Abstract
Background
Previous studies using EEG (electroencephalography) as biomarker for dementia have attempted to research, but results have been inconsistent. Most of the studies have extremely small number of samples (average N = 15) and studies with large number of data do not have control group. We identified EEG features that may be biomarkers for dementia with 120 subjects (dementia 10, MCI 33, against control 77).
Methods
We recorded EEG from 120 patients with dementia as they stayed in relaxed state using a single-channel EEG device while conducting real-time noise reduction and compared them to healthy subjects. Differences in EEG between patients and controls, as well as differences in patients’ severity, were examined using the ratio of power spectrum at each frequency.
Results
In comparing healthy controls and dementia patients, significant power spectrum differences were observed at 3 Hz, 4 Hz, and 10 Hz and higher frequencies. In patient group, differences in the power spectrum were observed between asymptomatic patients and healthy individuals, and between patients of each respective severity level and healthy individuals.
Conclusions
A study with a larger sample size should be conducted to gauge reproducibility, but the results implied the effectiveness of EEG in clinical practice as a biomarker of MCI (mild cognitive impairment) and/or dementia.
Similar content being viewed by others
Background
The objective of this study was to propose a novel dementia diagnosis system and biomarkers for early MCI (mild cognitive impairment) detection and dementia by utilizing a simple electroencephalography (EEG) device.
Dementia has become a public issue in Japan. It is said that one in seven elderly people of 65 years old or more is affected with dementia, and it was predicted that the total dementia patients in Japan will reach around 6.5 million to 7 million people in 2025 [1]. Dementia is different from other illness in that it requires both public and family care. In total, the cost for the dementia care is estimated to be approximately 14.5 trillion Japanese Yen (JPY) per year and it is very taxing for the Japanese society. As a comparison, cancer is estimated to cost around 9.7 trillion JPY per year [2].
Recently, research projects are focused on prevention and early detection of dementia. These are especially important because there is no cure or treatment for dementia yet. Popular methodologies for early dementia detection utilize neuroimaging techniques such as Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET). Although they are accurate, neuroimaging techniques are both expensive and time consuming. Additionally, conventional neuroimaging techniques can only detect dementia when it has been sufficiently developed – i.e., the patient’s brain has suffered irreversible structural damage. As a result, the usage of EEG as simpler, cheaper, and easier alternative to neuroimaging is starting to become popular.
EEG features has been successfully used to detect Huntington disease and epilepsy, proving their reliability. Moreover, as EEG reflects functional changes in the cerebral cortex, detecting dementia before permanent structural damage in brain might be possible using EEG [3]. In addition to dementia, Mild Cognitive Impairment (MCI) detection is also an important task. MCI is said to be the early stages of dementia, and MCI-afflicted patients are more likely to progress into dementia, compared to healthy people.
Most of conventional studies that utilized EEG for dementia diagnosis system used multi-channel EEG devices and did not consider the diagnosis of MCI [4]. Additionally, previous studies related to EEG biomarkers suffered from low number of samples of lack of control groups. Moreover, one of the conventional studies that utilize EEG to assess MCI and AD is from Meghdadi et al. [5], which successfully built a machine learning for dementia / MCI diagnosis system using multi-channel EEG setup; unfortunately, multichannel EEG device is hard to wear and burdensome to patients. Here, this study aims to propose a novel dementia-MCI diagnosis system and the possibility of utilizing a comfortable, single-channel EEG device as biomarkers for clinical screening.
As the proposed system utilized a single-channel EEG device, it required less preparation time and also less burdensome to the experimental subjects; it can be said that the proposed system was an improvement from the conventional systems. Another objective of this research was to verify the validity of the dementia diagnosis system using the simple measurement by evaluating the feature values using statistical analysis and machine learning.
Methods
Participants
The participants of this study were 120 people (67% female) aged 40 years old up to 91 years old. The study was conducted from year 2016 to 2019. The mean age of the participants was 67.0 ± 9.19 years old. EEG, MRI (in particular, FA-BHQ and GM-BHQ values were obtained), and cognitive screening tests were conducted to screen the participants. However only MMSE is utilized in this study as variable. This study was approved by the ethics committees of Keio University and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants or their legal guardian. This study is a case–control study and is observational in nature; all obtained data were made available to the caregiver and / or the participant. The study protocol was approved by the Keio University Ethics Review Board with approval no.: 28–20, 28–59, 29–33, 30–96, 31–56.
Exclusion criteria for patients were: 1) persons who have physical or psychiatric disorders that impede the use of EEG; 2) persons who have comorbid psychiatric disorders other than dementia; 3) persons who have comorbidities that could interfere with EEG recordings, such as brain tumors, stroke, or epilepsy.
For the comparison, reference data for healthy individuals that were obtained separately from this study were used. Inclusion criteria for healthy individuals were: 1) no history of mental illness; 2) legal adult defined by Japanese law (age ≥ 20 years). The healthy volunteer’s age is matched as closely as possible to the dementia patients. It was also required that they do not meet the exclusion criteria for dementia patients listed above.
All participants were Japanese (Asian) and were divided into three groups: dementia (N = 10), MCI (N = 33), and control (N = 77). From all of the participants, 7 were diabetic, 7 were obese, 24 had hyperlipidemia, 4 were diagnosed as clinically depressed, 3 had history of neurological diseases, 35 had hypertension, 2 had history of stroke, 1 had history of myocardial infarction, 14 had history of allergic rhinitis, 1 had history of COPD, 4 had asthma, 12 had skin condition, 9 had arthritis, 20 had low back pain, 54 had osteoporosis, 4 had history of kidney problem, and 2 had history of cancer. Dementia patients and MCI patients in this study were all Alzheimer-type.
EEG acquisition
A Participants were asked to wear a single channel EEG device (NeuroSky Single Channel EEG, Original noise reduction BMD version). EEG was taken during closed-eye relaxed state for a total of 100 s. The location of the electrode was Fp1 according to the 10–20 international system (left prefrontal region) and the measurement device was MindWave Mobile II BMD II ver. with sampling rate of 512 Hz. Mini-mental state examination (MMSE) was utilized as the cognitive screening test. MMSE is a 30-points question which is commonly used for assessing dementia. Subjects who scored 24 points or lower were labeled as dementia while patients with score of 25 – 27 is labeled as MCI. Subjects with score of 28 or higher is labelled as healthy.
Analysis
Data preprocessing
For the EEG device used in this study, independent verification has already demonstrated that the device can reliably remove environmental noise and unintended frequencies [6]. Signals acquired from Fp1 using a monopole EEG were passed through a bandpass filter of 1–30 Hz to extract EEG components [7]. However, even if non-target frequencies can be removed, the acquired data come with noise caused by muscle movement or blinking. To remove these noises, a filter created for this purpose was used. This filter acquires the patterns of body movement and blinking in advance, and the threshold value is automatically set according to the situation. We adopted conventional methods as noise reduction [6, 8, 9] This procedure reduces computation costs and removes blinks, body movements, and electrical noise in real time.
In order to account for individual differences in EEG amplitude, normalization was performed with an average of 0 and a dispersion of 1 for the filtered signal. Subsequently, a fast Fourier transform was performed to calculate the power spectrum. Also, in order to clarify the difference between healthy individuals and each patient group with dementia and MCI, the average power spectrum of the comparison target group was set as 1; i.e., a relative power spectrum value was used.
The sampling interval of the EEG device was set to 512 Hz. Therefore, the amount of data for each individual was 51,200 samples of 100 × 512. Each individual’s EEG data were translated to the frequency domain by Fourier transform per second.
Statistical analysis
First, all acquired data were divided into three groups according to their MMSE score: patients with dementia, patients with MCI, and healthy controls. Descriptive statistics were used to describe the study participants. Distributions of all variables were inspected using histograms, q-q plots, and Shapiro-Wilks tests before conducting statistical analyses. Statistical significance was set at two-tailed p < 0.05, and we used false discovery rate (FDR) to control for multiple comparisons. Demographic variables for patients with dementia, MCI, and healthy individuals were compared by two-sample t test and/or chi-square test. For the EEG comparisons, patients’ EEG power spectra are expressed as a ratio when the average of the power spectra of healthy individuals is 1, as mentioned above.
Signal processing
As a preprocessing, noise removal was performed to the obtained EEG data and then transformed to the frequency-domain. The noise removal was performed using Summation of Derivatives within Windows (SDW) algorithm and Ensemble Empirical Mode Decomposition (EEMD).
The SDW method detects noise by using the sum of first derivatives within a window [10]. The window selected in this study is 2 s, according to the conventional methods, shown in Table 1. The signal was then decomposed into several intrinsic mode functions (IMFs) by applying EEMD in the interval detected by the SDW method.
The components with a mean cross-correlation function between IMFs greater than 0.5 were defined as noise and removed. Remaining components were then summed up to reconstruct the clean signal. For frequency transformation, the short-time Fourier transform (STFT) was used. The frequency features were the frequency bins 1-45 Hz, averaged between the windows, and then normalized. The average of the power spectra for each EEG band was also calculated and used as the frequency features. The specification of EEG bands were as follows: Delta (δ): 1–4 Hz, Theta (θ) 4–8 Hz, Alpha (α) 8–13 Hz, Alpha-1 (α1) 8–9 Hz, Alpha-2 (α2) 9–11 Hz, Alpha-3 (α3) 11–13 Hz, Beta (β) 13–30 Hz, Beta-1 (β1) 13–20 Hz, Beta-2 (β2) 20–30 Hz, and Gamma (γ) 30–45 Hz.
Next, the subjects were labelled according to their MMSE score: dementia, MCI, and healthy. As there was imbalance in the number of samples, Synthetic Minority Over-sampling Technique (SMOTE) algorithm was applied to artificially create new samples [30].
In order to verify whether the frequency features used were effective in discriminating between classes, a significant difference test between classes was conducted. First, a nonparametric test, Kruska-wallis test was performed. Then, multiple comparison with Bonferroni correction was performed. The significance level was set as 5%.
Next, the statistically significant features were utilized as predictors for Support Vector Machines (SVM) to solve the classification problem. Although SVM is known to be able to handle linearly separable data, by utilizing kernel trick and computing the maximum margin, it can sufficiently handle nonlinear data.
In this study, SVM with radial basis function kernel was utilized as the classifier. In order to evaluate the classification performance, tenfold cross-validation was performed.
Results
Feature selection results
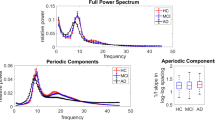
The power spectra comparison for each band was shown in Fig. 1a. The significance testing for the three groups showed significantly increased delta band for the healthy group compared to dementia group and MCI group, as shown in Fig. 1b, and significantly increased alpha-1 band for the dementia group compared to MCI group and healthy group as shown in Fig. 1c.
Results of accuracies
The classification results for the three classes of dementia group, MCI group, and healthy group are shown in Table 2. The classification accuracy rate, sensitivity, and specificity for the training data were all 100%, while the accuracy rate, sensitivity, and specificity for the training data were 86.32%, 78.91%, and 85.36%, respectively.
Discussion
Feature selection results
The findings were in line with conventional studies of higher power in low-frequencies range for healthy subjects and higher power in high-frequencies range for dementia and MCI patients [31]. Many of frequency bins also showed significant differences between the classes and were chosen as predictors. These predictors might also be utilized as EEG biomarkers for dementia and MCI in clinical scene.
The statistical analysis result showed that the power in the low-frequency region of the dementia and MCI patients was higher than that of the healthy subjects, and that the power in the high-frequency region was lower, similar to conventional studies which utilized multi-channel EEG device.
Results of accuracies
It was confirmed that the power spectra of the frequency bands and frequency bins selected as features were sufficient to discriminate among the three classes of dementia, MCI, and normal. These results were comparable in accuracy to previous studies using multichannel EEG device with accuracy of 88.89% [32]. From these results, it was shown that it is possible to obtain a new biomarker for dementia and early MCI by using a simple EEG device that acquires only single channel.
Conclusion
The objective of this study was to develop a system for diagnosing dementia and early detection of dementia using only EEG. The experimental subjects were divided into three classes according to their MMSE scores: dementia group, MCI group, and healthy group. Feature extraction was performed, and the validity of the features were verified by constructing a machine learning model that can differentiate and label the subjects into the three classes.
The statistical analysis result showed that the power in the low-frequency region of the dementia and MCI patients was higher than that of the healthy subjects, and that the power in the high-frequency region was lower, similar to conventional studies which utilized multi-channel EEG device.
Features other than EEG frequencies and different classifiers might be considered as a future work to improve the classification accuracy.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to restrictions from IRB (identifying and potentially sensitive information) but are available from the corresponding author on reasonable request.
Abbreviations
- EEG:
-
Electroencephalography
- EEMD:
-
Ensemble Empirical Mode Decomposition
- IMFs:
-
Intrinsic Mode Functions
- JPY:
-
Japanese Yen
- MCI:
-
Mild Cognitive Impairment
- MMSE:
-
Mini-mental state examination
- MRI:
-
Magnetic Resonance Imaging
- PET:
-
Positron Emission Tomography
- SDW:
-
Summation of Derivatives within Windows
- SMOTE:
-
Synthetic Minority Over-sampling Technique
- STFT:
-
Short-time Fourier transform
- SVM:
-
Support Vector Machines
References
Ministry of Health Labour and Welfare Japan. Research on the future estimation of the elderly population with dementia in Japan. 2014.
Ministry of Health Labour and Welfare Japan. Research on the economic impact of dementia in Japan. 2014.
Paraskevaidi M, Martin-Hirsch PL, Martin FL. Progress and challenges in the diagnosis of dementia: a critical review. ACS Chem Neurosci. 2018;9(3):446–61.
Cassani R, Estarellas M, San-Martin R, Fraga FJ, Falk TH. Systematic review on resting-state EEG for Alzheimer’s disease diagnosis and progression assessment. Dis Markers. 2018;2018(2018):5174815.
Meghdadi AH, Karić MS, McConnell M, Rupp G, Richard C, Hamilton J, et al. Resting state EEG biomarkers of cognitive decline associated with Alzheimer’s disease and mild cognitive impairment. PLOS ONE. 2021;16(2):e0244180.
Kanoga S, Mitsukura Y. A study of pattern recognition in children using single-channel electroencephalogram for specialized electroencephalographic devices. Electron Commun Japan. 2017;100(11):43–53.
Ratti E, Waninger S, Berka C, Ruffini G, Verma A. Comparison of medical and consumer wireless EEG systems for use in clinical trials. Front Hum Neurosci. 2017;11:398.
Grin-Yatsenko VA, Baas I, Ponomarev VA, Kropotov JD. Independent component approach to the analysis of EEG recordings at early stages of depressive disorders. Clin Neurophysiol. 2010;121(3):281–9.
Ogino M, Mitsukura Y. Portable drowsiness detection through use of a prefrontal single-channel electroencephalogram. Sensors. 2018;18(12):4477.
Chang WD, Cha HS, Kim K, Im CH. Detection of eye blink artifacts from single prefrontal channel electroencephalogram. Comput Methods Programs Biomed. 2016;124:19–30.
Nishida K, Yoshimura M, Isotani T, Yoshida T, Kitaura Y, Saito A, et al. Differences in quantitative EEG between frontotemporal dementia and Alzheimer’s disease as revealed by LORETA. Clin Neurophysiol. 2011;122(9):1718–25.
Neto E, Allen EA, Aurlien H, Nordby H, Eichele T. EEG spectral features discriminate between Alzheimer’s and Vascular Dementia. Front Neurol. 2015;6:25.
Neto E, Biessmann F, Aurlien H, Nordby H, Eichele T. Regularized linear discriminant analysis of EEG features in Dementia patients. Front Aging Neurosci. 2016;8:273.
Kim JS, Lee SH, Park G, Kim S, Bae SM, Kim DW, et al. Clinical Implications of quantitative electroencephalography and current source density in patients with Alzheimer’s disease. Brain Topogr. 2012;25(4):461–74.
Anghinah R, Kanda PAM, Lopes HF, Basile LFH, Machado S, Ribeiro P, et al. Alzheimer’s disease qEEG: spectral analysis versus coherence. which is the best measurement? Arq Neuro-Psiquiatri. 2011;69(6):871–4.
Triggiani AI, Bevilacqua V, Brunetti A, Lizio R, Tattoli G, Cassano F, et al. Classification of healthy subjects and Alzheimer’s disease patients with Dementia from cortical sources of resting state EEG rhythms: a study using artificial neural networks. Front Neurosci. 2017;10:604.
Moretti DV, Prestia A, Fracassi C, Binetti G, Zanetti O, Frisoni GB. Specific EEG changes associated with Atrophy of Hippocampus in subjects with Mild Cognitive Impairment and Alzheimer’s disease. Int J Alzheimers Dis. 2012;2012:253153.
Moretti D. Electroencephalography reveals lower regional blood perfusion and atrophy of the temporoparietal network associated with memory deficits and hippocampal volume reduction in mild cognitive impairment due to Alzheimer’s disease. Neuropsychiatr Dis Treat. 2015;11:461–70.
Babiloni C, Triggiani AI, Lizio R, Cordone S, Tattoli G, Bevilacqua V, et al. Classification of single normal and Alzheimer’s disease individuals from Cortical sources of resting state EEG rhythms. Front Neurosci. 2016;10:47.
Miraglia F, Vecchio F, Bramanti P, Rossini PM. EEG characteristics in “eyes-open” versus “eyes-closed” conditions: Small-world network architecture in healthy aging and age-related brain degeneration. Clin Neurophysiol. 2016;127(2):1261–8.
Moretti DV, Frisoni GB, Fracassi C, Pievani M, Geroldi C, Binetti G, et al. MCI patients’ EEGs show group differences between those who progress and those who do not progress to AD. Neurobiol. 2011;32(4):563–71.
Moretti DV, Paternicò D, Binetti G, Zanetti O, Frisoni GB. EEG markers are associated to gray matter changes in thalamus and basal ganglia in subjects with mild cognitive impairment. NeuroImage. 2012;60(1):489–96.
Moretti DV. Mild Cognitive Impairment: Structural, Metabolical, and Neurophysiological Evidence of a Novel EEG Biomarker. Front Neurol. 2015;6:152.
Moretti D. Association of EEG, MRI, and regional blood flow biomarkers is predictive of prodromal Alzheimer’s disease. Neuropsychiatr Dis Treat. 2015;11:2779–91.
Babiloni C, Del Percio C, Caroli A, Salvatore E, Nicolai E, Marzano N, et al. Cortical sources of resting state EEG rhythms are related to brain hypometabolism in subjects with Alzheimer’s disease: an EEG-PET study. Neurobiol Aging. 2016;48:122–34.
Hata M, Kazui H, Tanaka T, Ishii R, Canuet L, Pascual-Marqui RD, et al. Functional connectivity assessed by resting state EEG correlates with cognitive decline of Alzheimer’s disease – an eLORETA study. Clinic Neurophysiol. 2016;127(2):1269–78.
Vecchio F, Miraglia F, Piludu F, Granata G, Romanello R, Caulo M, et al. “Small World” architecture in brain connectivity and hippocampal volume in Alzheimer’s disease: a study via graph theory from EEG data. Brain Imaging Behav. 2017;11(2):473–85.
Moretti DV, PaternicoG D, Binetti G, Zanetti O, Frisoni GB. Analysis of Grey matter in Thalamus and Basal Ganglia Based on EEG α3/α2 frequency ratio reveals specific changes in subjects with Mild Cognitive Impairment. ASN Neuro. 2012;4(7):e00103.
Moretti DV. Understanding early dementia: EEG, MRI, SPECT and memory evaluation. Transl Neurosci. 2015;6(1):32–46.
Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP. SMOTE: Synthetic Minority Over-sampling Technique. J Artif Intell Res. 2002;16:321-57. https://doi.org/10.1613/jair.953.
Babiloni C, Carducci F, Lizio R, Vecchio F, Baglieri A, Bernardini S, et al. Resting state cortical electroencephalographic rhythms are related to gray matter volume in subjects with mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp. 2013;34(6):1427.
Yang S, Bornot JMS, Wong-Lin K, Prasad G. M/EEG-Based bio-markers to predict the MCI and Alzheimer’s disease: a review from the ML Perspective. IEEE Trans Biomed. 2019;66(10):2924–35.
Acknowledgements
N/A.
Funding
This study is supported by grant: JSPS Kakenhi Kiban S (17H06151).
Author information
Authors and Affiliations
Contributions
Experiments were conceived and designed by YM, TN, and TI. Experiments and data analysis were performed by YM, TN, HW. This paper was written by YM, BS, TI, and TN. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committees of Keio University and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants. The study protocol was approved by the Keio University Ethics Review Board with approval no.: 28–20, 28–59, 29–33, 30–96, 31–56.
Consent to publication
N/A.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mitsukura, Y., Sumali, B., Watanabe, H. et al. Frontotemporal EEG as potential biomarker for early MCI: a case–control study. BMC Psychiatry 22, 289 (2022). https://doi.org/10.1186/s12888-022-03932-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-022-03932-0