Abstract
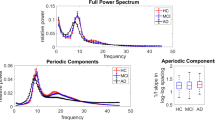
Distinguishing between Alzheimer’s disease (AD) and frontotemporal dementia (FTD) presents a clinical challenge. Inexpensive and accessible techniques such as electroencephalography (EEG) are increasingly being used to address this challenge. In particular, the potential relevance between aperiodic components of EEG activity and these disorders has gained interest as our understanding evolves. This study aims to determine the differences in aperiodic activity between AD and FTD and evaluate its potential for distinguishing between the two disorders. A total of 88 participants, including 36 patients with AD, 23 patients with FTD, and 29 healthy controls (CN) underwent cognitive assessment and scalp EEG acquisition. Neuronal power spectra were parameterized to decompose the EEG spectrum, enabling comparison of group differences in different components. A support vector machine was employed to assess the impact of aperiodic parameters on the differential diagnosis. Compared with the CN group, both the AD and FTD groups showed varying degrees of increased alpha power (both periodic and raw power) and theta alpha power ratio. At the channel level, theta power (both periodic and raw power) in the frontal regions was higher in the AD group compared to the FTD group, and aperiodic parameters (both exponents and offsets) in the frontal, temporal, central, and parietal regions were higher in the AD group than in the FTD group. Importantly, the inclusion of aperiodic parameters led to improved performance in distinguishing between the two disorders. These findings highlight the significance of aperiodic components in discriminating dementia-related diseases.









Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available in the ds004504 repository, https://github.com/OpenNeuroDatasets/ds004504. The custom codes used for the current study are available at https://github.com/dcgggg/ds004504_Aperdiodic.
Abbreviations
- AD :
-
Alzheimer’s disease
- ANOVA :
-
Analysis of variance
- AUC :
-
Area under curve
- CN :
-
Healthy controls
- EEG :
-
Electroencephalography
- E/I :
-
Excitation/inhibition
- FDR :
-
False discovery rate
- FTD :
-
Frontotemporal dementia
- MMSE :
-
Mini-Mental State Examination
- NFTs :
-
Neurofibrillary tangles
- PSD :
-
Power spectral density
- SMOTE :
-
Synthetic Minority Oversampling Technique
- SVM :
-
Support vector machine
- TAR :
-
Theta alpha power ratio
References
Lattante S, Ciura S, Rouleau GA, Kabashi E. Defining the genetic connection linking amyotrophic lateral sclerosis (ALS) with frontotemporal dementia (FTD). Trends Genet. 2015;31:263–73. https://doi.org/10.1016/j.tig.2015.03.005.
Mendez MF, Perryman KM, Miller BL, Cummings JL. Behavioral differences between frontotemporal dementia and Alzheimer’s disease: a comparison on the BEHAVE-AD rating scale. Int Psychogeriatr. 1998;10:155–62. https://doi.org/10.1017/s1041610298005262.
Piguet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol. 2011;10:162–72. https://doi.org/10.1016/S1474-4422(10)70299-4.
Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer’s disease. Neurology. 2000;54:2277–84. https://doi.org/10.1212/WNL.54.12.2277.
Reul S, Lohmann H, Wiendl H, Duning T, Johnen A. Can cognitive assessment really discriminate early stages of Alzheimer’s and behavioural variant frontotemporal dementia at initial clinical presentation? Alzheimers Res Ther. 2017;9:61. https://doi.org/10.1186/s13195-017-0287-1.
Minoshima S, Mosci K, Cross D, Thientunyakit T. Brain [F-18]FDG PET for clinical dementia workup: differential diagnosis of Alzheimer’s disease and other types of dementing disorders. Semin Nucl Med. 2021;51:230–40. https://doi.org/10.1053/j.semnuclmed.2021.01.002.
Talbot PR, Snowden JS, Lloyd JJ, Neary D, Testa HJ. The contribution of single photon emission tomography to the clinical differentiation of degenerative cortical brain disorders. J Neurol. 1995;242:579–86. https://doi.org/10.1007/BF00868810.
Yu Q, Mai Y, Ruan Y, Luo Y, Zhao L, Fang W, et al. An MRI-based strategy for differentiation of frontotemporal dementia and Alzheimer’s disease. Alzheimers Res Ther. 2021;13:23. https://doi.org/10.1186/s13195-020-00757-5.
Nardone R, Sebastianelli L, Versace V, Saltuari L, Lochner P, Frey V, et al. Usefulness of EEG techniques in distinguishing frontotemporal dementia from Alzheimer’s disease and other dementias. Dis Markers. 2018;2018:6581490. https://doi.org/10.1155/2018/6581490.
Huang C, Wahlund L, Dierks T, Julin P, Winblad B, Jelic V. Discrimination of Alzheimer’s disease and mild cognitive impairment by equivalent EEG sources: a cross-sectional and longitudinal study. Clin Neurophysiol. 2000;111:1961–7. https://doi.org/10.1016/s1388-2457(00)00454-5.
Jiao B, Li R, Zhou H, Qing K, Liu H, Pan H, et al. Neural biomarker diagnosis and prediction to mild cognitive impairment and Alzheimer’s disease using EEG technology. Alzheimers Res Ther. 2023;15:32. https://doi.org/10.1186/s13195-023-01181-1.
Mattia D, Babiloni F, Romigi A, Cincotti F, Bianchi L, Sperli F, et al. Quantitative EEG and dynamic susceptibility contrast MRI in Alzheimer’s disease: a correlative study. Clin Neurophysiol. 2003;114:1210–6. https://doi.org/10.1016/s1388-2457(03)00085-3.
Meghdadi AH, StevanovićKarić M, McConnell M, Rupp G, Richard C, Hamilton J, et al. Resting state EEG biomarkers of cognitive decline associated with Alzheimer’s disease and mild cognitive impairment. PLoS ONE. 2021;16:e0244180. https://doi.org/10.1371/journal.pone.0244180.
Lindau M, Jelic V, Johansson S-E, Andersen C, Wahlund L-O, Almkvist O. Quantitative EEG abnormalities and cognitive dysfunctions in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2003;15:106–14. https://doi.org/10.1159/000067973.
Passant U, Rosén I, Gustafson L, Englund E. The heterogeneity of frontotemporal dementia with regard to initial symptoms, qEEG and neuropathology. Int J Geriatr Psychiatry. 2005;20:983–8. https://doi.org/10.1002/gps.1388.
Milstein J, Mormann F, Fried I, Koch C. Neuronal shot noise and Brownian 1/f2 behavior in the local field potential. PLoS ONE. 2009;4:e4338. https://doi.org/10.1371/journal.pone.0004338.
He BJ. Scale-free brain activity: past, present, and future. Trends Cogn Sci. 2014;18:480–7. https://doi.org/10.1016/j.tics.2014.04.003.
Voytek B, Knight RT. Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol Psychiatry. 2015;77:1089–97. https://doi.org/10.1016/j.biopsych.2015.04.016.
Gao R. Interpreting the electrophysiological power spectrum. J Neurophysiol. 2016;115:628–30. https://doi.org/10.1152/jn.00722.2015.
Gao R, Peterson EJ, Voytek B. Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage. 2017;158:70–8. https://doi.org/10.1016/j.neuroimage.2017.06.078.
Voytek B, Kramer MA, Case J, Lepage KQ, Tempesta ZR, Knight RT, et al. Age-related changes in 1/f neural electrophysiological noise. J Neurosci. 2015;35:13257–65. https://doi.org/10.1523/JNEUROSCI.2332-14.2015.
Schaworonkow N, Voytek B. Longitudinal changes in aperiodic and periodic activity in electrophysiological recordings in the first seven months of life. Dev Cogn Neurosci. 2021;47:100895. https://doi.org/10.1016/j.dcn.2020.100895.
Miskovic V, MacDonald KJ, Rhodes LJ, Cote KA. Changes in EEG multiscale entropy and power-law frequency scaling during the human sleep cycle. Hum Brain Mapp. 2019;40:538–51. https://doi.org/10.1002/hbm.24393.
Lendner JD, Helfrich RF, Mander BA, Romundstad L, Lin JJ, Walker MP, et al. An electrophysiological marker of arousal level in humans. Elife 2020;9. https://doi.org/10.7554/eLife.55092.
Pertermann M, Bluschke A, Roessner V, Beste C. The modulation of neural noise underlies the effectiveness of methylphenidate treatment in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2019;4:743–50. https://doi.org/10.1016/j.bpsc.2019.03.011.
Racz F, Farkas K, Stylianou O, Kaposzta Z, Czoch A, Csukly G, et al. Separating scale-free and oscillatory components of neural activity in schizophrenia. Brain Behav 2021;11. https://doi.org/10.1002/brb3.2047.
Wang Z, Mo Y, Sun Y, Hu K, Peng C, Zhang S, et al. Separating the aperiodic and periodic components of neural activity in Parkinson’s disease. Eur J Neurosci. 2022. https://doi.org/10.1111/ejn.15774.
Ouyang G, Hildebrandt A, Schmitz F, Herrmann CS. Decomposing alpha and 1/f brain activities reveals their differential associations with cognitive processing speed. Neuroimage. 2020;205:116304. https://doi.org/10.1016/j.neuroimage.2019.116304.
Cross ZR, Corcoran AW, Schlesewsky M, Kohler MJ, Bornkessel-Schlesewsky I. Oscillatory and aperiodic neural activity jointly predict language learning. J Cogn Neurosci. 2022;1–20. https://doi.org/10.1162/jocn_a_01878.
Donoghue T, Haller M, Peterson EJ, Varma P, Sebastian P, Gao R, et al. Parameterizing neural power spectra into periodic and aperiodic components. Nat Neurosci. 2020;23:1655–65. https://doi.org/10.1038/s41593-020-00744-x.
Miltiadous A, Tzimourta KD, Afrantou T, Ioannidis P, Grigoriadis N, Tsalikakis DG, et al. A dataset of 88 EEG recordings from: Alzheimer’s disease, frontotemporal dementia and healthy subjects 2023. https://doi.org/10.18112/OPENNEURO.DS004504.V1.0.4.
Miltiadous A, Tzimourta KD, Giannakeas N, Tsipouras MG, Afrantou T, Ioannidis P, et al. Alzheimer’s disease and frontotemporal dementia: a robust classification method of EEG signals and a comparison of validation methods. Diagnostics. 2021;11:1437. https://doi.org/10.3390/diagnostics11081437.
Tzimourta KD, Afrantou T, Ioannidis P, Karatzikou M, Tzallas AT, Giannakeas N, et al. Analysis of electroencephalographic signals complexity regarding Alzheimer’s disease. Comput Electr Eng. 2019;76:198–212. https://doi.org/10.1016/j.compeleceng.2019.03.018.
Miltiadous A, Tzimourta KD, Afrantou T, Ioannidis P, Grigoriadis N, Tsalikakis DG, et al. A dataset of scalp EEG recordings of Alzheimer’s disease, frontotemporal dementia and healthy subjects from routine EEG. Data. 2023;8:95. https://doi.org/10.3390/data8060095.
Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009.
Chang C-Y, Hsu S-H, Pion-Tonachini L, Jung T-P. Evaluation of artifact subspace reconstruction for automatic artifact components removal in multi-channel EEG recordings. IEEE Trans Biomed Eng. 2020;67:1114–21. https://doi.org/10.1109/TBME.2019.2930186.
Winkler I, Haufe S, Tangermann M. Automatic classification of artifactual ICA-components for artifact removal in EEG signals. Behav Brain Funct. 2011;7:30. https://doi.org/10.1186/1744-9081-7-30.
Chang C-C, Lin C-J. LIBSVM: A library for support vector machines. ACM Trans Intell Syst Technol. 2011;2:27:1-27:27. https://doi.org/10.1145/1961189.1961199.
Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP. SMOTE: synthetic minority over-sampling technique. J Artif Intell Res. 2002;16:321–57. https://doi.org/10.1613/jair.953.
Donoghue T, Dominguez J, Voytek B. Electrophysiological frequency band ratio measures conflate periodic and aperiodic neural activity. eNeuro 2020;7. https://doi.org/10.1523/ENEURO.0192-20.2020.
Caso F, Cursi M, Magnani G, Fanelli G, Falautano M, Comi G, et al. Quantitative EEG and LORETA: valuable tools in discerning FTD from AD? Neurobiol Aging. 2012;33:2343–56. https://doi.org/10.1016/j.neurobiolaging.2011.12.011.
Ma C, M N, M B, O G, S C, M R, et al. The spectral exponent of the resting EEG indexes the presence of consciousness during unresponsiveness induced by propofol, xenon, and ketamine. NeuroImage 2019;189. https://doi.org/10.1016/j.neuroimage.2019.01.024.
Zsido RG, Molloy EN, Cesnaite E, Zheleva G, Beinhölzl N, Scharrer U, et al. One-week escitalopram intake alters the excitation-inhibition balance in the healthy female brain. Hum Brain Mapp. 2022. https://doi.org/10.1002/hbm.25760.
Maestú F, de Haan W, Busche MA, DeFelipe J. Neuronal excitation/inhibition imbalance: core element of a translational perspective on Alzheimer pathophysiology. Ageing Res Rev. 2021;69:101372. https://doi.org/10.1016/j.arr.2021.101372.
Ghosh I, Liu CS, Swardfager W, Lanctôt KL, Anderson ND. The potential roles of excitatory-inhibitory imbalances and the repressor element-1 silencing transcription factor in aging and aging-associated diseases. Mol Cell Neurosci. 2021;117:103683. https://doi.org/10.1016/j.mcn.2021.103683.
Merkin A, Sghirripa S, Graetz L, Smith AE, Hordacre B, Harris R, et al. Do age-related differences in aperiodic neural activity explain differences in resting EEG alpha? Neurobiol Aging. 2022. https://doi.org/10.1016/j.neurobiolaging.2022.09.003.
Knafo S, Alonso-Nanclares L, Gonzalez-Soriano J, Merino-Serrais P, Fernaud-Espinosa I, Ferrer I, et al. Widespread changes in dendritic spines in a model of Alzheimer’s disease. Cereb Cortex. 2009;19:586–92. https://doi.org/10.1093/cercor/bhn111.
León-Espinosa G, DeFelipe J, Muñoz A. Effects of amyloid-β plaque proximity on the axon initial segment of pyramidal cells. J Alzheimers Dis. 2012;29:841–52. https://doi.org/10.3233/JAD-2012-112036.
Garcia-Marin V. Diminished perisomatic GABAergic terminals on cortical neurons adjacent to amyloid plaques. Front Neuroanat 2009;3. https://doi.org/10.3389/neuro.05.028.2009.
Merino-Serrais P, Benavides-Piccione R, Blazquez-Llorca L, Kastanauskaite A, Rábano A, Avila J, et al. The influence of phospho-tau on dendritic spines of cortical pyramidal neurons in patients with Alzheimer’s disease. Brain. 2013;136:1913–28. https://doi.org/10.1093/brain/awt088.
Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet. 2015;386:1672–82. https://doi.org/10.1016/S0140-6736(15)00461-4.
Lendner JD, Harler U, Daume J, Engel AK, Zöllner C, Schneider TR, et al. Oscillatory and aperiodic neuronal activity in working memory following anesthesia. Clin Neurophysiol. 2023;150:79–88. https://doi.org/10.1016/j.clinph.2023.03.005.
Garn H, Waser M, Deistler M, Schmidt R, Dal-Bianco P, Ransmayr G, et al. Quantitative EEG in Alzheimer’s disease: cognitive state, resting state and association with disease severity. Int J Psychophysiol Off J Int Organ Psychophysiol. 2014;93:390–7. https://doi.org/10.1016/j.ijpsycho.2014.06.003.
Ibarra Chaoul A, Siegel M. Cortical correlation structure of aperiodic neuronal population activity. Neuroimage. 2021;245:118672. https://doi.org/10.1016/j.neuroimage.2021.118672.
Ranjan B, Sun W, Park J, Mishra K, Schmidt F, Xie R, et al. DUBStepR is a scalable correlation-based feature selection method for accurately clustering single-cell data. Nat Commun. 2021;12:5849. https://doi.org/10.1038/s41467-021-26085-2.
Acknowledgements
We acknowledge the support of the 2nd Department of Neurology of AHEPA General University Hospital of Thessaloniki for graciously sharing the datasets. All the participants who volunteered for this study deserve our gratitude. We also thank ChatGPT (version 3.5, Open AI, San Francisco, CA, USA) for its assistance in the visualization of the results.
Author information
Authors and Affiliations
Contributions
Z.W. and W.Z. provided the concept and design; Z.W., A.L., J.Y., P.W., and Y.B. analyzed and explained the data; J.Z. and S.X. guided the data processing in this study; Z.W. and W.Z. wrote the main manuscript; W.Z., B.G., and J.Z. supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Wang, Z., Liu, A., Yu, J. et al. The effect of aperiodic components in distinguishing Alzheimer’s disease from frontotemporal dementia. GeroScience 46, 751–768 (2024). https://doi.org/10.1007/s11357-023-01041-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-01041-8