Abstract
Background
Glycogen storage disease (GSD) type IXb is one of the rare variants of GSDs. It is a genetically heterogeneous metabolic disorder due to deficient hepatic phosphorylase kinase activity. Diagnosis of GSD can be difficult because of overlapping manifestations. Mutation analysis of the genes related to each type of GSD is supposed to be problem-solving, however, the presence of novel mutations can be confusing. In this case report, we will describe our experience with a young girl with the diagnosis of GSD and two novel mutations related to GSD type IXb.
Case presentation
A 3-year- old girl presented with short stature, hepatomegaly, and liver cirrhosis. No specific diagnosis was made based on laboratory data, so liver biopsy and targeted-gene sequencing (TGS) were performed to find out the specific molecular basis of her disease. It was confirmed that the patient carries two novel variants in the PHKB gene. The variant in the PHKB gene was classified as pathogenic.
Conclusions
This is the first reported case of a dual molecular mutation of glycogen storage disease type IXb in the same patient. Two novel variants in PHKB were identified and one of them was a pathogenic split-site mutation. In conclusion, for the first time, identification of the novel variants in this patient expands the molecular and the phenotype basis of dual variants in GSD-IXb.
Similar content being viewed by others
Background
Glycogen storage disease (GSD) type IX is caused by phosphorylase b kinase (PhK) deficiency (EC 2.7.1.38), a key enzyme in glycogen degradation [1, 2]. This enzyme is expressed in the liver and muscle tissue, though liver PhK deficiency is more common than muscle [3]. PhK is a complex enzyme including four different subunits. The α subunit is encoded by the PHKA2 gene (MIM 306,000), namely GSD IXa, which is liver-specific with X-linked inheritance. The PHKB gene (MIM 261,750) indicating GSD IXb encodes the β subunit, and the PHKG2 gene (MIM 613,027) indicating GSD IXc encodes the γ subunit [4]. Both of these subunits are autosomal recessive.
Reports on the PHKB gene mutations resulting in deficient phosphorylase kinase in both liver and muscle are very infrequent [5, 6]. GSD IXb is characterized by hepatomegaly, hypoglycemia, growth retardation, as well as motor developmental delays [7]. Unlike other hepatic GSDs, symptoms of GSD IXb are often mild, and patients may even become asymptomatic as they grow up [8,9,10].
So far, there has not been any case report of GSD type IXb from Iran. We present the first case diagnosed in our center, i.e. a child with GSD IXb, whose specific symptoms are related to a dual mutation in the PHKB gene.
Case presentation
A 3-year-old girl was referred to the pediatrician with hepatomegaly and developmental delay. The girl was the first child of consanguineous marriage in an Iranian family. She was delivered following a normal and term pregnancy with a birth weight of 2.95 kg, and height of 47 cm. No hypoglycemia was noted in the perinatal period and the postnatal transition.
At the age of 1, she developed abdominal distension. However, abdominal ultrasound has been reported as unremarkable. During childhood, she frequently experienced morning nausea, vomiting, and lethargy.
At the age of 2, she was referred to our center because of developmental delay. Biochemical lab tests revealed high hepatic transaminases (ALT 375 U/L, AST 495 U/L), mild neutropenia (750 per microliter), microcytic hypochromic anemia (hemoglobin of 10.3 g/dl and hematocrit of 34.1 %, mean corpuscular volume of 73.81 fl., and low mean corpuscular hemoglobin 25.54 pg), as well as high triglyceride (498 mg/dl), and cholesterol (268 mg/dl).
A abdominal ultrasound revealed hepatomegaly with mild diffuse heterogeneous echogenicity of the liver parenchyma. A liver biopsy was performed, which revealed cirrhosis with severe swelling of the hepatocytes with clear cytoplasm. Portal tracts showed very mild lymphocytic infiltration (Fig. 1). According to clinical findings and liver biopsy, a hepatic form of GSD was suspected, so treatment with frequent feeds was initiated. Nevertheless, hepatic transaminase elevation persisted and ketosis with hyperlactatemia were developed. Therefore, at the age of 3, an aggressive regimen with uncooked cornstarch (5 times per day) and protein (2.5 g/kg/day) was initiated. After the diet therapy, her morning vomiting, and lethargy improved, and hepatic enzyme (ALT 80 U/L, AST 86 U/L) were decreased, but her cholesterol level increased (313 mg/dl).
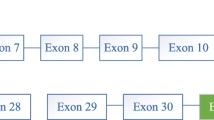
Targeted gene sequencing (TGS) with a custom-targeted Ion AmpliSeq panel was performed. The panel included 7219 amplicons covering 450 genes associated with Inborn Metabolic Diseases consisting of glycogen storage disorders genes with hepatic involvement. Sanger sequencing validated identified the variants, using an ABI Prism 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Analyses were done using an Ion Torrent 540 chip (Life Technologies, Guilford, CT, South San Francisco, CA). The human genome 19 was used as the reference. Polyphen2, SIFT, and Mutation Taster were used for in silico analysis, GERP and Phastcons scores were used to evaluate the conservation of the variants. The population frequency of each variation was evaluated, using data from the gnomAD database. ACMG guidelines were used for variant interpretation [11]. The sequence variants were described according to the Human Genome Variation Society Nomenclature [12]. Interestingly, TGS findings showed that the patient carried two novel variants, which consisted of a homozygous variant c.1127-2 A > G (p.?) in exon 12, a homozygous missense variant c.2840 A > G (p.Gln947Arg) in exon 28 of the PHKB gene. In silico analysis revealed that the novel variant, c.1127-2 A > G (p.?) is pathogenic, which would have possibly damaged the splice site, and two other ones are variants of uncertain significance (VUS). However, samples from the parents were not available for the zygosity determination of these novel variants. It should be mentioned that both parents were asymptomatic.
Discussion and conclusions
Herein we are reporting novel homozygous variants in the PHKB gene, leading to loss of function, in a 3-year-old girl born to a consanguineous family of Iranian descent.
To the best of our knowledge, our patient is the only and the first case of dual homozygote variants in GSD-IXb, with severe liver involvement. Compared with other GSD-IXb patients with a single mutation in PHKB, our patient who has dual variants showed a more severe short stature and liver dysfunction. To find and carefully evaluate all reported mutations and effects on the presentation of GSD IXb, we did a literature search in August 2020. To date, 23 variants were identified in PHKB gene in 18 patients with GSD-IXb (Table 1). A comparison of the literature showed that manifestation of GSD-IXb was highly variable, ranging from benign mild to moderate, and sometimes aggressive [2, 3, 6,7,8,9,10]. The age of GSD-IXb onset can be in young children with a mean age of 3.8 years. It is noteworthy that the majority of patients shown in Table 1 presented with hepatomegaly (92.85 %) and elevated hepatic transaminase (35.71 %). However, less than half of the 18 patients showed signs of hypoglycemia (27.7 %), hyperlipidemia (21.42 %), and short stature (14.28 %). Just in our case, liver cirrhosis (5.6 %) is reported.
Molecular method confirmed a dual mutation in GSD-IXb. Co-occurrence of two different mutations in GSD subtype in one patient is exceedingly rare and has never been reported so far. Posey et al. [13] reported that out of 7374 patients only 101 (4.9 %) were diagnosed with more than one locus for the disease by performing next-generation sequencing (NGS). So, NGS is an efficient, accurate, and cost-effective method for identifying disease genes. For clinically and genetically heterogeneous diseases caused by a group of genes involving a common metabolic pathway, TGS can also be used for simultaneous sequencing of the group of candidate genes [14]. Our case demonstrates that molecular analysis especially using TGS is an essential method in the diagnosis of GSD subtypes. An early genetic diagnosis by TGS has many benefits including time and cost-effectiveness, right treatment, accurate recurrence risk advice, and where appropriate, screening of patients [15].
In conclusion, our study describes an Iranian patient who suffered from GSD-IXb. Two novel variants in the PHKB gene were identified, one of which is pathogen. The report of these variants could expand the mutation spectrum of GSD-IXb. Dual mutations in the GSD subtype in one patient is rare; however, with the progress in molecular diagnostic methods, we may be able to identify more patients with multiple mutations in different genes, and because of that, our knowledge about inherited human diseases will be improved.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALT:
-
: Alanine transaminase
- AST:
-
Aspartate transaminase
- GSD:
-
Glycogen storage disease
- NGS:
-
Next-generation sequencing
- PAS:
-
Periodic acid–Schiff
- PhK:
-
Phosphorylase b kinase
- TGS:
-
Targeted Gene Sequencing
- VUS:
-
Variants of uncertain significance
References
Brushia RJ, Walsh DA. Phosphorylase kinase: the complexity of its regulation is reflected in the complexity of its structure. Front Biosci. 1999;4:D618–41.
Beauchamp NJ, Dalton A, Ramaswami U, Niinikoski H, Mention K, Kenny P, Kolho KL, Raiman J, Walter J, Treacy E, Tanner S, Sharrard M. Glycogen storage disease type IX: High variability in clinical phenotype. Mol Genet Metab. 2007;92(1–2):88–99.
Burwinkel B, Moses SW, Kilimann MW. Phosphorylase-kinase deficient liver glycogenosis with an unusual biochemical phenotype in blood cells associated with a missense mutation in the beta subunit gene (PHKB). Hum Genet. 1997;101:170–4.
Beyzaei Z, Geramizadeh B. Molecular diagnosis of glycogen storage disease type I: a review. EXCLI J. 2019;18:30–46.
Burwinkel B, Hu B, Schroers A, Clemens PR, Moses SW, Shin YS, Pongratz D, Vorgerd M, Kilimann MW. Muscle glycogenosis with low phosphorylase kinase activity: mutations in PHKA1, PHKG1 or six other candidate genes explain only a minority of cases. Eur J Hum Genet. 2003;11:516–26.
Burwinkel B, Maichele AJ, Aagenaes O, Bakker HD, Lerner A, Shin YS, Strachan JA, Kilimann MW. Autosomal glycogenosis of liver and muscle due to phosphorylase kinase deficiency is caused by mutations in the phosphorylase kinase beta subunit (PHKB). Hum Mol Genet. 1997;6:1109–15.
van den Berg IE, van Beurden EA, de Klerk JB, van Diggelen OP, Malingre HE, Boer MM, Berger R. Autosomal recessive phosphorylase kinase deficiency in liver, caused by mutations in the gene encoding the beta subunit (PHKB). Am J Hum Genet. 1997;61:539–46.
Roscher A, Hewson S, Nagy L. The natural history of glycogen storage disease types VI and IX: Long-term outcome from the largest metabolic center in Canada. Mol Genet Metab. 2014;113:171–6.
Davit-Spraul A, Piraud M, Dobbelaere D, Valayannopoulos V, Labrune P, et al. Liver glycogen storage diseases due to phosphorylase system deficiencies: Diagnosis thanks to non invasive blood enzymatic and molecular studies. Mol Genet Metab. 2011;104:137–43.
Degrassi I, Deheragoda M, Creegen D, Mundy H, Mustafa A, Vara R, Hadzic N. Liver histology in children with glycogen storage disorders type VI and IX. Digestive Liver Dis. 2020. https://doi.org/10.1016/j.dld.2020.04.017.
Richards S, Aziz N, Bale S, Bick D. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–23.
Den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mut. 2000;15:7–12.
Posey JE, Harel T, Liu P, Rosenfeld JA, James RA, Akdemir ZH, et al. Resolution of disease phenotypes resulting from multilocus genomic variation. N Engl J Med. 2017;376(1):21–31.
Beyzaei Z, Geramizadeh B, Karimzadeh S. Diagnosis of hepatic Glycogen storage disease patients with overlapping clinical symptoms by massively parallel sequencing: a systematic review of the literature. Orphanet J Rare Dis. 2020;15:217.
Beyzaei Z, Ezgu F, Geramizadeh B, Imanieh MH, Haghighat M, Honar N, et al. Clinical and genetic spectrum of glycogen storage disease in Iranian population using targeted gene sequencing. Scientific reports. 2021;11:7040.
Acknowledgements
Not applicant.
Funding
This study was financially supported by the Transplant Research Center, affiliated with Shiraz University of Medical Sciences, Shiraz, Iran (Grant No.1396-01-106-15748) and National Institute for Medical Research Development (Grant No. 976961).
Author information
Authors and Affiliations
Contributions
ZB contributed to the review of the literature, review of the patient’s information, and drafting of the manuscript. BG contributed to the examination of the pathology slides, review of the manuscript, and provision of guidance on the approach to the topic. FE and ZB performed the mutation analysis. ARA and ARS contributed to the clinical evaluation of the patient and search of the databases. All authors have reviewed the manuscript and approved the content for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Parents of patients or legal guardians provided a written informed consent form for participation in the study. Parents of the participants gave written informed consent. The ethics committee of Shiraz University of Medical Sciences approved this study (Approval #: IR.SUMS.REC.1396.S805).
Consent for publication
A consent was obtained from the parents of the patient whose case is being reported in this manuscript. Also, the case report is anonymized to protect the identity of the patient in the study. The patient’s parents provided written informed consent for the publication of potentially identifying images and clinical details.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Beyzaei, Z., Ezgu, F., Geramizadeh, B. et al. Novel mutations in the PHKB gene in an iranian girl with severe liver involvement and glycogen storage disease type IX: a case report and review of literature. BMC Pediatr 21, 175 (2021). https://doi.org/10.1186/s12887-021-02648-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-021-02648-6