Abstract
Background
During the first period of life, critically ill as well as healthy newborn infants experience recurrent painful procedures. Parents are a valuable but often overlooked resource in procedural pain management in newborns. Interventions to improve parents’ knowledge and involvement in infants’ pain management are essential to implement in the care of the newborn infant. Neonatal pain research has studied a range of non-pharmacological pain alleviating strategies during painful procedures, yet, regarding combined multisensorial parent-driven non-pharmacological pain management, research is still lacking.
Methods/design
A multi-center randomized controlled trial (RCT) with three parallel groups with the allocation ratio 1:1:1 is planned. The RCT “Parents as pain management in Swedish neonatal care – SWEpap”, will investigate the efficacy of combined pain management with skin-to-skin contact, breastfeeding and live parental lullaby singing compared with standard pain care initiated by health care professionals, during routine metabolic screening of newborn infants (PKU-test).
Discussion
Parental involvement in neonatal pain management enables a range of comforting parental interventions such as skin-to-skin contact, breastfeeding, rocking and soothing vocalizations. To date, few studies have been published examining the efficacy of combined multisensorial parent-driven interventions. So far, research shows that the use of combined parent-driven pain management such as skin-to-skin contact and breastfeeding, is more effective in reducing behavioral responses to pain in infants, than using the pain-relieving interventions alone. Combined parental soothing behaviors that provide rhythmic (holding/rocking/vocalizing) or orogustatory/orotactile (feeding/pacifying) stimulation that keep the parent close to the infant, are more effective in a painful context. In the SWEpap study we also include parental live lullaby singing, which is an unexplored but promising biopsychosocial, multimodal and multisensory pain alleviating adjuvant, especially in combination with skin-to-skin contact and breastfeeding.
Trial registration
ClinicalTrials.gov (NCT04341194) 10 April 2020.
Similar content being viewed by others
Background
Parents are a valuable but often overlooked resource in procedural pain management in newborn infants. The influence of the parent is particularly salient in early infancy and in extremely distressful situations such as painful procedures, because infants lack the resources to successfully self-regulate. Therefore, infants need an emotionally available and stable parent who responds adequately to the infant’s distress signals and who is able to soothe, regulate and share the infant’s states [1]. In the Nordic countries today, the concept of family-centered care (FCC) is considered best practice in the care of the newborn [2, 3]. Since the 1990s, FCC has been and still is, part of an ongoing paradigm shift where family involvement in the infant’s care and the parent-infant relationship are of central importance, a cornerstone in current neonatal and pediatric health care [4, 5]. Nonseparation of parents and infants is a protective measure in decreasing stress in both parents and infants [6] and should also be applied in painful procedures [7].
As part of routine postnatal care, all infants experience recurrent painful procedures early in life with blood sampling for newborn metabolic screening and immunization programs. Infants in the neonatal intensive care unit (NICU) receive the highest amount of pain exposure, on average between 7 and 17 painful procedures per day [8]. Far from all infants receive adequate pain management during procedures (Shah & Siu 2019, [9, 10]). Untreated pain leads to unnecessary suffering but also leaves the newborn infant at risk for long term negative consequences from the pain. Research have shown detrimental effects of repeated painful experiences in the newborn infant including altered cortical development [11, 12], altered pain processing, increased internalizing behavior [13] and long-term effects on cortisol response [14]. For infants born preterm, neonatal pain-related stress was associated with alterations in both early and in later developmental outcomes [15].
During the last decade, parents’ participation in infant pain management has become a focus for research in nursing pain science (e.g. [16,17,18,19,20]). Parental presence has been shown to increase documentation of nursing pain assessment and of the use of non-pharmacological pain-relieving methods such as skin-to-skin contact (SSC) and breastfeeding, and decrease the infant’s pain intensity and behavioral distress [21, 22]. Parents have a unique knowledge and perspective of their infant’s needs and personality and can be effective partners in the care of the infant, including pain management, if they are acknowledged by the health professionals [20, 23]. Not all parents are able to provide emotional and physical closeness, many parents do not know how to effectively comfort their child during a medical procedure, and some do not feel confident doing so [24]. But most parents experience a feeling of helplessness in their inability to protect their infant from pain during procedures [25, 26]. Therefore, health professionals need to safeguard parent-infant proximity, reciprocity, and establishment of parental responsibility, which are all essential factors for the parent-infant attachment process [26]. Interventions to improve parents’ knowledge and involvement in infants’ pain management are essential to implement in the care of the newborn infant [26]. Parents need to and want to participate actively in their infant’s pain management, and parents should receive education and guidance in various format, not just verbal information, on how to mitigate their infant’s pain [23, 24, 27,28,29,30,31].
Pain alleviating pharmacological agents such as oral sweet solutions, i.e. sucrose and glucose, are today part of standard care in neonatal and postnatal care and are considered to have a calming and analgesic effect on infants during painful procedures [32]. Sweet solutions have been extensively investigated and found to reduce procedural pain from blood-sampling procedures in preterm, term infants and infants ≤12 months old, without serious side effects [33]. However, there is a consensus that non-pharmacological strategies should be the first choice in procedural pain management for infants because there are no adverse effects and parents can be involved using these approaches safely [23, 34, 35]. Non-pharmacological pain management involves interventions driven by nurses and/or parents. Just like oral sweet solutions, swaddling, containment or facilitated tucking, positioning, non-nutritive sucking and recorded music stimulation, are nurse-initiated pain alleviating interventions where parents can be included but parental presence is not required [32, 36]. Among the non-pharmacological approaches, the biopsychosocial perspective strongly supports parent-driven interventions [37]. In the parent-driven non-pharmacological interventions, the parent herself/himself is a mediator for pain relief [36]. Parent-driven non-pharmacological interventions are consistent with modern family-centered care in which the best interests of the infant and family are put ahead of staff convenience [36]. SSC, breastfeeding and live singing are simple and cost-effective evidence-based interventions that may modify the infant’s pain and stress if the strategies are well-timed [38,39,40,41]. SSC is a method widely used in postnatal care globally. A significant amount of research has also shown the pain-relieving effect of skin-to-skin contact during painful procedures in newborn infants [19, 42]. Breastfeeding has demonstrated efficacy that is equal to, or greater than, sweet taste interventions in reducing behavioral and physiological responses to pain in full-term infants undergoing venipuncture with no demonstrated adverse outcomes [38]. Live parental infant-directed lullaby singing, informed by music therapy research, has not been previously investigated during painful procedures. It has been confirmed in previous research in non-painful contexts that live parental lullaby singing is an individually tailored, non-verbal, multisensory, affective, relationship-based tool useful in regulating the infant, augmenting the parent’s focus on the infant in the moment, enhancing the parent’s emotional availability and responsiveness and decreasing stress in the dyad [43,44,45,45]. Previous research with recorded music stimulation with instrumental lullaby music, selected in consultation with an accredited music therapist, has shown similar effect to the current gold standard of oral sucrose [46], and the combination of music stimulation with sucrose provides better pain relief during blood sampling than when sucrose or music is used alone [46]. Recorded mother’s voice has also shown similar pain alleviating effects as oral sweet solutions [47, 48].
Neonatal pain research has studied a range of non-pharmacological pain alleviating strategies during painful procedures. However, there is a dearth of research about combined multisensorial parent-driven non-pharmacological pain management, which encompasses a combination of individual pain-relieving interventions such as parental closeness and SSC, olfactory, oral and auditory stimulation. Therefore, a multi-center randomized controlled trial with the aim to investigate the efficacy of combined pain management with SSC, breastfeeding and live parental lullaby singing, is planned in postnatal care.
Methods: hypothesis, participants, interventions, measures and outcomes
Hypothesis
No previous research has assessed the efficacy of combined parent-driven pain management with SSC, breastfeeding and live parental lullaby singing in newborn infants during routine newborn metabolic screening. In this research study, “Parents as pain management in Swedish neonatal care – SWEpap”, we hypothesize that parent-driven pain management such as SSC in combination with breastfeeding and live parental lullaby singing, will provide a more effective pain management during venipuncture in newborn infants compared to standard pain care initiated by health care professionals.
Study design
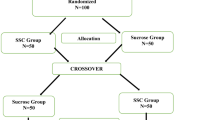
This is a multi-center, randomized controlled trial (RCT) with three parallel groups with the allocation ratio 1:1:1.
Study setting
This RCT involves parent-infant dyads recruited from four health care regions in Sweden during the routine newborn metabolic screening. In the Swedish context, the healthy newborn infant is often discharged soon after birth and returns at 48 h of age for a newborn screening test and medical examination. The involved regions represent both university hospitals and regional hospitals.
Eligibility criteria
Healthy newborn infants, who will be screened with the routine newborn metabolic screening test and their parent are enrolled. Infants treated with sedatives or analgesics within the last 24 h are excluded. A written informed consent is acquired from the infant’s parents. Parents who understand Swedish or English are eligible for inclusion. The infant-parent dyad is randomly assigned to one of three treatment groups; one standard of care group with glucose (n = 75), one group with skin-to-skin contact (n = 75), and one group where skin-to-skin contact is combined with breastfeeding (if applicable) and live parental lullaby singing (n = 75), (Table 1).
Interventions
The treatment groups in the RCT are described in Table 1. Group 2, comprising SSC, is divided into two subgroups, one with only SSC and one where the mother also chooses to breastfeed her infant during the test. The reason for these subgroups is that standard of care in one of the hospitals does not include glucose but comprises only SSC and breastfeeding when applicable. To ensure intervention fidelity in the live parental lullaby singing, a short video, showing a parent who sings according to the treatment description of the lullaby singing, will be played for the parents who are randomly assigned to group 3. These parents will also be verbally guided in how to sing before the intervention starts. The verbal instructions are simplified and delivered in dialogue with the parent who can ask questions.
Outcomes
The primary outcome is infant pain expression measured with Premature Infant Pain Profile Revised (PIPP-R) [50], which is a pain assessment instrument that has also been translated into Swedish [51].
Secondary outcomes in the RCT are: a) changes in galvanic skin response (GSR) (area small peaks, area huge peaks, peaks per second, average rise time, average peak), which is obtained via three electrodes on the infant’s foot [52], b) parents’ rating of the infant’s pain, c) parents’ rating of their own stress during the procedure and d) parents’ rating of how beneficial the pain management they were involved in felt to them and their infant.
Study measures
The PIPP-R has been tested for reliability, construct validity and clinical utility, all with results indicating good psychometrics [50]. The PIPP-R evaluates three behavioral facial actions (brow bulge, eye squeeze and nasolabial furrow), two physiological items (heart rate, oxygen saturation), and two contextual items (gestational age and behavioral state). The PIPP-R gives a weighted and higher score for the youngest infants to adjust for their lesser ability to show signs of pain. Scores can range from 0 to 21 where a higher score signifies a higher level of rated pain. A PIPP-R pain assessment includes a 15 s baseline measurement of heart rate and oxygen saturation together with an observation of the infant’s behavioral state and gestational age. Changes in physiological and behavioral indicators from baseline are then assessed during the first 30 s of the painful procedure. PIPP-R scores will be assessed from video recordings after the procedure by a trained nurse and a subset will also be assessed by one of the researchers for interrater reliability [50].
Galvanic skin response (GSR) refers to changes in sweat gland activity in response to a sensory stimulus. GSR measurements detects changes in electrical (ionic) activity resulting from changes in sweat gland activity. The increase in skin conductance reflects the infant’s arousal intensity in response to the sensory stimulus. As an indicator of pain, skin conductance measurements have detected increased sweating in newborn infants < 28 + 0 postnatal age submitted to heel lancing [53]. GSR have been tested in several neonatal pain studies on term and preterm infants, measuring skin conductance that reflects pain-related activation of the sympathetic nervous system [52–55]. Analyses of GSR are presented with the following variables; area small peaks, area huge peaks (both representing forcefulness of sympathetic nerve firing), peaks per second (the rate of firing in the sympathetic nerves), average rise time, average peak. Higher values in any of the above GSR parameters are indicative of more stress [52].
For the parents’ ratings, a visual analogue scale (VAS) with a 10-cm line anchored at the ends is used; from “no pain” on the left end point, up to “worst imaginable pain” on the right end point, from “no stress” on the left end point, up to “worst imaginable stress” on the right end point and finally from “not beneficial” on the left end point, up to “most beneficial possible” on the right end point of the VAS scale. The parents will perform the rating on the VAS scales right after the blood sampling procedure.
Sample size
No previous RCT has examined the effects of parent-driven pain management in newborn infants by combining SSC, breastfeeding and live parental lullaby singing. Based on previous studies using PIPP-R or its predecessor PIPP in pain alleviation projects, a difference of two PIPP-R points between standard care (group 1) and the full parent driven pain management (group 3) is considered clinically important. Based on those studies we also assume a standard deviation around 2 points. A power calculation sets the number of infants to include in the study to 63 in each group, with a power of 0.8 and a significance level set to 0.05 (www.clincalc.com). To compensate for possible dropouts, incomplete data, such as equipment malfunction or blood sample collection failure, 75 infants per group will be enrolled, making a total of 225 infants.
Recruitment and allocation
The researcher in each region will present the study and provide written information about the study to eligible parents before they are discharged from the hospital (Fig. 1. Timeline SWEpap study). When the parent-infant dyads return to the postnatal care unit for the metabolic screening test, they will be asked to participate and to sign the consent form (both the mother and her partner). The parents who have accepted to participate are then randomly assigned to one of the three interventions groups using the sealed opaque envelope system with randomly generated treatment allocations administered by an impartial researcher. Once the parents have consented to enter the trial, the researcher opens the envelope and the allocated treatment regimen is offered to the parents. The researchers will then guide the parents in each group how to position themselves and the infant and if applicable how to use live parental lullaby singing. If the parent does not adhere to the protocol in the allocated treatment group, the infant will be excluded from the study.
Blinding will not be possible for the nurse and parents during the procedures. Since only the infant’s face and pulse oximetry will be videotaped, the subsequent PIPP-R pain assessment and GSR measurements will be blinded using muted recordings. The parents’ participation in the study is voluntary and they are informed in advance that they can withdraw at any time without giving any reason.
Methods: data collection, data analyses and management, data monitoring
Data collection
Demographic data of the parent-infant dyad is registered before the procedure starts, including registration of potential music and singing activities during pregnancy for the parents in group 3. In all groups: before, during and after the procedure the infant will have a saturation probe attached to one foot making it possible to measure heart rate and oxygen saturation and electrodes measuring galvanic skin response on the other foot. The infant’s face and pulse oximetry values will be videotaped for later pain assessment with PIPP-R. An experienced midwife/nurse will perform the venipuncture. Venipuncture is routinely used in all hospitals in Swedish neonatal and postnatal care since it is considered less painful for the infant than heel lance [56]. A tourniquet using a soft cotton band is applied, the skin is disinfected, and a 21–23 Gauge needle is used for the blood sampling. The infant will be monitored until the treatment is concluded. In case of failed attempts, only the first venipuncture will be used in the study. In the breastfeeding condition, the midwife/nurse will make sure that the infant is latched and sucking well before the blood test is performed to safeguard the analgesic effect of breastfeeding. The infants’ behavioral responses to pain together with oxygen saturation and heart rate values are videotaped with two digital video cameras and will be assessed subsequently from the video films.
Data analyses, management and monitoring
Methods for statistical analyses are chosen depending on scales of measurement and normal distribution. The data will be analyzed with descriptive and comparative statistics. Continuous variables that are normally distribute will be presented with mean and standard deviation and compared with t-test for two groups and ANOVA for multiple groups. For data that doesn’t fulfil those criteria we will use median with interquartile range and Mann-Whitney U-test or Kruskall-Wallis one way analysis of variance, respectively. Intention-to-treat will be used, including all randomized parent-infant dyads in the analysis. The division of group 2 into 2a and 2b, which is a nomenclature to distinguish breastfeeding from no breastfeeding, will be taken into account in the comparative analyses and differences between the two subgroups will be scrutinized. Cohens kappa will be used to calculate interrater reliability between PIPP-R scorers.
The data collected in the study is safely stored on a secure server at Örebro University. The project folder on the server is only available to the researchers in the project and is automatically backed-up. The principal investigator is responsible for the data monitoring, ensuring that the researchers in the study are carrying out the research according to the research protocol, ensuring intervention fidelity in the live parental lullaby singing via recurring spot checks with microanalysis of the live singing, ensuring the research is keeping to time and budget and that the research is being conducted ethically. The research group will meet regularly to ensure consistency. During the original development of the project proposal of the SWEpap-study, the research group consulted with parents with previous extensive experience of NICU care. These parents will continue to give feedback on demand.
Adverse events
Adverse events are not collected specifically but noted when observed and reported to the research team for discussion. Since parent-driven interventions are non-pharmacological, safe and have no known side effects we are not expecting any adverse events.
Ethics and dissemination
The study is approved by the Swedish Ethical Review Authority (Dnr 2020–01562) and registered at ClinicalTrials. Gov (NCT04341194) in April 2020. Parents will receive both written and verbal information about the study. Written informed consent will be acquired from both parents of each participant. The Swedish Patient Injury Insurance is valid. The parent-driven interventions are non-pharmacological, safe and have no known side effects. The study complies with the ethical conduct of neonatal pain trials [57] by providing pain management for all involved infants and avoiding separation of the newborn infant and her/his parent. The results from the SWEpap-study will be published in scientific journals, in a doctoral thesis and presented at scientific conferences. In order to make our research more accessible to the public, the results will also be presented at health care related workshops and seminars, published in popular science journals and family and parenting magazines and communicated with the media (newspapers, radio, television and social media).
Discussion
This study protocol describes a randomized controlled trial where the pain reliving effects of combined parent-driven pain management during venipuncture on newborn infants will be studied. Parents are a valuable resource in procedural pain management in newborn infants, a resource which has not been addressed in research until the last decade. In the SWEpap study, we emphasize the importance of parent involvement in newborn pain management, but the key question is to address the research gap in the literature of the efficacy of combined non-pharmacological parent-driven pain management. Combined parental soothing behaviors that provide rhythmic (holding/rocking/vocalizing) or orogustatory/orotactile (feeding/pacifying) stimulation that keep the parent close to the infant, are effective in a painful context [58]. Consequently, in the SWEpap study, we include parental live lullaby singing, which is an unexplored but promising biopsychosocial, multimodal and multisensory pain alleviating adjuvant, especially in combination with SSC and breastfeeding [37]. Newborn infants are highly responsive to social and affective communication. In live parental infant-directed singing, the parent is attuned to the moment-to-moment psychological experience of the infant [43, 59]. The mainly “physical” parent-driven interventions with SSC and breastfeeding are therefore accompanied by a relationship-based intervention, the live parental lullaby singing, which may assist in modifying the painful situation for both the infant and the parent before, during and after the painful procedure [37].
In research, SSC is most often provided by mothers [60]. There are few studies exploring gender differences, mothers versus fathers, or pain alleviating differences in SSC between the mother and her partner or a non-related woman. When testing paternal versus maternal SSC to reduce pain from heel lance, mothers were marginally more effective than fathers in decreasing pain response [61]. Non-related women who provide SSC for procedural pain in preterm neonates are marginally less effective than mothers at decreasing pain response [60, 62].
Within the interdisciplinary research field of neonatal pain, there is a small number of research studies that have addressed the parents’ voice and musical presence during painful procedures [63, 64]. However, the nature of the parents’ vocal and musical engagement with their infants was not systematically reported. The parental live singing was not described and differentiated from “babytalk”, reciting of nursery rhymes or reading a story [64]. In general, live infant-directed singing is considerably more effective than infant-directed speech in lowering infants’ elevated arousal levels and ameliorating distress [65]. Analyses of parents’ singing to their infant in a non-painful context, have revealed similarities in the singing style of mothers and fathers, for example fathers, like mothers, adjust their singing with slower tempo and with warmer vocal tone when singing to infants than in other contexts [66, 67]. The soothing, comforting and emotion regulating properties of a lullaby are well-known cross-culturally and historically [65]. To date, research about parental infant-directed singing has focused primarily on the importance of the mother’s voice, but the possibility for fathers and partners to partake in live lullaby singing with their infant may facilitate a more equal parenthood [68, 69].
In the SWEpap study, multiple methods will be used when assessing the infants’ pain; GSR which comprises neurophysiological signs of pain and stress, and PIPP-R which is a widely used and validated pain assessment instrument. In addition, according to the parent-driven practice where parents are integrated in procedural support, the parents in the SWEpap study will also be assessing their infant’s pain. Pain assessment is as subjective as the pain experience itself and it is important to recognize the reciprocal relationship between infant and parent in a pain assessment context [39]. Parents are acknowledged as having a crucial role in assessing the infant’s pain and taking appropriate action to manage it [70]. Research has also shown that the parent’s assessment is not always based on the infant’s behavior [71] and may therefore differ from the health care professionals’ assessment [72].
To reduce the risk for selection bias in this study, broad inclusion criteria will be used including also parent-infant dyads from other cultures who understands English or Swedish, which strengthens the results and generalizability. A strength in this study is that data collection will take place in four hospitals in diverse health care regions in Sweden which will hopefully increase the scientific quality by recruiting a large sample size within a feasible time period, increasing the generalizability to other settings and reduce the risk of recruitment bias. The allocation with group 2a and b, referring to two different groups receiving skin-to-skin contact, might be considered as problematic. However, performing clinical research sometimes makes it difficult to use standardized procedures. It is not ethically feasible to defer from already existing guidelines and, in this study, preventing mothers from breastfeeding if applicable. It is important to focus on relevant clinical situations where the results can be useful for the patients, families and various health care settings and not only conducting research in laboratory surroundings. An aspect that must be taken into consideration is how to empower parents to use their voice and sing in conjunction with painful procedures. Parents might feel shy and inhibited about singing, especially in unfamiliar situations among unfamiliar people. On the other hand, research shows that most parents (60%) already sing spontaneously to their newborn during hospitalization [73]. By starting the lullaby singing together with SSC 10 min before the venipuncture and giving the parents clear instructions how the singing should be performed, we might modulate possible concerns. The use of technical equipment during the venipuncture will add a few minutes to the procedure, however the research group has previous extensive experience of similar research projects that will ensure the implementation of the study protocol.
The results generated in the SWEpap study will hopefully contribute to the interdisciplinary endeavor world-wide of involving and integrating parents in neonatal pain management and presumably also inform pain management practice in the neonatal intensive care context, where the critically ill and vulnerable hospitalized infants suffer the most from repeated, cumulative and inadequately treated procedural pain in addition to separation from the parents.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- FCC:
-
Family-centered care
- GSR:
-
Galvanic skin response
- NICU:
-
Neonatal intensive care unit
- PIPP-R:
-
Premature infant pain profile-revised
- RCT:
-
Randomized controlled trial
- SSC:
-
Skin to skin contact
- PIDS:
-
Live parental infant-directed lullaby singing
- VAS:
-
Visual analogue scale
References
Pillai Riddell R, Campbell L, Flora DB, Racine N, Osmun LD, Garfield H, Greenberg S. The relationship between caregiver sensitivity and infant pain behaviors across the first year of life. Pain. 2011;152(12):2819–26.
Raiskila S, Axelin A, Toome L, Caballero S, Tandberg BS, Montirosso R, Lehtonen L. Parents’ presence and parent–infant closeness in 11 neonatal intensive care units in six European countries vary between and within the countries. Acta Paediatr. 2017;106(6):878–88.
Tandberg BS, Flacking R, Markestad T, Grundt H, Moen A. Parent psychological wellbeing in a single-family room versus an open bay neonatal intensive care unit. PLoS One. 2019;14(11):e0224488.
Davidson JE, Aslakson RA, Long AC, Puntillo KA, Kross EK, Hart J, Netzer G. Guidelines for family-centered care in the neonatal, pediatric, and adult ICU. Crit Care Med. 2017;45(1):103–28.
Ullsten A, Söderström T, Mangersnes J. Development of family-centred care informing Nordic neonatal music therapy. In: Bonde LO, Johansson K, editors. Music in paediatric hospitals – Nordic perspectives. Oslo: Norwegian Academy of Music; 2019. p. 1–25. http://hdl.handle.net/11250/2623062.
Francis K, Pugsley L. Care through the Newborn’s eyes. J Perinat Neonatal Nurs. 2018;32(1):80–90.
Ullsten A, Eriksson M, Axelin AO. Parent, where art thou? Paediatr Neonatal Pain. 2019. https://doi.org/10.1002/pne2.12010.
Cruz MD, Fernandes AM, Oliveira CR. Epidemiology of painful procedures performed in neonates: a systematic review of observational studies. Eur J Pain. 2016;20(4):489–98.
Shah P, Siu A. Considerations for neonatal and pediatric pain management. Am J Health Syst Pharm. 2010;76(19):1511–20.
Balice-Bourgois C, Zumstein-Shaha M, Vanoni F, Jaques C, Newman CJ, Simonetti GD. A systematic review of clinical practice guidelines for acute procedural pain on neonates. Clin J Pain. 2020;36(5):390–8.
Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, Miller SP. Procedural pain and brain development in premature newborns. Ann Neurol. 2012;71(3):385–96.
Ranger M, Chau CM, Garg A, Woodward TS, Beg MF, Bjornson B, Poskitt K, Fitzpatrick K, Synnes AR, Miller SP, Grunau RE. Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLoS One. 2013;8:e76702.
Ranger M, Grunau RE. Early repetitive pain in preterm infants in relation to the developing brain. Pain Manage. 2014;4(1):57–67.
Brummelte S, Chau CM, Cepeda IL, Degenhardt A, Weinberg J, Synnes AR, Grunau RE. Cortisol levels in former preterm children at school age are predicted by neonatal procedural painrelated stress. Psychoneuroendocrinology. 2015;51:151–63.
Valeri Beatriz O, Holsti L, Linhares Maria BM. Neonatal pain and developmental outcomes in children born preterm: a systematic review. Clin J Pain. 2015;31(4):355–62.
Axelin A, Lehtonen L, Pelander T, Salanterä S. Mothers’ different styles of involvement in preterm infant pain care. J Obstet Gynecol Neonatal Nurs. 2010;39(4):415–24.
Axelin A, Anderzén-Carlsson A, Eriksson M, Pölkki T, Korhonen A, Franck LS. Neonatal intensive care nurses’ perceptions of parental participation in infant pain management: a comparative focus group study. J Perinat Neonatal Nurs. 2015;29(4):363–74.
Franck LS, Oulton K, Bruce E. Parental involvement in neonatal pain management: an empirical and conceptual update. J Nurs Scholarsh. 2012;44(1):45–54.
Olsson E, Ahlsén G, Eriksson M. Skin‐to‐skin contact reduces near‐infrared spectroscopy pain responses in premature infants during blood sampling. Acta Paediatr. 2015;105(4):376–80.
Palomaa AK, Korhonen A, Pölkki T. Factors influencing parental participation in neonatal pain alleviation. J Pediatr Nurs. 2016;31(5):519–27.
Courtois E, Cimerman P, Dubuche V, Goiset MF, Orèvre C, Lagarde A, Carbajal R. The burden of venipuncture pain in neonatal in-tensive care units: EPIPPAIN 2, a prospective observational study. Int J Nurs Stud. 2016;57:48–59.
Johnston CC, Barrington KJ, Taddio A, Carbajal R, Filion F. Pain in Canadian NICUs: have we improved over the past 12 years? Clin J Pain. 2011;27(3):225–32.
Pölkki T, Korhonen A, Laukkala H. Parents’ use of nonpharmacologic methods to manage procedural pain in infants. J Obstet Gynecol Neonatal Nurs. 2018;47(1):43–51.
Gates A, Shave K, Featherstone R, Buckreus K, Ali S, Scott SD, Hartling L. Procedural pain: systematic review of parent experiences and information needs. Clin Pediatr. 2018;57(6):672–88.
Sikorova L, Kucova J. The needs of mothers to newborns hospitalised in intensive care units. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2012;156(4):330–6.
Skene C, Franck CP, Gerrish K. Parental involvement in neonatal comfort care. J Obstet Gynecol Neonatal Nurs. 2012;41(6):786–97.
Balice-Bourgois C, Zumstein-Shaha M, Simonetti GD, Newman CJ. Interprofessional collaboration and involvement of parents in the management of painful procedures in newborns. Front Pediatr. 2020;8(394):1–10.
Campbell-Yeo M, Dol J, Disher T, Benoit B, Chambers CT, Sheffield K, et al. The power of a parent’s touch. J Perinat Neonatal Nurs. 2017;31(4):341–9.
Korki de Candido L, Harrison D, de La Ó, Ramallo Veríssimo M, Bueno M. Effectiveness of a parent‐targeted video on neonatal pain management: nonrandomized pragmatic trial. Paediatr Neonatal Pain. 2020. https://doi.org/10.1002/pne2.12023.
Lavin Venegas C, Taljaard M, Reszel J, Dunn S, Graham ID, Harrold J, et al. A parent-targeted and mediated video intervention to improve uptake of pain treatment for infants during newborn screening: a pilot randomized controlled trial. J Perinat Neonatal Nurs. 2019;33(1):74–81.
Richardson B, Falconer A, Shrestha J, Cassidy C, Campbell-Yeo M, Curran JA. Parent-targeted education regarding infant pain management delivered during the perinatal period: a scoping review. J Perinat Neonatal Nurs. 2020;34(1):56–65.
Fernandes A, Campbell-Yeo M, Johnston CC. Procedural pain management for neonates using nonpharmacological strategies: part 1: sensorial interventions. Adv Neonatal Care. 2011;11(4):235–41.
Stevens B, Yamada J, Ohlsson A, Haliburton S, Shorkey A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev. 2016;7. https://doi.org/10.1002/14651858.CD001069.pub5.
Anand KJS, Hall RW. Pharmacological therapy for analgesia and sedation in the newborn. Arch Dis Child Fetal Neonatal Ed. 2006;91(6):F448–53.
Swedish Medical Products Agency. Behandling av barn i samband med smärtsamma procedurer i hälso och sjukvård – Kunskapsdokument. Inform Läkemedelsverket. 2014;25(3):9–22.
Campbell-Yeo M, Fernandes A, Johnston CC. Procedural pain management for neonates using nonpharmacological strategies: part 2 mother-driven interventions. Adv Neonatal Care. 2011;11(5):312–8.
Ullsten A, Eriksson M, Klässbo M, Volgsten U. Singing, sharing, soothing –biopsychosocial rationales for parental infant-directed singing in neonatal pain management: a theoretical approach. Music Sci. 2018. https://doi.org/10.1177/2059204318780841.
Benoit B, Martin-Misener R, Latimer M, Campbell-Yeo M. Breast-feeding analgesia in infants. J Perinat Neonatal Nurs. 2017;31(2):145–59.
Pillai Riddell R, Chambers CT. Parenting and pain during infancy. In: Anand KJS, Stevens BJ, McGrath PJ, editors. Pain in neonates and infants. Toronto: Elsevier; 2007. p. 289–98.
Ullsten A, Eriksson M, Klässbo M, Volgsten U. Live music therapy with lullaby singing as affective support during painful procedures: a case study with microanalysis. Nord J Music Ther. 2016;26(2):142–66.
Ullsten A, Hugoson P, Forsberg M, Forzelius L, Klässbo M, Olsson E, Eriksson M. Efficacy of live lullaby singing during procedural pain in preterm and term neonates. Music Med. 2017;9(2):73–85.
Johnston CC, Campbell‐Yeo M, Disher T, Benoit B, Fernandes A, Streiner D, Zee R. Skin‐to‐skin care for procedural pain in neonates. Cochrane Database Syst Rev. 2017;2:1–110.
Fancourt D, Perkins R. The effects of mother–infant singing on emotional closeness, affect, anxiety, and stress hormones. Music Sci. 2018;1. https://doi.org/10.1177/2059204317745746.
Loewy J, Stewart K, Dassler A-M, Telsey A, Homel P. The effects of music therapy on vital signs, feeding, and sleep in premature infants. Pediatrics. 2013;131(5):902–18. https://doi.org/10.1542/peds.2012-1367.
Persico G, Antolini L, Vergani P, Costantini W, Nardi MT, Bellotti L. Maternal singing of lullabies during pregnancy and after birth: effects on mother–infant bonding and on newborns’ behaviour. concurrent cohort study. Women Birth. 2017;30(4):e214–20.
Shah SR, Kadage S, Sinn J. Trial of music, sucrose, and combi-nation therapy for pain relief during heel prick procedures in neonates. J Pediatr. 2017;190:153–8.
Chirico G, Cabano R, Villa G, Bigogno A, Ardesi M, Dioni E. Randomised study showed that recorded maternal voices reduced pain in preterm infants undergoing heel lance procedures in a neonatal in-tensive care unit. Acta Paediatr. 2017;106(10):1564–8.
Filippa M, Poisbeau P, Mairesse J, Monaci MG, Baud O, Hüppi P, Grandjean D, Kuhn P. Pain, parental involvement, and oxytocin in the neonatal intensive care unit. Front Psychol. 2019;10(715):1–15.
Neal DO, Lindeke LL. Music as a nursing intervention for preterm infants in the NICU. Neonatal Netw. 2008;27(5):319–27.
Stevens BJ, Gibbins S, Yamada J, Dionne K, Lee G, Johnston C, Taddio A. The premature infant pain profile-revised (PIPP-R): initial validation and feasibility. Clin J Pain. 2014;30(3):238–43.
Olsson E, Anderzén-Carlsson A, Atladóttir SM, Axelin A, Campbell-Yeo M, Eriksson M, Dovland AR. Cultural adaptation and harmonization of four Nordic translations of the revised Premature Infant Pain Profile (PIPP-R). BMC Pediatr. 2018;18(349):1–9.
Hu J, Modanloo S, Squires JE, Harrold J, Harrison D. The validity of skin conductance for assessing acute pain in infants: a scoping review. Clin J Pain. 2019. https://doi.org/10.1097/ajp.0000000000000721.
Munsters J, Wallström L, Ågren J, Norsted T, Sindelar R. Skin conductance measurements as pain assessment in newborn infants born at 22–27 weeks gestational age at different postnatal age. Early Hum Dev. 2012;88(1):21–6.
Eriksson M, Storm H, Fremming A, Schollin J. Skin conductance compared to a combined behavioural and physiological pain measure in newborn infants. Acta Paediatr. 2008;97(1):27–30.
Pettersson M, Olsson E, Ohlin A, Eriksson M. Neurophysiological and behavioral measures of pain during neonatal hip examination. Paediatr Neonatal Pain. 2019;1(1):15–20.
Shah VS, Ohlsson A. Venepuncture versus heel lance for blood sampling in term neonates. Cochrane Database Syst Rev. 2011;10.
Campbell-Yeo M. ‘First, do no harm’– the use of analgesia or placebo as control for babies in painful clinical trials. Acta Paediatr. 2016;105(2):119–20.
Jahromi LB, Putnam SP, Stifter CA. Maternal regulation of infant reactivity from 2 to 6 months. Dev Psychol. 2004;40(4):477–87.
Loewy J. NICU music therapy: song of kin as critical lullaby in research and practice. Ann N Y Acad Sci. 2015;1337:178–85. https://doi.org/10.1111/nyas.12648.
Johnston CC, Byron J, Filion F, Campbell‐Yeo M, Gibbins S, Ng E. Alternative female kangaroo care for procedural pain in preterm neonates: a pilot study. Acta Paediatr. 2012;101(11):1147–50.
Johnston CC, Campbell-Yeo M, Filion F. Paternal vs maternal kangaroo care for procedural pain in preterm neonates: a randomized crossover trial. Arch Pediatr Adolesc Med. 2011;165(9):792–6.
Murmu J, Venkatnarayan K, Thapar RK, Shaw SC, Dalal SS. When alternative female kangaroo care is provided by other immediate postpartum mothers, it reduces postprocedural pain in preterm babies more than swaddling. Acta Paediatr. 2017;106(3):411–5.
Johnston CC, Filion F, Campbell-Yeo M, Goulet C, Bell L, McNaughton K, Byron J. Enhanced kangaroo mother care for heel lance in preterm neonates: a crossover trial. J Perinatol. 2009;29(1):51–6.
Johnston CC, Rennick JE, Filion F, Campbell-Yeo M, Goulet C, Bell L, Tucci M, Ranger M. Maternal touch and talk for invasive procedures in infants and toddlers in the pediatric intensive care unit. J Pediatr Nurs. 2012;27(2):144–53.
Trehub SE. Nurturing infants with music. Int J Music Early Child. 2019;14(1):9–15. https://doi.org/10.1386/ijmec.14.1.9_1.
Trehub SE, Unyk AM, Kamenetsky SB, Hill DS, Trainor LJ, Henderson JL, Saraza M. Mothers’ and fathers’ singing to infants. Dev Psychol. 1997;33:500–7.
Trehub SE. The maternal voice as a special signal for infants. In: Filippa M, Kuhn P, Westrup B, editors. Early vocal contact and preterm infant brain development. Cham: Springer International Publishing; 2017. p. 39–54.
Ettenberger M, Rojas Cárdenas C, Parker M, Odell-Miller H. Family-centred music therapy with preterm infants and their parents in the Neonatal Intensive Care Unit (NICU) in Colombia–a mixed-methods study. Nord J Music Ther. 2017;26(3):207–34.
Mondanaro JF, Ettenberger M, Park L. Mars rising: music therapy and the increasing presence of fathers in the NICU. Music Med. 2016;8(3):96–107.
Pillai Riddell R, Psych C, Racine N. Assessing pain in infancy: the caregiver context. Pain Res Manag. 2009;14:117–32.
Pillai Riddell R, Flora D, Stevens S, Greenberg S, Garfield H. The role of infant pain behaviour in predicting parent pain ratings. Pain Res Manag. 2014;19(5):e124–32.
Olsson E, Pettersson M, Eriksson M, Ohlin A. Sweet solution to prevent pain during neonatal hip examination: a randomized controlled trial. Acta Paediatr. 2018;108(4):626–9.
Shoemark H, Arnup S. A survey of how mothers think about and use voice with their hospitalized newborn infant. J Neonatal Nurs. 2014;20(3):115–21.
Acknowledgements
Not applicable.
Funding
This study is supported by financial grants from Uppsala-Örebro Regional Research Council (LIVFOU-930105), Centre for Clinical Research Region Värmland, Örebro University. The funders had no role in the conception, design or planning of the study and will not have a role during conduct of the study; data collection, management, analysis, interpretation of the data or decision to publish. The funders had no role in the preparation of the manuscript. Open Access funding provided by Örebro Unversity.
Author information
Authors and Affiliations
Contributions
AU, EO, ME, MCM, YTB, RDA, JE were involved in the conception and in the design of the study. All authors have made substantial contributions to the planning of the study. AU and EO drafted the manuscript and all authors contributed to refinement of the study protocol. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study is approved by the Swedish Ethical Review Authority (Dnr 2020–01562).
Trial registration: ClinicalTrials.gov (NCT04341194) 10 April 2020.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Olsson, E., Carlsen Misic, M., Dovland Andersen, R. et al. Study protocol: parents as pain management in Swedish neonatal care – SWEpap, a multi-center randomized controlled trial. BMC Pediatr 20, 474 (2020). https://doi.org/10.1186/s12887-020-02356-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-020-02356-7