Abstract
Background
The purpose of this study was to investigate the typing of adenovirus (AdV) infection in children hospitalized with acute respiratory tract infection (ARTI) and its clinical characteristics.
Methods
Samples from 7832 hospitalized children with ARTIs from January 2021 to June 2022 were tested by multiplex PCR for AdV. AdV hex neighborhood genes were amplified and sequenced for typing by nested PCR.
Results
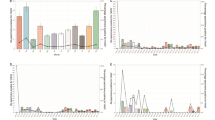
Three hundred twenty-eight cases were positive for AdV with rate of 4.48% (328/7832). No statistical difference in the rate of AdV detection was observed in different ages (P > 0.05). Among the 328 cases, 305 cases underwent amplification and sequence determination of AdV five-neighborhood, six-neighborhood and fibronectin genes. Only 237 cases were sequenced successfully for all 3 genetic fragments. The typing results of 231 cases with 3 genes were consistent, with 49.78% (115/231) of type 3, 41.56% (96/231) of type 7 and 8.66% (20/231) of other types identified. The main clinical symptoms in 231 children hospitalized with ARTI who were AdV positive were cough, sputum not easily coughable, Wheezing or shortness of breath and fever. Clinical diagnoses of 231 cases included: acute bronchitis 3.03% (7/231), capillary bronchitis 16.45% (38/231), pneumonia (mild/severe) 76.62% (177/231) (68.40% (158/231) in mild and 8.23% (19/231) in severe cases), bronchial asthma combined with pulmonary infection 3.46% (8/231). Higher percentage of shortness of breath, multilobar infiltration, and pleural effusion were found in type 7. Calcitoninogen in type 7 were significantly higher than those of type 3 and other types, and the white blood cell count was lower than those of type 3 and other types, and the difference was statistically significant (P < 0.05).
Conclusion
AdV type 3 and 7 were frequently found in hospitalized children with acute lower respiratory tract involvement. AdV type 7 seems to be associated with more severe outcome.
Similar content being viewed by others
Introduction
According to relevant statistics, ARTI account for the highest morbidity and mortality among children under 5 years of age, with 120–156 million illnesses per year, of which about 920,000 die, and about 95% of the deaths are in low- and middle-income countries [1, 2]. Thus, ARTI are one of the leading causes of morbidity and mortality in children worldwide, and respiratory diseases caused by AdV infections account for 5–7% [3]. AdV are capable of causing a range of diseases, including ARTI, acute gastroenteritis, conjunctivitis, hemorrhagic cystitis, and meningoencephalitis [4, 5]. Previous evidence points out that AdV serotypes 1, 2, 3, 4, 5, 6, 7, 11, 14, and 21 are mainly associated with respiratory diseases, with AdV types 3, 4, 7, and 14 being the main types causing ARTI in children [6]. In recent years, the newly emerged type 14PI and recombinant type 55 and AdV types 3, 4, 7 and 14 often cause severe lower respiratory tract infections and are the main pneumonia agents of fatal incendiary infections in infants and children. Differences in viruses lead to different diseases, while small changes in the genes of the same virus can have an important impact on the viral pathogenicity [7]. Among the same virus, there are differences in clinical symptoms, disease severity and prognosis triggered by different types [8]. Therefore, investigation of the typing and clinical characteristics of AdV infection in children is a hot topic of clinical research, however, there are few clinical studies on this topic. Based on this, this study will investigate the typology and clinical characteristics of AdV infection in children.
Materials and methods
Clinical data
Retrospective analysis of 7832 respiratory specimens sent from children admitted to our hospital with ARTI from January 2021 to June 2022 were tested for AdV positivity. The study was in accordance with the relevant requirements of the World Medical Association Declaration of Helsinki [9]. Inclusion criteria: all children met the diagnostic criteria for ARTI in Zhu Fu-Tang Practical Pediatrics [10]; all children hospitalized with ARTI in the Department of Respiratory Medicine and the Department of Critical Care Medicine of our hospital; respiratory specimens collected on the day of admission or on day 2 were tested positive for AdV antigen by direct immunofluorescence assay (DFA, Diagnostic Hybrids, USA) or AdV nucleic acid positivity by respiratory pathogen detection kit (RPP, Luminex, USA); informed consent signed by the guardians of the children. Exclusion criteria: guardians who did not want to participate in this study; children with severe cardiopulmonary disease, immunosuppression, or immune ischemic disease.
The study protocol was approved by the Ethics Committee of Xi’an Jiaotong University Health Science Center. Informed consent was obtained from all the legal representatives or the guardian of the children participants involved in the study.
Processing of specimens
Specimens such as nasopharyngeal swabs, nasopharyngeal secretions, sputum and alveolar lavage fluid were collected from the children, 2 ml of Hank’s fluid was added to the blown and mixed, and centrifuged at 1200 g for 10 min. The supernatant was partially used for viral nucleic acid extraction for RPP detection, and the remainder was stored at − 80 °C; the precipitate (mainly exfoliated respiratory epithelial cells) was resuspended and spotted onto slides for DFA detection. RPP and DFA were performed according to the kit instructions.
Nucleic acid extraction and AdV typing
Specimens tested positive for AdV by DFA and RPP were selected, 140 μl of specimen supernatant was taken, nucleic acid extraction kit from QIAGEN, total nucleic acid was extracted according to the instructions, and finally dissolved in 60 μl of buffer and stored at − 20 °C. PCR was performed to amplify each of the major coat protein genes of AdV five-neighbor, six-neighbor and fibronectin. The PCR products were sequenced using the ABI13730xI DNA analyzer (Beijing sinogenomax Co.)
Phylogenetic analysis
After the sequencing results were returned, Chromas Lite 2.22 software was applied to determine the correct reading of the chromatogram of the sequencing results, and NCBI BLAST was used to determine whether the amplification products were AdV, and the preliminary determination of the type; Editseq and Seqman in DNA Star were used to splice and edit the sequences. Clustal W in MECA X software was used to compare the obtained sequences with the reference strains of the identified type in GenBank, and the neighbor-joining method was used to construct a phylogenetic tree based on gene distances.
Collection of clinical data of patients
Including the age, gender, clinical diagnosis, admission time, discharge time, length of stay, discharge department, and imaging data of the children.
Statistics
SPSS 23.0 software was applied to analyze the data. Continuous variables with normal distribution were described by mean ± sd, and independent samples t-test was used for comparison between groups; continuous variables with non-normal distribution were described by M(Q1,Q3), and U-test was used for comparison between groups; count data were described by cases (%), and chi-square χ2 test was used. Differences were considered statistically significant at P < 0.05.
Results
AdV positivity rate in children hospitalized with ARTI
Among the respiratory specimens sent from children hospitalized with ARTI, 328 cases were positive for AdV, with a positive detection rate of 4.48% (328/7832). The detection rate was 4.81% (194/4032) for boys and 3.53% (134/3800) for girls, with a positive detection rate of 2.97% (38/1281) for AdV ≤6 months of age, 4.86% (39/802) for AdV > 6 months of age to 1 year of age, 6.67% (83/ 1245), 6.38% for > 2–5 years (131/2054), and 2.16% for > 5 years (53/2450), with no statistically significant difference (P > 0.05).
AdV genotyping
Among the 328 AdV-positive respiratory samples, 23 had insufficient volume for testing and the remaining 305 underwent amplification and sequencing of AdV genotypes. The 3 gene fragments of AdV were successfully sequenced in 237 cases. The results of phylogenetic tree typing showed that the typing results of 3 gene fragments in 6 cases were inconsistent and could not be determined. The genotyping results of 3 gene fragments in the remaining 231 cases were consistent., among which 49.78% (115/231) of type 3 AdV, 41.56% (96/231) of type 7, and 8.66% (20/231) of other types were identified.
Main clinical symptoms of AdV positivity in children hospitalized with ARTI
The main clinical symptom of AdV positivity in 231 children hospitalized with ARTI was cough 96.54% (223/231), followed by having sputum that could not be easily coughed up 71.43% (165/231), then shortness of breath 74.03% (171/231), fever 47.19% (109/231), in addition to a few cases of nasal congestion 14.72% (34 /231), runny nose 15.15% (35/231), vomiting 12.99% (30/231), diarrhea 9.52% (22/231), convulsions 6.06% (14/231), and rash 2.60% (6/231).
Type 7 wheezing or shortness of breath proportion was significantly higher than type 3 and other types, and the white blood cell count was lower than type 3 and other types, and the difference was statistically significant (P < 0.05); with no difference when comparing gender, age, peak body temperature, cough, having sputum not easily coughing up, fever, nasal congestion, runny nose, vomiting, diarrhea, convulsions, and rash of type 7 and other types in the 3 groups, see Table 1.
Clinical diagnosis of AdV positivity in children hospitalized with s
The 231 AdV-positive clinical diagnoses in children hospitalized with ARTI included: acute bronchitis 3.03% (7/231), capillary bronchitis 16.45% (38/231), pneumonia (mild/severe) 76.62% (177/231) (68.40% (158/231) in mild and 8.23% (19/231) in severe cases), bronchial asthma combined with pulmonary infection 3.46% (8/231).
Type 7 multilobar infiltration, pleural effusion proportion and calcitoninogen were significantly higher than type 3 and other types, and white blood cell count was lower than type 3 and other types, and the difference was statistically significant (P < 0.05); There was no difference in comparing with acute bronchitis, capillary bronchitis and pneumoniaamong 3 groups, see Table 2.
Discussion
In our study, it was found that the adenoviruses infected in hospitalized children with ARTI were mainly type 3 and type 7. Higher percentage of shortness of breath, multilobar infiltration, and pleural effusion were observed in clinical symptoms of type 7. A study noted that the main type of AdV infections among inpatients and outpatients in south China were type 7 [11]. A study by Yali Duan [12] et al. noted that a cross-sectional study of nine hospitals in different provinces of China pointed out that the predominant types in the northern local epidemic were types 3 and 7, and the predominant types in the southern region were types 2 and 3. In addition, a shift in the predominant AdV endemic type from type 3 to type 7 was monitored in Guangdong, and this change in type may have caused the AdV outbreak in the southern region in 2018 [13]. The findings of these studies are generally consistent with our results, implying that there are no significant differences in the AdV types leading to ARTI across Chinese provinces.
ARTI is a common disease in children, which can occur throughout the year and has a high morbidity and mortality rate, seriously affecting the lives of people worldwide, and AdV infection is one of the important pathogens causing ARTI in children [14]. AdV is commonly found in nature and AdV infections have been reported worldwide. Since 1953, when Rowe first isolated AdV, and 1958, when research on AdV infections began in China, previous reports indicated that 100 serotypes have been identified, divided into two genera, mammalian AdV and avian AdV, while human AdV is divided into 7 subgroups A-G, with more than 80 different serotypes, which can exist as regional, epidemic and sporadic infections with worldwide, or outbreaks epidemics [15]. Different types of human AdV have different characteristics such as histophilia, pathogenicity, and endemic areas, and are mainly transmitted through droplets, contact, and fecal-oral, etc. Patients with AdV infection and those with occult infection are the main sources of infection, which can cause pneumonia, pharyngeal-conjugate fever, tonsillitis, cystitis, encephalitis, and enteritis, etc. Most patients have mild or insignificant symptoms, but the infection can also spread to the whole body and severe pneumonia, and even death; in addition, AdV infection can leave different degrees of sequelae, and most patients have a poor prognosis, which has a serious impact on the quality of life of the children [16]. Therefore, it is necessary to analyze the typology and clinical characteristics of AdV infection in children with ARTI in order to provide a basis for clinical prevention and treatment.
As AdV recombination leads to the continuous emergence of novel types, the International AdV Organization believes that the acquisition of the three gene sequences, five-neighborhood, six-neighborhood and fibronectin, is necessary to determine the AdV type [13]. The results of the present study showed consistent results of typing of 231 cases with 3 genes, where 49.78% (115/231) of AdV type 3, 41.56% (96/231) of type 7 and 8.66% (20/231) of other types were identified, which is consistent with the results of the above study. The results of Lin [17] et al. pointed out that type 7 infections have lower white blood cell counts than type 3 infected children and higher calcitonin pro and C-reactive protein levels were high. The results of the present study showed that type 7 wheezing or shortness of breath, multilobar infiltrates, pleural effusion proportion and calcitoninogen were significantly higher than type 3 and other types, and leukocyte count was lower than type 3 and other types (P < 0.05), suggesting that the inflammatory response is higher in children with ARTI with AdV-positive gene type 7 who have more symptoms of wheezing or shortness of breath and a reduced leukocyte count and higher levels of calcitoninogen. The results of the present study differ from the above-mentioned studies in that the results of the present study did not find any difference in C-reactive protein levels between type 3 and type 7 infections. In addition, the comparison of critical AdV pneumonia, admission to pediatric intensive care unit (PICU), invasive mechanical ventilation and death among type 3, type 7 and other types in this study (P > 0.05), but type 7 critical AdV pneumonia, admission to PICU, invasive mechanical ventilation and death were slightly higher than type 3 and other types, which may be analyzed because type 7 AdV infection is more likely to progress to severe disease. The study by Xie [18] et al. noted that type 7 AdV infections are more likely to progress to severe pneumonia, with higher oxygen requirements and longer hospital stays. Similarly, Fu [19] et al. showed through in vivo, in vitro and clinical correlation analysis that type 7 AdV has a greater replication capacity compared to type 3 AdV and can promote and exacerbate cytokine responses, resulting in a more severe respiratory inflammatory response.
There are shortcomings in this study. The results of this study are retrospective and only represent the typology and clinical symptoms of AdV infection in children admitted to our hospital with ARTI. In addition, significant differences in the distribution of AdV infection subtypes between children in the north and south of China have been previously found, and subsequent multicenter studies need to take into account sampling in the north and south. Moreover, the recently discovered association of severe acute hepatitis with AdV and SARS-CoV-2 should be included in subsequent studies if possible.
Conclusion
In summary, type 3, 7 and other types of AdV infection are common in children hospitalized with ARTI. Type 7 has a higher proportion of shortness of breath, multilobar infiltration, pleural effusion, significantly higher calcitoninogen, and lower in white blood cell count. These results indicating that type 7 is more severe and should be given clinical attention and timely and useful treatment. Therefore, clinical attention should be paid to differentiate AdV types, and timely monitoring of AdV helps clinicians to diagnose, treat and judge the prognosis of the disease.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DFA:
-
direct immunofluorescence assay
- RPP:
-
respiratory pathogen detection kit
References
Synowiec A, Szczepański A, Barreto-Duran E, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a systemic infection. Clin Microbiol Rev. 2021;34(2):e00133–20.
Petrarca L, Nenna R, Frassanito A, et al. Human bocavirus in children hospitalized for acute respiratory tract infection in Rome. World J Pediatr. 2020;16(3):293–8.
Kong D, Zheng Y, Jiang C, et al. Analysis of adenovirus infection in acute respiratory tract infection cases in Shanghai from 2015 to 2019. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(5):733–7.
Gu Y, Huang R, Wang M, et al. Epidemiological characteristics of adenovirus infection in hospitalized children with acute respiratory tract infection in Kunming during 2019. Zhonghua er ke za zhi= Chinese. J Pediatr. 2021;59(9):772–6.
Brodin P, Arditi M. Severe acute hepatitis in children: investigate SARS-CoV-2 superantigens. Lancet Gastroenterol Hepatol. 2022;7(7):594–5.
Song J, Lee H, Cho E. Epidemiological investigation of the outbreak of acute respiratory infection caused by adenovirus type B55 in a physical education school in 2017. Infect Chemother. 2019;51(2):119–29.
Sun J, Xiao Y, Zhang M, et al. Serum inflammatory markers in patients with adenovirus respiratory infection. Med Sci Monit. 2018;24:3848.
Wang F, Yang C, Qian Y, et al. Clinical characteristics of human adenovirus infection in hospitalized children with acute respiratory infection in Beijing. Zhonghua Er Ke Za Zhi. 2022;60(1):30–5.
Williams JR. The declaration of Helsinki and public health. Bull World Health Organ. 2008;86:650–2.
Hu Y, Jiang Z, Shen K. Zhu Futang practical pediatrics. Beijing: People’s Health Publishing House; 2002. p. 970–1014.
Zhao S, Wan C, Ke C, et al. Re-emergent human adenovirus genome type 7d caused an acute respiratory disease outbreak in southern China after a twenty-one year absence. Sci Rep. 2014;4(1):1–10.
Duan Y, Zhu Y, Xu B, et al. Multicenter study of human adenovirus infection in pediatric community-acquired pneumonia in China. Zhonghua er ke za zhi= Chinese. J Pediatr. 2019;57(1):27–32.
Chen Y, Liu F, Wang C, et al. Molecular identification and epidemiological features of human adenoviruses associated with acute respiratory infections in hospitalized children in southern China, 2012-2013. PLoS One. 2016;11(5):e0155412.
Merera AM. Determinants of acute respiratory infection among under-five children in rural Ethiopia. BMC Infect Dis. 2021;21(1):1–12.
Dagne H, Andualem Z, Dagnew B, et al. Acute respiratory infection and its associated factors among children under-five years attending pediatrics ward at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia: institution-based cross-sectional study. BMC Pediatr. 2020;20(1):1–7.
Davidson JA, Banerjee A, Smeeth L, et al. Risk of acute respiratory infection and acute cardiovascular events following acute respiratory infection among adults with increased cardiovascular risk in England between 2008 and 2018: a retrospective, population-based cohort study. Lancet Digit Health. 2021;3(12):e773–83.
Lin M-R, Yang S-L, Gong Y-N, et al. Clinical and molecular features of adenovirus type 2, 3, and 7 infections in children in an outbreak in Taiwan, 2011. Clin Microbiol Infect. 2017;23(2):110–6.
Xie L, Zhang B, Xiao N, et al. Epidemiology of human adenovirus infection in children hospitalized with lower respiratory tract infections in Hunan, China. J Med Virol. 2019;91(3):392–400.
Fu Y, Tang Z, Ye Z, et al. Human adenovirus type 7 infection causes a more severe disease than type 3. BMC Infect Dis. 2019;19(1):1–11.
Acknowledgements
None.
Funding
This study was funded by Shaanxi province social development science and technology project (NO.2016SF-210).
Author information
Authors and Affiliations
Contributions
LW and WX contributed to the conception and design of the study; XH,YZ, XZ, XL, HM and ZH performed the experiments, collected and analyzed data; HH,LW and WX wrote the manuscript; LW and WX revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study designed in accordance with Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Xi’an Jiaotong University Health Science Center. Informed consent was obtained from all the legal representatives or the guardian of the children participants involved in the study..
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, L., Hu, X., Huang, Z. et al. Analysis of the typing of adenovirus and its clinical characteristics in children with acute respiratory tract infection. BMC Pediatr 23, 25 (2023). https://doi.org/10.1186/s12887-023-03840-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-023-03840-6