to which these measurements reflect brain activity remains unknown, despite the potential involvement of leptin resistance mechanisms [35].
Conclusion
Our study demonstrated a significant increase in peripheral blood leptin levels in ASD patients compared to healthy controls. Additionally, a meta-analysis involving 19 studies provided evidence of altered leptin levels in ASD. Furthermore, progranulin exhibited a contrasting trend, suggesting the significant potential of adipokines as biomarkers for ASD, albeit requiring further analysis. Future investigations into adipokines, particularly leptin, as potential diagnostic markers and therapeutic targets for ASD are essential.
Availability of data and materials
Data and materials are available on request. The email of corresponding author is yongcheng@muc.edu.cn.
References
Jeste SS. Neurodevelopmental behavioral and cognitive disorders. Continuum (Minneap Minn). 2015;21(3 Behavioral Neurology and Neuropsychiatry):690–714.
Mukherjee SB. Autism spectrum disorders - diagnosis and management. Indian J Pediatr. 2017;84(4):307–14.
Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383(9920):896–910.
Smaga I, Niedzielska E, Gawlik M, Moniczewski A, Krzek J, Przegalinski E, Pera J, Filip M. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol Rep. 2015;67(3):569–80.
Raghavan R, Zuckerman B, Hong X, et al. Fetal and infancy growth pattern, cord and early childhood plasma leptin, and development of autism spectrum disorder in the Boston birth cohort. Autism Res. 2018;11(10):1416–31.
Zhang F, Chen Y, Heiman M, Dimarchi R. Leptin: structure, function and biology. Vitam Horm. 2005;71:345–72.
Zupancic ML, Mahajan A. Leptin as a neuroactive agent. Psychosom Med. 2011;73(5):407–14.
Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A. 1996;93(16):8374–8.
Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68(4):437–46.
Zhang F, Basinski MB, Beals JM, et al. Crystal structure of the obese protein leptin-E100. Nature. 1997;387(6629):206–9.
Takayanagi Y, Cascella NG, Santora D, Gregory PE, Sawa A, Eaton WW. Relationships between serum leptin level and severity of positive symptoms in schizophrenia. Neurosci Res Sep-Oct. 2013;77(1–2):97–101.
Cakici N, Bot M, Lamers F, et al. Increased serum levels of leptin and insulin in both schizophrenia and major depressive disorder: A cross-disorder proteomics analysis. Eur Neuropsychopharmacol. 2019;29(7):835–46.
Burrows K, McNaughton BA, Figueroa-Hall LK, et al. Elevated serum leptin is associated with attenuated reward anticipation in major depressive disorder independent of peripheral C-reactive protein levels. Sci Rep. 2023;13(1):11313.
Fernandes BS, Dash S, Jacka F, et al. Leptin in bipolar disorder: A systematic review and meta-analysis. Eur Psychiatry. 2016;35:1–7.
Atmaca M, Kuloglu M, Tezcan E, Ustundag B, Bayik Y. Serum leptin and cholesterol levels in patients with bipolar disorder. Neuropsychobiology. 2002;46(4):176–9.
Pan W, Kastin AJ. Adipokines and the blood-brain barrier. Peptides. 2007;28(6):1317–30.
Fujita-Shimizu A, Suzuki K, Nakamura K, et al. Decreased serum levels of adiponectin in subjects with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(3):455–8.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Chen L, Shi XJ, Liu H, Mao X, Gui LN, Wang H, Cheng Y. Oxidative stress marker aberrations in children with autism spectrum disorder: a systematic review and meta-analysis of 87 studies (N = 9109). Transl Psychiatry. 2021;11(1):15.
Zheng Z, Zhang L, Li S, et al. Association among obesity, overweight and autism spectrum disorder: a systematic review and meta-analysis. Sci Rep. 2017;7(1):11697.
Hill AP, Zuckerman KE, Fombonne E. Obesity and autism. Pediatrics. 2015;136(6):1051–61.
Hyman SL, Stewart PA, Schmidt B, et al. Nutrient intake from food in children with autism. Pediatrics. 2012;130 Suppl 2(Suppl 2):S145-153.
Blardi P, de Lalla A, Ceccatelli L, Vanessa G, Auteri A, Hayek J. Variations of plasma leptin and adiponectin levels in autistic patients. Neurosci Lett. 2010;479(1):54–7.
Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63(7):824–30.
Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25(1):40–5.
Goines PE, Ashwood P. Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol Teratol. 2013;36:67–81.
Bouret SG, Simerly RB. Minireview: Leptin and development of hypothalamic feeding circuits. Endocrinology. 2004;145(6):2621–6.
Huang CW, Tsai MH, Chen NC, et al. Clinical significance of circulating vascular cell adhesion molecule-1 to white matter disintegrity in Alzheimer’s dementia. Thromb Haemost. 2015;114(6):1230–40.
Davis C, Mudd J, Hawkins M. Neuroprotective effects of leptin in the context of obesity and metabolic disorders. Neurobiol Dis. 2014;72 Pt A:61–71.
Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67–81.
Valleau JC, Sullivan EL. The impact of leptin on perinatal development and psychopathology. J Chem Neuroanat. 2014;61–62:221–32.
Ashwood P, Kwong C, Hansen R, et al. Brief report: plasma leptin levels are elevated in autism: association with early onset phenotype? J Autism Dev Disord. 2008;38(1):169–75.
Parimisetty A, Dorsemans AC, Awada R, Ravanan P, Diotel N, Lefebvre d’Hellencourt C. Secret talk between adipose tissue and central nervous system via secreted factors-an emerging frontier in the neurodegenerative research. J Neuroinflammation. 2016;13(1):67.
Al-Zaid FS, Alhader AA, Al-Ayadhi LY. Altered ghrelin levels in boys with autism: a novel finding associated with hormonal dysregulation. Sci Rep. 2014;4:6478.
Caro JF, Kolaczynski JW, Nyce MR, et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348(9021):159–61.
Acknowledgements
Not applicable.
Funding
Hunan Provincial Health High-Level Talent Scientific Research Project (R2023159), National Natural Science Foundation of China (82071676).
Author information
Authors and Affiliations
Contributions
Y.C. conceived and designed this study; X.-Y. X. provide the samples. L.C., L.-M. L, Y. D. and Y.-W. C. extracted the data; L.C., M. G. and X.-Y. X. performed statistical analyses; Y.C. and L.C. drafted the manuscript; all the authors interpreted the data and provided critical revisions for the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Approved by the Biological and Medical Ethics Committee, Minzu University of China.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, L., Liu, LM., Guo, M. et al. Altered leptin level in autism spectrum disorder and meta-analysis of adipokines. BMC Psychiatry 24, 479 (2024). https://doi.org/10.1186/s12888-024-05936-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-024-05936-4