Abstract
Background
To assess the efficacy of 577 nm subthreshold micropulse laser (SML) treatment for acute central serous chorioretinopathy (CSC).
Methods
This retrospective comparative case-series included 34 eyes of 34 patients with acute CSC who received either 577 nm SML treatment (SML group, n = 16 eyes) or were only monitored (observation group, n = 18 eyes). Acute CSC was defined as disease course < 3 months. Eyes with any history of treatment in the past were excluded. Data were collected over a period of 6 months. The best corrected visual acuity (BCVA), central macular thickness (CMT), and subfoveal choroidal thickness (SFCT) were observed.
Results
SML group showed significantly greater improvement in the BCVA (logMAR) compared to observation group at 1 month (0.20 ± 0.10 vs 0.30 ± 0.12, P < 0.01), 3 months (0.13 ± 0.06 vs 0.21 ± 0.06, P < 0.01) and 6 months (0.01 ± 0.06 vs 0.09 ± 0.66, P < 0.01). The CMT reduction was significantly greater in the SML group at 1 month (337.19 ± 62.96 µm vs 395.11 ± 91.30 µm, P < 0.05), 3 months (312.94 ± 49.50 µm vs 364.50 ± 70.30 µm, P < 0.05) and 6 months (291.38 ± 26.46 µm vs 348.56 ± 54.65 µm, P < 0.05). In the SML group, the SFCT did not show a significant decrease at 1 month (468.88 ± 42.19 µm, P > 0.05) but showed a significant reduction at 3 months (451.75 ± 39.36 µm, P < 0.05) and 6 months (450.50 ± 34.24 µm, P < 0.05) from baseline (489.94 ± 45.86 µm). In the observation group, there was no significant change in SFCF during follow-up. No adverse events occurred in the SML group.
Conclusions
Although some patients with acute CSC show spontaneous healing, timely intervention with 577 nm SML can shorten the disease course, improve visual acuity, and reduce the risk of chronic transformation without adverse events.
Similar content being viewed by others
Background
Central serous chorioretinopathy (CSC) is a disease of the retina characterized by serous detachment of the neurosensory retina, secondary to at least one focal lesion of the retinal pigment epithelium [1]. The disease most commonly affects middle-age men (mean age at presentation: 41–51 years) [2]. The identified risk factors for CSC include type A personality, psychological stress, exogenous steroid use, smoking, alcohol consumption, uncontrolled hypertension, use of psychopharmacologic medications, pregnancy, autoimmune diseases, gastroesophageal reflux, and Helicobacter pylori infection [3, 4]. Patients with CSC often develop reduced visual acuity, metamorphopsia, central scotoma, and loss of contrast sensitivity with progression of the disease [5]. There are two main forms of disease presentation: acute CSC and chronic CSC. Acute CSC is generally regarded as a self-limiting disease which resolves spontaneously within 3–4 months in most patients [6]; however, the reported incidence of recurrence of CSC is 33–50% [7, 8]. Cases that do not show spontaneous resolution may develop into chronic CSC, with progressive damage to the retinal pigment epithelium and photoreceptors, leading to irreversible visual impairment [9, 10].
Till date, there is no standardized treatment strategy for acute CSC [11]. Some have argued that acute CSC should only be monitored and does not require treatment [12]. However, some treatment modalities such as focal laser photocoagulation [13], photodynamic therapy (PDT) [14,15,16], intravitreal injection of anti-vascular endothelial growth factor (Anti-VEGF) [17], oral medication [18] and subthreshold micropulse laser (SML) [19, 20] have also been used to treat acute CSC. The clinical application of focal laser and PDT is limited owing to their serious side effects, such as central scotoma, retinal pigment epithelium atrophy, and choroidal neovascularization (CNV). According to Gawecki et al. [21], that SML should be considered in acute cases to achieve good visual results. Maruko et al. [22] and Özmert et al. [23] demonstrated the efficacy and safety of 577 nm SML for treatment of chronic CSC. However, there is a paucity of studies on the treatment of acute CSC with 577 nm SML [19, 24]. The purpose of this study was to assess the efficacy of 577 nm SML treatment for acute CSC.
Methods
Study design and participants
This retrospective comparative study included 34 eyes of 34 patients with acute CSC who were either treated with 577 nm SML (SML group, n = 16 eyes) or were only monitored (observation group, n = 18 eyes) at the Wuhan Aier Eye Hanyang Hospital between August 2019 and December 2020. The observation group consisted of patients with acute CSC who received no treatment during the same period. The treatment strategy was chosen by the surgeons in agreement with the patient. This study was conducted in accordance with the Declaration of Helsinki.
The diagnosis of acute CSC was confirmed by clinical examination, fundoscopy, optical coherence tomography (Cirrus HD-OCT 5000, Carl Zeiss Meditec AG, Germany), and fundus fluorescein angiography (FFA). Some patients underwent indocyanine green angiography (ICGA) and fundus autofluorescence (FAF) imaging examination (Spectralis, Heidelberg Retina Angiograph, Germany). Only eyes with acute CSC (disease duration: < 3 months) were included in this study. If both eyes were eligible for the study, the right eye was chosen in the analysis. The exclusion criteria were: (1) Acute CSC with previous history of treatment with PDT, focal photocoagulation, or intravitreal anti-VEGF injections; (2) systemic steroid therapy; (3) other retinal diseases such as pathological myopia, CNV, polypoidal choroidal vasculopathy (PCV), or history of intraocular surgery; (4) Incomplete follow-up and case data.
Data collection
All patients were examined at baseline and at 1 month, 3 months, and 6 months after treatment, including slit-lamp biomicroscopy, dilated fundus examination, intraocular pressure measurements, best corrected visual acuity (BCVA), and spectral- domain optical coherence tomography (SD-OCT). FFA was performed at baseline and repeated as needed. ICGA and FAF results were available for 56% and 41% patients, respectively. The BCVA was measured with a standard visual acuity chart, and the decimal BCVA was converted to the logarithm of the minimum angle of resolution (logMAR) units for the statistical analyses. Central macular thickness (CMT) was defined as the distance between the internal limiting membrane and the inner border of the RPE by OCT [19, 25], which included the subretinal fluid (SRF), if present. Using a virtual caliper the subfoveal choroidal thickness (SFCT) was measured from enhanced depth imaging (EDI) scan by taking the vertical distance between the hyper-reflective line of Bruch’s membrane and the innermost hyper-reflective line of the choroid scleral interface, and the average of the two scans (vertical and horizontal) was regarded as the SFCT [26].
577 nm micropulse laser treatment
The pupil was dilated with 0.5% tropicamide phenylephrine eye drops (Mydrin-P, Santian Pharmaceutical Co. Ltd., China). Then, 0.4% oxybuprocaine hydrochloride eye drops (Benoxil, Santian Pharmaceutical Co. Ltd., China) were used for superficial anesthesia. Iridex IQ 577 nm SML treatment (IQ 577; IRIDEX, America) was performed by the same experienced surgeon (Xin Li). The parameters for 577 nm SML treatment were: spot diameter, 200 μm; exposure time, 200 ms; duty cycle, 5%; TxCell fusion laser matrix, 7 × 7; laser spot spacing, 0. Energy titration was performed using a continuous wavelength laser in the peripapillary nasal 2DD area. The initial titration energy was 50 mW, and the energy was gradually increased until the appearance of the retinal spot (light gray spot of grade I). Quadruple of the titration energy is the treatment energy of the SML. Then, the SML multi-point mode was used to perform full-coverage laser photocoagulation therapy on the serous retinal detachment area. The number of laser envelopes, laser power energy, and photocoagulation sessions were recorded. At one month review, the same experienced surgeon (Xin Li) decided whether or not to repeat SML therapy. The criterion for repeat treatment was the presence of SRF in the macular area, and the criteria for termination of treatment were the complete absorption of SRF or completion of three treatments. Complete resolution was said to have been achieved if the macula showed no SRF on OCT images.
Statistical analyses
The primary outcome measure was the change in the BCVA on follow-up. Secondary outcome measures were change in CMT and SFCT at 6 months. The BCVA was converted to the logarithm of the minimum angle of resolution (logMAR) units for statistical analyses. Statistical analysis included comparison of the baseline data and follow-up data in each group. All statistical analyses were performed using SPSS software (version 23.0, IBM SPSS). Continuous variables were presented as mean ± standard deviation and between-group differences assessed using independent t tests (parametric data distribution). Levene test was used to test homogeneity of variance. Fisher test was used to compare categorical data between the two groups. Two tailed P values < 0.05 were considered indicative of statistical significance.
Results
Baseline demographic and clinical characteristics
Thirty-four patients (23 men, 11 women) were enrolled in this study. The mean age of patients was 40.47 ± 6.77 years. There was no significant difference between the SML group and observation group with respect to the mean duration of symptoms until baseline visit (6.99 ± 1.75 days versus 6.54 ± 1.54 days; P = 0.306). The mean number of 577 nm SML treatment sessions in the SML group was 2.0 ± 0.82. At baseline, there were no significant between-group differences with respect to sex, age, duration of symptoms, BCVA (logMAR), CMT, or SFCT (P > 0.05 for all) (Table 1).
Changes in visual acuity
The changes in BCVA (LogMAR) at different time-points are shown in Fig. 1. In the 577 nm SML group, BCVA (logMAR) improved significantly from 0.48 ± 0.20 at baseline to 0.20 ± 0.10 at 1 month, 0.13 ± 0.06 at 3 months, and 0.01 ± 0.06 at 6 months (P < 0.01). Significant improvement in the BCVA (logMAR) was also observed in the observation group from baseline (0.56 ± 0.22) to 1 month, 3 months, and 6 months (0.30 ± 0.12, 0.21 ± 0.06, 0.09 ± 0.06, respectively) (P < 0.01). Improvement in visual acuity was more significant in the SML group than in the observation group (0.20 ± 0.10 vs 0.30 ± 0.12, 0.13 ± 0.06 vs 0.21 ± 0.06, 0.01 ± 0.06 vs 0.09 ± 0.06, respectively) (P < 0.05).
Changes in central macular thickness
In the 577 nm SML group, mean CMT decreased from 485.38 ± 151.44 µm at baseline to 337.19 ± 62.96 µm at 1 month, 312.94 ± 49.50 µm at 3 months, and 291.38 ± 26.46 µm 6 months (P < 0.01) (Fig. 2). In the observation group, mean CMT was 483.50 ± 110.89 µm at baseline, 395.11 ± 91.30 µm at 1 month, 364.50 ± 70.30 µm at 3 months, and 348.56 ± 54.65 µm at 6 months (P < 0.05) (Fig. 2). During follow-up, compared with the observation group, the SML group showed a more obvious decrease in CMT, and the difference was statistically significant (P < 0.05).
Changes in subfoveal choroidal thickness
In the SML group, the mean SFCT decreased from 489.94 ± 45.86 µm at baseline to 468.88 ± 42.19 µm at 1 month (P = 0.186), 451.75 ± 39.36 µm at 3 months (P = 0.017), and 450.50 ± 34.24 µm at 6 months (P = 0.10) (Fig. 3). In the observation group, the mean SFCT was 493.78 ± 44.02 µm at baseline, 486.06 ± 46.12 µm at 1 month (P = 0.611), 482.22 ± 44.64 µm at 3 months (P = 0.440), and 478.78 ± 41.02 µm at 6 months (P = 0.298) (Fig. 3). The decrease in SFCT in the observation group was not statistically significant. In the 577 nm SML group, the reduction in SFCT was significant at 3 months (P = 0.044) and 6 months (P = 0.038), compared with the observation group.
Safety
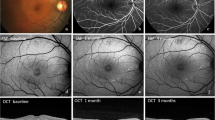
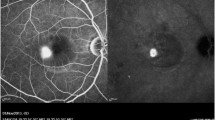
No structural changes in RPE, photoreceptor layer, or inner and outer retinal layers were observed on fundoscopy, OCT imaging, or on FFA at the end of follow-up (Fig. 4).
Fundus fluorescein angiography (FFA) and optical coherence tomography (OCT) of a patient with acute CSC at baseline, 1 month, and 3 months. A) A hot spot of leakage is visible on FFA at baseline. B and C show subretinal fluid (SRF) on OCT at baseline and 1 month after SML treatment, respectively. D) The SRF has completely resolved 3 months after SML treatment; no signs of laser-induced damage are seen
Discussion
CSC is the fourth most frequent cause of retinopathy, after age-related macular degeneration, diabetic retinopathy, and retinal vein occlusion [27], and the condition is usually classified as acute or chronic. Most episodes of CSC are self-limiting and typically show spontaneous resolution within 3 to 4 months [28]; therefore, it was generally believed that patients with acute CSC should be initially monitored for at least 3 months before considering treatment. However, up to 50% patients with CSC may develop recurrence and in up to 20% patients, the disease becomes chronic [29, 30]. Recurrent episodes of CSC, persistent SRF and/or pigment epithelium detachment, and RPE atrophy may lead to permanent visual impairment [7]. Scholz et al. [31] suggested that patients should be treated as early as possible while they are still in the acute stage in order to prevent any permanent structural damage. In our study, to differentiate the efficacy of 577 nm SML from the natural course of the disease, we enrolled a control group of patients with acute CSC during the same time-period who received no treatment. From baseline to 6-month follow-up, the 577 nm SML group showed better BCVA (logMAR) compared to the observation group, and the CMT also decreased more obviously. Similar results were reported by Zhou et al. [19] and Arora et al. [20] Zhou et al. [19]. reported that at 3 months, 577 nm SML with 50% threshold power and 25% threshold power improved BCVA (logMAR) and reduced CMT compared to baseline in patients with acute CSC. In the study by Arora et al. [20]. 810 nm SML group showed significantly higher BCVA (logMRA) at 2, 4, 8, and 16 weeks, and six months compared with the observation group for acute CSC. During follow-up, the changes in contrast sensitivity, the height of neurosensory detachment, and the width of the neurosensory detachment in the 810 nm SML group were better than those in the observation group. 577 nm SML is a quite different modality than 810 nm SML. The 577 nm SML is absorbed by both melanin and oxyhemoglobin, which leads to maximum absorption in the pigment epithelium and choriocapillaris [32], and minimizes the absorption of yellow light by lutein in the inner and outer cluster layers of macula; therefore, the treatment of macular fovea and its vicinity is relatively safe [33].
In addition, our study also showed a decrease in SFCT beginning at 3 months after 577 nm SML treatment. However, there was no significant change in SFCT in the observation group. In the study by Rocal et al. [34], 577 nm SML and half-dose PDT treatment for chronic CSC significantly diminished SFCT. Sun et al. [35] also observed a significant reduction in the mean SFCT from baseline to week 12 in the SML group and in the threshold conventional laser group. Acute CSC has a short disease course of approximately 3 months and does not result in any irreversible structural damage; spontaneous healing over time results in reduced choroidal vascular permeability and recovery of choroidal thickness. Since acute CSC spontaneously heals by approximately 3 months, the decrease in SFCT at 6 months should be the result of 577 nm SML therapy rather than spontaneous absorption. However, we found that the amplitude of SFCT reduction in the 577 nm SML group was small, which may be related to the fact that SML mainly acts on the RPE.
The mechanism by which SML therapy induces changes in SFCT is unclear. According to Arora et al. [20], 810 nm SML causes resolution of CSC by normalization of RPE function and retinal autoregulation by means of sublethal RPE heat-shock protein activation or possibly by causing cytokine expression which decreases choroidal hyper-permeability. We believe that the decrease in SFCT is attributable to the following reasons: CSC is a pachychoroid disease characterized by localized or diffuse choroidal thickening and/or dilatation of the outer choroidal vessels (Haller layer), atrophy of the inner choroidal capillary layer and sattler layer, damage to RPE, and hyperpermeability of the choroidal vessels. A previous study documented a decrease in SFCT after therapy, further illustrating the role of choroidal vasculature in the pathogenesis of CSC [36,37,38,39]. SML targets RPE and exerts heat stress on RPE cells, eliciting their biological response rather than causing cell death. Heating of the RPE complex with high-density light spots induces the expression of HSP70, inhibits cell apoptosis, and down-regulates the expression of inflammatory factors. At the same time, stimulation of sublethal RPE cells by light induces the production of pigment epithelial-derived factors and VEGF inhibitory factors, restoring the pumping function and external barrier. These changes reduce the choroidal permeability to achieve the therapeutic effect [20, 33, 40, 41].
Finally, in our study, no adverse effects were noted during or after 577 nm SML treatment, and none of the patients reported any adverse effects related to 577 nm SML. Our results are consistent with those of Maruko et al. [22] and Özmert et al. [23] who used 577 nm SML to treat chronic CSC. Although focal laser [13], PDT [14,15,16] and intravitreal injection Anti-VEGF [17] have been shown to be effective in the treatment of acute CSC, focal laser may lead to permanent scotoma, which enlarges over time with RPE scar expansion, and may potentially cause choroidal neovascularization [42]. PDT may also cause several adverse effects such as RPE atrophy, choroidal ischemia and secondary CNV [43, 44]. In addition, PDT is expensive and the lack of relevant drugs in China is a barrier to its wider use. Anti-VEGF agents have shown promising results only in the subgroup of patients with CNVs secondary to CSC [45]; in addition, the treatment is costly and requires repeated intravitreal injection, which affects patient compliance. Therefore, 577 nm SML would be an effective and safe alternative therapy for acute CSC.
Some potential limitations of our study should be acknowledged. First, the study was a retrospective study with a small sample size and a relatively short follow-up period. Second, the conversion of decimal BCVA to logMAR units may lead to overestimation of its true value according to a previous report [46], especially in patients with poorer acuity. Thus, the significant improvement in BCVA (logMAR) in the 577 nm SML group should be interpreted with caution. Third, Tan et al. [47] demonstrated a significant and consistent diurnal variation in SFCT in normal individuals. SFCT measurements in this study did not take into account the time of day at which the OCT scan was performed, which requires further study. In addition, we measured the SFCT manually, which may affect the accuracy of measurement. In some patients, the scleral border was insufficiently detectable on EDI-OCT imaging, which may have hindered SFCT analysis.
In conclusion, this retrospective study demonstrated the efficacy of 577 nm SML as a treatment option for acute CSC. No adverse events related to 577 nm SML were observed in this cohort. Prompt treatment of acute CSC with a 577 nm SML may result in better visual outcomes. Larger, prospective, randomized controlled clinical trials with longer follow-up are required to provide more robust evidence.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CSC:
-
Central serous chorioretinopathy
- RPE:
-
Retinal pigment epithelial
- PDT:
-
Photodynamic therapy
- Anti-VEGF:
-
Anti-vascular endothelial growth factor
- SML:
-
Subthreshold micropulse laser
- CNV:
-
Choroidal neovascularization
- FFA:
-
Fundus fluorescein angiography
- ICGA:
-
Indocyanine green angiography
- FAF:
-
Fundus autofluorescence
- PCV:
-
Polypoidal choroidal vasculopathy
- BCVA:
-
Best corrected visual acuity
- SD-OCT:
-
Spectral-domain optical coherence tomography
- logMAR:
-
Logarithm of the minimum angle of resolution
- CMT:
-
Central macular thickness
- SFCT:
-
Subfoveal choroidal thickness
- SRF:
-
Subretinal fluid
- EDI:
-
Enhanced depth imaging
References
Wang M, Munch IC, Hasler PW, Prünte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86(2):126–45.
Azad AD, Zhou M, Afshar AR, Bakri SJ, Pershing S. Systemic Corticosteroid Use after Central Serous Chorioretinopathy Diagnosis. Ophthalmology. 2021;128(1):121–9.
Haimovici R, Koh S, Gagnon DR, Lehrfeld T, Wellik S. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111(2):244–9.
Eom Y, Oh J, Kim SW, Huh K. Systemic factors associated with central serous chorioretinopathy in Koreans. Korean journal of ophthalmology : KJO. 2012;26(4):260–4.
Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980–2002. Ophthalmology. 2008;115(1):169–73.
Piccolino FC, de la Longrais RR, Ravera G, Eandi CM, Ventre L, Abdollahi A, Manea M. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005;139(1):87–99.
Loo RH, Scott IU, Flynn HW Jr, Gass JD, Murray TG, Lewis ML, Rosenfeld PJ, Smiddy WE. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina (Philadelphia, Pa). 2002;22(1):19–24.
Bujarborua D. Long-term follow-up of idiopathic central serous chorioretinopathy without laser. Acta Ophthalmol Scand. 2001;79(4):417–21.
Arora S, Maltsev DS, Sahoo NK, Parameshwarappa DC, Iovino C, Arora T, Kulikov AN, Tatti F, Venkatesh R, Reddy NG et al: Correction to: Visual acuity correlates with multimodal imaging-based categories of central serous chorioretinopathy. Eye (London, England) 2021.
Mrejen S, Balaratnasingam C, Kaden TR, Bottini A, Dansingani K, Bhavsar KV, Yannuzzi NA, Patel S, Chen KC, Yu S, et al. Long-term Visual Outcomes and Causes of Vision Loss in Chronic Central Serous Chorioretinopathy. Ophthalmology. 2019;126(4):576–88.
Wong KH, Lau KP, Chhablani J, Tao Y, Li Q, Wong IY. Central serous chorioretinopathy: what we have learnt so far. Acta Ophthalmol. 2016;94(4):321–5.
Iacono P, Battaglia Parodi M, Falcomatà B, Bandello F. Central Serous Chorioretinopathy Treatments: A Mini Review. Ophthalmic Res. 2015;55(2):76–83.
Ambiya V, Khodani M, Goud A, Narayanan R, Tyagi M, Rani PK, Chhablani J. Early Focal Laser Photocoagulation in Acute Central Serous Chorioretinopathy: A Prospective, Randomized Study. Ophthalmic Surg Lasers Imaging Retina. 2017;48(7):564–71.
Zhao M, Zhang F, Chen Y, Dai H, Qu J, Dong C, Kang X, Liu Y, Yang L, Li Y, et al. A 50% vs 30% dose of verteporfin (photodynamic therapy) for acute central serous chorioretinopathy: one-year results of a randomized clinical trial. JAMA ophthalmology. 2015;133(3):333–40.
Smretschnig E, Ansari-Shahrezaei S, Moussa S, Glittenberg C, Krebs I, Binder S. Half-fluence photodynamic therapy in acute central serous chorioretinopathy. Retina (Philadelphia, Pa). 2012;32(10):2014–9.
Chan WM, Lai TY, Lai RY, Liu DT, Lam DS. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008;115(10):1756–65.
Kim M, Lee SC, Lee SJ. Intravitreal ranibizumab for acute central serous chorioretinopathy. Ophthalmologica Journal international d’ophtalmologie International journal of ophthalmology Zeitschrift fur Augenheilkunde. 2013;229(3):152–7.
Sun X, Shuai Y, Fang W, Li J, Ge W, Yuan S, Liu Q. Spironolactone versus observation in the treatment of acute central serous chorioretinopathy. Br J Ophthalmol. 2018;102(8):1060–5.
Zhou L, Chong V, Lai K, Huang C, Xu F, Gong Y, Youlidaxi M, Li T, Lu L, Jin C. A pilot prospective study of 577-nm yellow subthreshold micropulse laser treatment with two different power settings for acute central serous chorioretinopathy. Lasers Med Sci. 2019;34(7):1345–51.
Arora S, Sridharan P, Arora T, Chhabra M, Ghosh B. Subthreshold diode micropulse laser versus observation in acute central serous chorioretinopathy. Clin Exp Optom. 2019;102(1):79–85.
Gawęcki M, Jaszczuk-Maciejewska A, Jurska-Jaśko A, Grzybowski A. Functional and morphological outcome in patients with chronic central serous chorioretinopathy treated by subthreshold micropulse laser. Graefe’s arch clinical exp ophthal. 2017;255(12):2299–306.
Maruko I, Koizumi H, Hasegawa T, Arakawa H, Iida T. Subthreshold 577 nm micropulse laser treatment for central serous chorioretinopathy. PloS one. 2017;12(8):e0184112.
Özmert E, Demirel S, Yanık Ö, Batıoğlu F. Low-Fluence Photodynamic Therapy versus Subthreshold Micropulse Yellow Wavelength Laser in the Treatment of Chronic Central Serous Chorioretinopathy. Journal of ophthalmology. 2016;2016:3513794.
Zhou L, Lai K, Jin L, Huang C, Xu F, Gong Y, Li L, Zhu Z, Lu L, Jin C. Subthreshold Micropulse Laser vs. Conventional Laser for Central Serous Chorioretinopathy: A Randomized Controlled Clinical Trial. Frontiers med. 2021;8:682264.
Roisman L, Magalhães FP, Lavinsky D, Moraes N, Hirai FE, Cardillo JA, Farah ME. Micropulse diode laser treatment for chronic central serous chorioretinopathy: a randomized pilot trial. Ophthalmic Surg Lasers Imaging Retina. 2013;44(5):465–70.
Ntomoka CG, Rajesh B, Muriithi GM, Goud A, Chhablani J. Comparison of photodynamic therapy and navigated microsecond laser for chronic central serous chorioretinopathy. Eye (Lond). 2018;32(6):1079–86.
Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Experiment Ophthalmol. 2013;41(2):201–14.
Yannuzzi LA. Type-A behavior and central serous chorioretinopathy. Retina (Philadelphia, Pa). 1987;7(2):111–31.
Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol. 1984;68(11):815–20.
Levine R, Brucker AJ, Robinson F. Long-term follow-up of idiopathic central serous chorioretinopathy by fluorescein angiography. Ophthalmology. 1989;96(6):854–9.
Scholz P, Altay L, Fauser S. Comparison of subthreshold micropulse laser (577 nm) treatment and half-dose photodynamic therapy in patients with chronic central serous chorioretinopathy. Eye (Lond). 2016;30(10):1371–7.
Mainster MA. Wavelength selection in macular photocoagulation. Tissue optics, thermal effects, and laser systems. Ophthal. 1986;93(7):952–8.
Scholz P, Altay L, Fauser S. A Review of Subthreshold Micropulse Laser for Treatment of Macular Disorders. Adv Ther. 2017;34(7):1528–55.
Roca JA, Wu L, Fromow-Guerra J, Rodríguez FJ, Berrocal MH, Rojas S, Lima LH, Gallego-Pinazo R, Chhablani J, Arevalo JF, et al. Yellow (577 nm) micropulse laser versus half-dose verteporfin photodynamic therapy in eyes with chronic central serous chorioretinopathy: results of the Pan-American Collaborative Retina Study (PACORES) Group. Br J Ophthalmol. 2018;102(12):1696–700.
Sun Z, Huang Y, Nie C, Wang Z, Pei J, Lin B, Zhou R, Zhang J, Chong V, Liu X. Efficacy and safety of subthreshold micropulse laser compared with threshold conventional laser in central serous chorioretinopathy. Eye (Lond). 2020;34(9):1592–9.
Daruich A, Matet A, Dirani A, Gallice M, Nicholson L, Sivaprasad S, Behar-Cohen F. Oral Mineralocorticoid-Receptor Antagonists: Real-Life Experience in Clinical Subtypes of Nonresolving Central Serous Chorioretinopathy With Chronic Epitheliopathy. Translational vision science & technology. 2016;5(2):2.
Gergely R, Kovács I, Schneider M, Resch M, Papp A, Récsán Z, Nagy ZZ, Ecsedy M. MINERALOCORTICOID RECEPTOR ANTAGONIST TREATMENT IN BILATERAL CHRONIC CENTRAL SEROUS CHORIORETINOPATHY: A COMPARATIVE STUDY OF EXUDATIVE AND NONEXUDATIVE FELLOW EYES. Retina (Philadelphia, Pa). 2017;37(6):1084–91.
Salz DA, Pitcher JD 3rd, Hsu J, Regillo CD, Fineman MS, Elliott KS, Vander JF, Fischer DH, Spirn MJ. Oral eplerenone for treatment of chronic central serous chorioretinopathy: a case series. Ophthalmic Surg Lasers Imaging Retina. 2015;46(4):439–44.
Bousquet E, Beydoun T, Rothschild PR, Bergin C, Zhao M, Batista R, Brandely ML, Couraud B, Farman N, Gaudric A, et al. SPIRONOLACTONE FOR NONRESOLVING CENTRAL SEROUS CHORIORETINOPATHY: A RANDOMIZED CONTROLLED CROSSOVER STUDY. Retina (Philadelphia, Pa). 2015;35(12):2505–15.
Wood EH, Karth PA, Sanislo SR, Moshfeghi DM, Palanker DV. NONDAMAGING RETINAL LASER THERAPY FOR TREATMENT OF CENTRAL SEROUS CHORIORETINOPATHY: What is the Evidence? Retina (Philadelphia, Pa). 2017;37(6):1021–33.
Gawęcki M. Micropulse Laser Treatment of Retinal Diseases. J clin med. 2019;8(2):242.
Burumcek E, Mudun A, Karacorlu S, Arslan MO. Laser photocoagulation for persistent central serous retinopathy: results of long-term follow-up. Ophthalmology. 1997;104(4):616–22.
Chan WM, Lam DS, Lai TY, Tam BS, Liu DT, Chan CK. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003;87(12):1453–8.
Tseng CC, Chen SN. Long-term efficacy of half-dose photodynamic therapy on chronic central serous chorioretinopathy. Br J Ophthalmol. 2015;99(8):1070–7.
Salehi M, Wenick AS, Law HA, Evans JR, Gehlbach P. Interventions for central serous chorioretinopathy: a network meta-analysis. Cochrane database syst rev. 2015;2015(12):Cd011841.
Mataftsi A, Koutsimpogeorgos D, Brazitikos P, Ziakas N, Haidich AB. Is conversion of decimal visual acuity measurements to logMAR values reliable? Graefe’s arch clin exp ophthalmo. 2019;257(7):1513–7.
Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(1):261–6.
Acknowledgements
We gratefully acknowledge all of the participants in this study.
Funding
The authors do not have any sources of support, including sponsorship or sources of material not available commercially.
Author information
Authors and Affiliations
Contributions
HL: concept and design, data acquisition and analysis, drafting of the manuscript, revision of the manuscript. MXL: contributed to the conception and design of the research, critical revision of the manuscript. QHH: critical revision of the manuscript. XL: concept and design, data acquisition and analysis, critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Wuhan Aier Eye Hanyang Hospital (HYEYE2021IRB01). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declares that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Long, H., Liu, M., Hu, Q. et al. 577 nm subthreshold micropulse laser treatment for acute central serous chorioretinopathy: a comparative study. BMC Ophthalmol 22, 105 (2022). https://doi.org/10.1186/s12886-022-02330-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-022-02330-0